Spontaneous Ultraslow Na+ Fluctuations in the Neonatal Mouse Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Tissue Slices
2.2. Fluorescence-Based Ion Imaging
2.3. Determination of Baseline [Na+]i and Electrophysiology
2.4. Data Analysis and Statistics
3. Results
3.1. Neonatal Cells Show Spontaneous Na+ Fluctuations
3.2. Fluctuations Are Developmentally Regulated
3.3. Fluctuations Are Not Causally Linked to Ca2+ Signalling
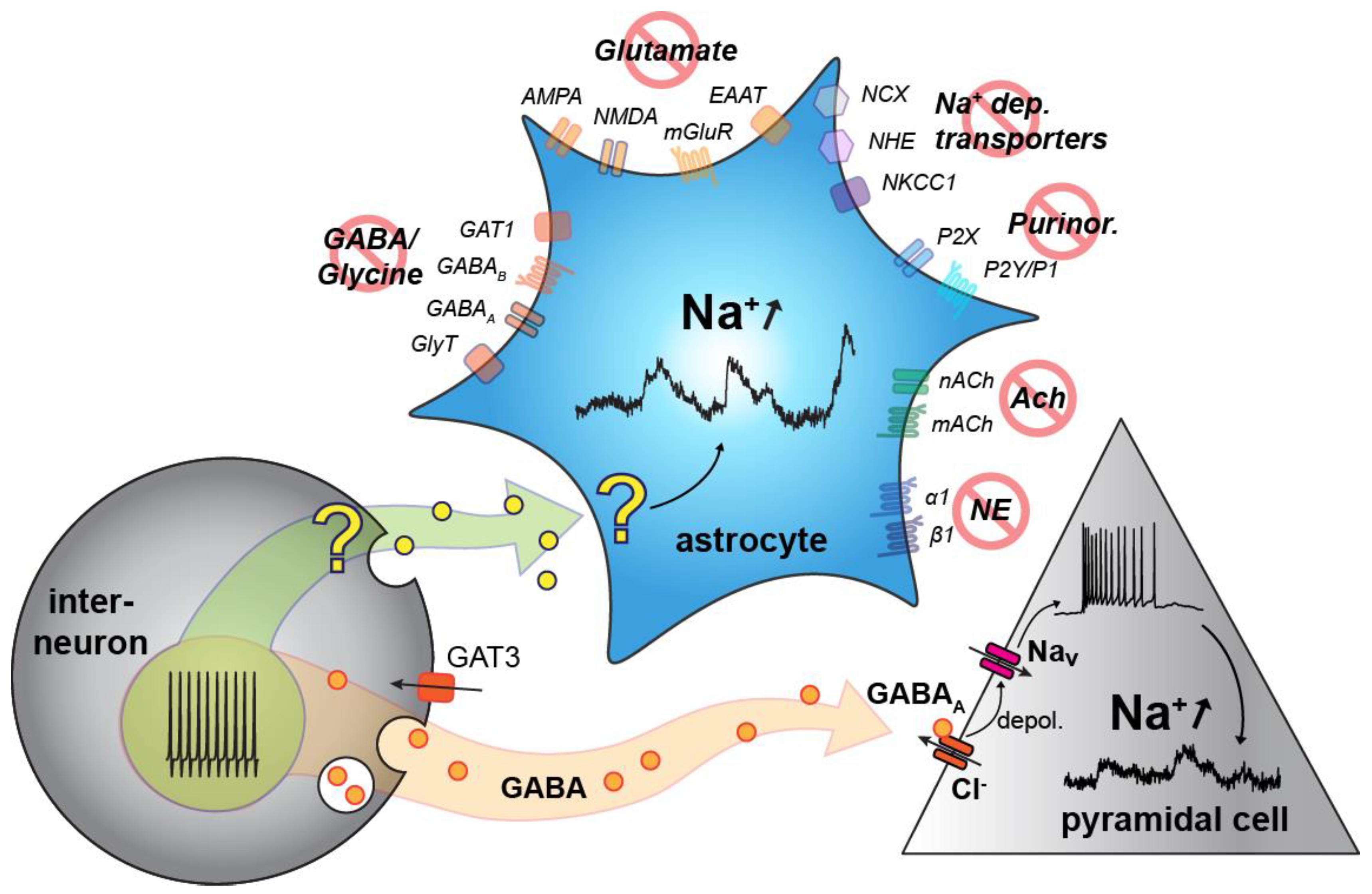
3.4. Origin of Neuronal Na+ Fluctuations
3.5. Pharmacology of Astrocytic Na+ Fluctuations
3.6. Astrocyte Fluctuations Are Not Bound to pH Regulation
4. Discussion
4.1. Neuronal Na+ Fluctuations
4.2. Astrocytic Na+ Fluctuations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spitzer, N.C. Electrical activity in early neuronal development. Nature 2006, 444, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, H.J.; Sinning, A.; Yang, J.W.; Reyes-Puerta, V.; Stuttgen, M.C.; Kirischuk, S.; Kilb, W. Spontaneous neuronal activity in developing neocortical networks: From single cells to large-scale interactions. Front. Neural Circuits 2016, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griguoli, M.; Cherubini, E. Early correlated network activity in the hippocampus: Its putative role in shaping neuronal circuits. Front. Cell. Neurosci. 2017, 11, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garaschuk, O.; Linn, J.; Eilers, J.; Konnerth, A. Large-scale oscillatory calcium waves in the immature cortex. Nat. Neurosci. 2000, 3, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Cherubini, E.; Corradetti, R.; Gaiarsa, J.L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 1989, 416, 303–325. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Khazipov, R.; Leinekugel, X.; Caillard, O.; Gaiarsa, J.L. GABAa, NMDA and AMPA receptors: A developmentally regulated ‘menage a trois’. Trends Neurosci. 1997, 20, 523–529. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Developing networks play a similar melody. Trends Neurosci. 2001, 24, 353–360. [Google Scholar] [CrossRef]
- Rivera, C.; Voipio, J.; Kaila, K. Two developmental switches in GABAergic signalling: The K+-Cl- cotransporter KCC2 and carbonic anhydrase cavii. J. Physiol. 2005, 562, 27–36. [Google Scholar] [CrossRef]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Bordey, A. The astrocyte odyssey. Prog. Neurobiol. 2008, 86, 342–367. [Google Scholar] [CrossRef]
- Schreiner, A.E.; Durry, S.; Aida, T.; Stock, M.C.; Rüther, U.; Tanaka, K.; Rose, C.R.; Kafitz, K.W. Laminar and subcellular heterogeneity of GLAST and GLT-1 immunoreactivity in the developing postnatal mouse hippocampus. J. Comp. Neurol. 2014, 522, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Koulakoff, A.; Roux, L.; Holcman, D.; Rouach, N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010, 11, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Kressin, K.; Kuprijanova, E.; Jabs, R.; Seifert, G.; Steinhauser, C. Developmental regulation of Na+ and K+ conductances in glial cells of mouse hippocampal brain slices. Glia 1995, 15, 173–187. [Google Scholar] [CrossRef]
- Bordey, A.; Sontheimer, H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J. Neurophysiol. 1997, 78, 461–477. [Google Scholar] [CrossRef]
- Seifert, G.; Huttmann, K.; Binder, D.K.; Hartmann, C.; Wyczynski, A.; Neusch, C.; Steinhauser, C. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J. Neurosci. 2009, 29, 7474–7488. [Google Scholar] [CrossRef]
- Zhou, M.; Schools, G.P.; Kimelberg, H.K. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: Mature astrocytes are electrophysiologically passive. J. Neurophysiol. 2006, 95, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Kafitz, K.W.; Meier, S.D.; Stephan, J.; Rose, C.R. Developmental profile and properties of sulforhodamine 101-labeled glial cells in acute brain slices of rat hippocampus. J. Neurosci. Methods 2008, 169, 84–92. [Google Scholar] [CrossRef]
- Meier, S.D.; Kafitz, K.W.; Rose, C.R. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia 2008, 56, 1127–1137. [Google Scholar] [CrossRef]
- Scimemi, A. Structure, function, and plasticity of GABA transporters. Front. Cell. Neurosci. 2014, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Volterra, A.; Liaudet, N.; Savtchouk, I. Astrocyte Ca(2+) signalling: An unexpected complexity. Nat. Rev. Neurosci. 2014, 15, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusakov, D.A. Disentangling calcium-driven astrocyte physiology. Nat. Rev. Neurosci. 2015, 16, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Aguado, F.; Espinosa-Parilla, J.; Carmona, M.; Soriano, E. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J. Neurosci. 2002, 22, 9430–9444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacar, B.; Young, S.Z.; Platel, J.C.; Bordey, A. Gap junction-mediated calcium waves define communication networks among murine postnatal neural progenitor cells. Eur. J. Neurosci. 2011, 34, 1895–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schousboe, A.; Sarup, A.; Bak, L.K.; Waagepetersen, H.S.; Larsson, O.M. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem. Int. 2004, 45, 521–527. [Google Scholar] [CrossRef]
- Chatton, J.Y.; Pellerin, L.; Magistretti, P.J. GABA uptake into astrocytes is not associated with significant metabolic cost: Implications for brain imaging of inhibitory transmission. Proc. Natl. Acad. Sci. USA 2003, 100, 12456–12461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziemens, D.; Oschmann, F.; Gerkau, N.J.; Rose, C.R. Heterogeneity of activity-induced sodium transients between astrocytes of the mouse hippocampus and neocortex: Mechanisms and consequences. J. Neurosci. 2019, 39, 2620–2634. [Google Scholar] [CrossRef] [Green Version]
- Karus, C.; Gerkau, N.J.; Rose, C.R. Differential contribution of GLAST and GLT-1 to network sodium signaling in the early postnatal hippocampus Opera Med. Physiol. 2017, 3, 71–83. [Google Scholar] [CrossRef]
- Close, B.; Banister, K.; Baumans, V.; Bernoth, E.M.; Bromage, N.; Bunyan, J.; Erhardt, W.; Flecknell, P.; Gregory, N.; Hackbarth, H.; et al. Recommendations for euthanasia of experimental animals: Part 2. Dgxt of the european commission. Lab. Anim. 1997, 31, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Gerkau, N.J.; Lerchundi, R.; Nelson, J.S.E.; Lantermann, M.; Meyer, J.; Hirrlinger, J.; Rose, C.R. Relation between activity-induced intracellular sodium transients and ATP dynamics in mouse hippocampal neurons. J. Physiol. 2019, 597, 5687–5705. [Google Scholar] [CrossRef] [Green Version]
- Wallraff, A.; Kohling, R.; Heinemann, U.; Theis, M.; Willecke, K.; Steinhauser, C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 2006, 26, 5438–5447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henneberger, C.; Rusakov, D.A. Monitoring local synaptic activity with astrocytic patch pipettes. Nat. Protoc. 2012, 7, 2171–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, J.; Rose, C.R. Synaptically induced sodium signals in hippocampal astrocytes in situ. J. Physiol. 2009, 587, 5859–5877. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Gerkau, N.J.; Derouiche, A.; Kleinhans, C.; Moshrefi-Ravasdjani, B.; Fredrich, M.; Kafitz, K.W.; Seifert, G.; Steinhauser, C.; Rose, C.R. Rapid sodium signaling couples glutamate uptake to breakdown of atp in perivascular astrocyte endfeet. Glia 2017, 65, 293–308. [Google Scholar] [CrossRef]
- Gerkau, N.J.; Rakers, C.; Durry, S.; Petzold, G.; Rose, C.R. Reverse NCX attenuates cellular sodium loading in metabolically compromised cortex. Cereb. Cortex 2018, 28, 4264–4280. [Google Scholar] [CrossRef]
- Mondragao, M.A.; Schmidt, H.; Kleinhans, C.; Langer, J.; Kafitz, K.W.; Rose, C.R. Extrusion versus diffusion: Mechanisms for recovery from sodium loads in mouse CA1 pyramidal neurons. J. Physiol. 2016, 594, 5507–5527. [Google Scholar] [CrossRef] [Green Version]
- Garaschuk, O.; Hanse, E.; Konnerth, A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J. Physiol. 1998, 507 Pt 1, 219–236. [Google Scholar] [CrossRef]
- Schipke, C.G.; Heidemann, A.; Skupin, A.; Peters, O.; Falcke, M.; Kettenmann, H. Temperature and nitric oxide control spontaneous calcium transients in astrocytes. Cell Calcium 2008, 43, 285–295. [Google Scholar] [CrossRef]
- Schools, G.P.; Zhou, M.; Kimelberg, H.K. Development of gap junctions in hippocampal astrocytes: Evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. J. Neurophysiol. 2006, 96, 1383–1392. [Google Scholar] [CrossRef]
- Rose, C.R.; Ransom, B.R. pH Regulation in Mammalian Glia; Wiley-Liss, Inc.: New York, NY, USA, 1998; pp. 253–275. [Google Scholar]
- Rose, C.R.; Ransom, B.R. Intracellular sodium homeostasis in rat hippocampal astrocytes. J. Physiol. 1996, 491, 291–305. [Google Scholar] [CrossRef]
- Theparambil, S.M.; Naoshin, Z.; Thyssen, A.; Deitmer, J.W. Reversed electrogenic sodium bicarbonate cotransporter 1 is the major acid loader during recovery from cytosolic alkalosis in mouse cortical astrocytes. J. Physiol. 2015, 593, 3533–3547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leinekugel, X.; Khalilov, I.; McLean, H.; Caillard, O.; Gaiarsa, J.L.; Ben-Ari, Y.; Khazipov, R. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv. Neurol. 1999, 79, 189–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Achilles, K.; Okabe, A.; Ikeda, M.; Shimizu-Okabe, C.; Yamada, J.; Fukuda, A.; Luhmann, H.J.; Kilb, W. Kinetic properties of Cl uptake mediated by Na+-dependent K+-2Cl cotransport in immature rat neocortical neurons. J. Neurosci. 2007, 27, 8616–8627. [Google Scholar] [CrossRef] [Green Version]
- Brumback, A.C.; Staley, K.J. Thermodynamic regulation of NKCC1-mediated Cl-cotransport underlies plasticity of GABA(a) signaling in neonatal neurons. J. Neurosci. 2008, 28, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Sipila, S.T.; Huttu, K.; Yamada, J.; Afzalov, R.; Voipio, J.; Blaesse, P.; Kaila, K. Compensatory enhancement of intrinsic spiking upon NKCC1 disruption in neonatal hippocampus. J. Neurosci. 2009, 29, 6982–6988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirmse, K.; Kummer, M.; Kovalchuk, Y.; Witte, O.W.; Garaschuk, O.; Holthoff, K. GABA depolarizes immature neurons and inhibits network activity in the neonatal neocortex in vivo. Nat. Commun. 2015, 6, 7750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilberter, M. Reality of inhibitory GABA in neonatal brain: Time to rewrite the textbooks? J. Neurosci. 2016, 36, 10242–10244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adelsberger, H.; Garaschuk, O.; Konnerth, A. Cortical calcium waves in resting newborn mice. Nat. Neurosci. 2005, 8, 988–990. [Google Scholar] [CrossRef]
- Vargas, E.; Petrou, S.; Reid, C.A. Genetic and pharmacological modulation of giant depolarizing potentials in the neonatal hippocampus associates with increased seizure susceptibility. J. Physiol. 2013, 591, 57–65. [Google Scholar] [CrossRef]
- Hartley, E.J.; Seeman, P. Development of receptors for dopamine and noradrenaline in rat brain. Eur. J. Pharmacol. 1983, 91, 391–397. [Google Scholar] [CrossRef]
- Hillman, K.L.; Lei, S.; Doze, V.A.; Porter, J.E. Alpha-1a adrenergic receptor activation increases inhibitory tone in CA1 hippocampus. Epilepsy Res. 2009, 84, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.W.; Hanganu-Opatz, I.L.; Sun, J.J.; Luhmann, H.J. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J. Neurosci. 2009, 29, 9011–9025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parri, H.R.; Gould, T.M.; Crunelli, V. Spontaneous astrocytic Ca2+ oscillations in situ drive nmdar-mediated neuronal excitation. Nat. Neurosci. 2001, 4, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Nett, W.J.; Oloff, S.H.; McCarthy, K.D. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J. Neurophysiol. 2002, 87, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Zur Nieden, R.; Deitmer, J.W. The role of metabotropic glutamate receptors for the generation of calcium oscillations in rat hippocampal astrocytes in situ. Cereb. Cortex 2006, 16, 676–687. [Google Scholar] [CrossRef]
- Wang, T.F.; Zhou, C.; Tang, A.H.; Wang, S.Q.; Chai, Z. Cellular mechanism for spontaneous calcium oscillations in astrocytes. Acta Pharmacol. Sin. 2006, 27, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Bindocci, E.; Savtchouk, I.; Liaudet, N.; Becker, D.; Carriero, G.; Volterra, A. Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science 2017, 356, eaai8185. [Google Scholar] [CrossRef] [Green Version]
- Shigetomi, E.; Bushong, E.A.; Haustein, M.D.; Tong, X.; Jackson-Weaver, O.; Kracun, S.; Xu, J.; Sofroniew, M.V.; Ellisman, M.H.; Khakh, B.S. Imaging calcium microdomains within entire astrocyte territories and endfeet with gcamps expressed using adeno-associated viruses. J. Gen. Physiol. 2013, 141, 633–647. [Google Scholar] [CrossRef]
- Srinivasan, R.; Huang, B.S.; Venugopal, S.; Johnston, A.D.; Chai, H.; Zeng, H.; Golshani, P.; Khakh, B.S. Ca(2+) signaling in astrocytes from IP3r2(-/-) mice in brain slices and during startle responses in vivo. Nat. Neurosci. 2015, 18, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Khakh, B.S.; McCarthy, K.D. Astrocyte calcium signaling: From observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol. 2015, 7, a020404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankenhaeuser, B.; Hodgkin, A.L. The action of calcium on the electrical properties of squid axons. J. Physiol. 1957, 137, 218–244. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.M. Distinguishing surface effects of calcium ion from pore-occupancy effects in Na+ channels. Proc. Natl. Acad. Sci. USA 1999, 96, 4158–4163. [Google Scholar] [CrossRef] [Green Version]
- Chesler, M. Regulation and modulation of pH in the brain. Physiol. Rev. 2003, 83, 1183–1221. [Google Scholar] [CrossRef]
- Lerma, J.; Herreras, O.; Herranz, A.S.; Munoz, D.; del Rio, R.M. In vivo effects of nipecotic acid on levels of extracellular gaba and taurine, and hippocampal excitability. Neuropharmacology 1984, 23, 595–598. [Google Scholar] [CrossRef]
- Kersante, F.; Rowley, S.C.; Pavlov, I.; Gutierrez-Mecinas, M.; Semyanov, A.; Reul, J.M.; Walker, M.C.; Linthorst, A.C. A functional role for both-aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J. Physiol. 2013, 591, 2429–2441. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tu, P.; Bonet, L.; Aubrey, K.R.; Supplisson, S. Cytosolic transmitter concentration regulates vesicle cycling at hippocampal GABAergic terminals. Neuron 2013, 80, 143–158. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Miyoshi, S.; Kaneko, A.; Copenhagen, D.R. Actions of nipecotic acid and skf89976a on GABA transporter in cone-driven horizontal cells dissociated from the catfish retina. Jpn. J. Physiol. 1995, 45, 457–473. [Google Scholar] [CrossRef]
- Madtes, P., Jr.; Redburn, D. Metabolism of [3 h] nipecotic acid in the rabbit retina. J. Neurochem. 1985, 44, 1520–1523. [Google Scholar] [CrossRef]
- Borden, L.A. GABA transporter heterogeneity: Pharmacology and cellular localization. Neurochem. Int. 1996, 29, 335–356. [Google Scholar] [CrossRef]
- Tritsch, N.X.; Granger, A.J.; Sabatini, B.L. Mechanisms and functions of GABA co-release. Nat. Rev. Neurosci. 2016, 17, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Nusbaum, M.P.; Blitz, D.M.; Swensen, A.M.; Wood, D.; Marder, E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001, 24, 146–154. [Google Scholar] [CrossRef]
- Jonas, P.; Bischofberger, J.; Sandkuhler, J. Corelease of two fast neurotransmitters at a central synapse. Science 1998, 281, 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattaneo, S.; Ripamonti, M.; Bedogni, F.; Sessa, A.; Taverna, S. Somatostatin-expressing interneurons co-release GABA and glutamate onto different postsynaptic targets in the striatum. BioRxiv 2019, 566984. [Google Scholar] [CrossRef]
- Choi, H.J.; Sun, D.; Jakobs, T.C. Astrocytes in the optic nerve head express putative mechanosensitive channels. Mol. Vis. 2015, 21, 749–766. [Google Scholar]
- Velasco-Estevez, M.; Mampay, M.; Boutin, H.; Chaney, A.; Warn, P.; Sharp, A.; Burgess, E.; Moeendarbary, E.; Dev, K.K.; Sheridan, G.K. Infection augments expression of mechanosensing piezo1 channels in amyloid plaque-reactive astrocytes. Front. Aging Neurosci. 2018, 10, 332. [Google Scholar] [CrossRef] [Green Version]
- Thrane, A.S.; Thrane, V.R.; Zeppenfeld, D.; Lou, N.; Xu, Q.; Nagelhus, E.A.; Nedergaard, M. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc. Natl. Acad. Sci. USA 2012, 109, 18974–18979. [Google Scholar] [CrossRef] [Green Version]
- Khazipov, R.; Luhmann, H.J. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006, 29, 414–418. [Google Scholar] [CrossRef]




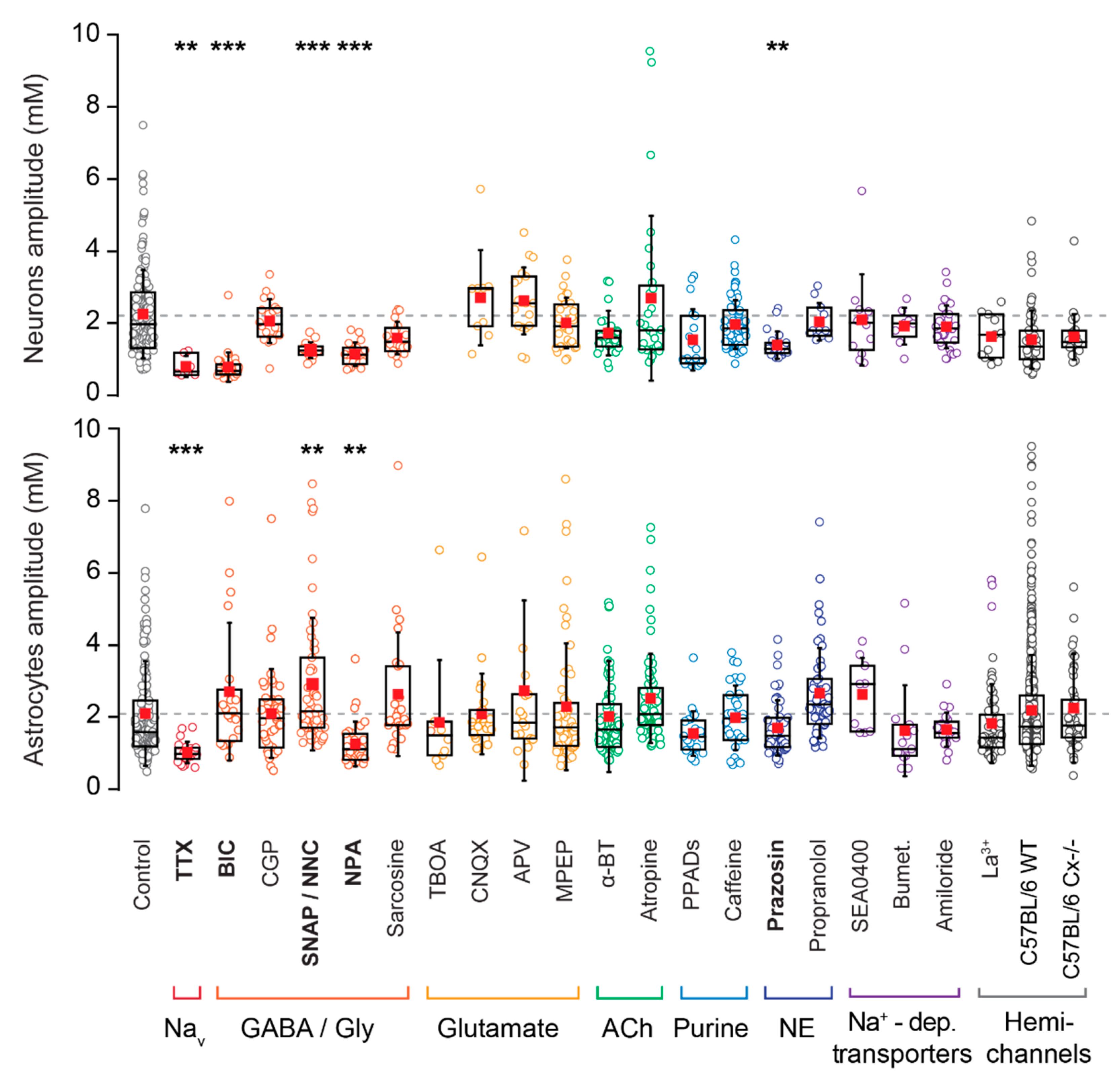

| Target Group | Blocker | Target | Solvent | Conc. (µM) | Manufacturer | Catalogue Number |
|---|---|---|---|---|---|---|
| Glutamatergic | MPEP | mGluR5 | DMSO | 25 | Tocris | 1212 |
| TFB-TBOA | EAATs | DMSO | 1 | Tocris | 2532 | |
| APV | NMDA | NaHCO3 | 100 | Cayman Chem. | 14,540 | |
| CNQX | AMPA | DMSO | 25 | Cayman Chem. | 14,618 | |
| GABAergic/ Glycinergic | Bicuculline | GABAA | σ H2O | 10 | Sigma-Aldrich | 14,343 |
| CGP-55845 | GABAB | σ H2O | 5 | Sigma-Aldrich | SML0594 | |
| NNC-711 | GAT1 | DMSO | 100 | Tocris | 1779 | |
| SNAP-5114 | GAT2/3 | DMSO | 100 | Sigma-Aldrich | S1069 | |
| NPA | GAT1/2/3 | σ H2O | 100 | Tocris | 0236 | |
| Sarcosine | GlyTs | σ H2O | 500 | Sigma-Aldrich | 131,776 | |
| Cholinergic | α-Bungarotoxin | α7 nAChR | σ H2O | 0.1 | Sigma-Aldrich | T0195 |
| Atropine | mAChR | σ H2O | 5 | Sigma-Aldrich | A0132 | |
| Purinergic | PPADs | P2X/Y | σ H2O | 20 | Sigma-Aldrich | P178 |
| Caffeine | P1 | σ H2O | 100 | Sigma-Aldrich | C0750 | |
| Adrenergic | Prazosin | α1 receptor | σ H2O | 0.2 | Sigma-Aldrich | P7791 |
| Propranolol | β receptor | σ H2O | 10 | Sigma-Aldrich | P0084 | |
| Na+ dependant transporters | TTX | Nav | σ H2O | 0.5 | BioTrend | BN0518 |
| SEA0400 | NCX | DMSO | 10 | MCE | HY15515 | |
| Bumetanide | NKCC1 | DMSO | 40 | BioTrend | BG0113 | |
| pH | Amiloride | NHE | σ H2O | 50 | Sigma-Aldrich | BP008 |
| Hemichannels | La3+ | TRP/Hemicha. | σ H2O | 50 | Merck | 203,521 |
| Neurons | Astrocytes | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells (n) | % Cells (n) Active | Fluctuation (n) | mM Mean | mM SD | p Values | Min Mean | Min SD | Cells (n) | % Active | Fluctuation (n) | mM Mean | mM SD | p Values | Min Mean | Min SD | |||
| Control | 412 | 21.6 | 166 | 2.2 | 1.2 | 9.2 | 4.1 | 213 | 43 | 166 | 2.1 | 1.4 | 8.6 | 3.2 | ||||
| TTX | 94 | 7.4 | 7 | 0.7 | 0.3 | ** | 8.2 | 3.7 | 39 | 35.9 | 23 | 1 | 0.3 | *** | 8.7 | 4.1 | ||
| BIC | 76 | 19.7 | 32 | 0.8 | 1 | *** | 6.6 | 2.3 | 22 | 59.1 | 20 | 2.7 | 1.9 | 0.06 | 8.1 | 2.3 | ||
| CGP | 101 | 12.9 | 20 | 2 | 0.6 | 0.48 | 10.9 | 3.5 | 49 | 44.9 | 42 | 2.1 | 1.2 | 0.78 | 9.6 | 4.6 | ||
| SNAP/NNC | 53 | 22.6 | 17 | 1.1 | 0.2 | *** | 10.2 | 3.1 | 86 | 34.9 | 33 | 3.2 | 2.0 | ** | 10.8 | 6.2 | ||
| NPA | 70 | 15.7 | 20 | 1 | 0.3 | *** | 10.7 | 3.8 | 43 | 32.6 | 31 | 1.3 | 0.6 | ** | 9.4 | 3.6 | ||
| Sarcosine | 74 | 20.3 | 20 | 1.5 | 0.5 | 0.02 | 11.3 | 3.7 | 62 | 21 | 25 | 2.6 | 1.7 | 0.06 | 8.5 | 3.5 | ||
| TFB-TBOA | 36 | 27.8 | 10 | 1.9 | 1.7 | 0.69 | 8.5 | 3.7 | ||||||||||
| CNQX | 130 | 6.2 | 9 | 2.6 | 1.3 | 0.29 | 9.6 | 4.1 | 45 | 37.8 | 25 | 2.1 | 1.1 | 0.86 | 8.9 | 3.1 | ||
| APV | 156 | 10.3 | 19 | 2.5 | 0.9 | 0.22 | 10.9 | 5.2 | 52 | 28.8 | 19 | 2.7 | 2.5 | 0.34 | 13.1 | 4.9 | ||
| MPEP | 117 | 16.2 | 30 | 1.9 | 0.7 | 0.28 | 9.5 | 3.8 | 26 | 46.2 | 53 | 3.9 | 2.2 | 0.36 | 9.1 | 6.2 | ||
| α-BT | 118 | 11.9 | 29 | 1.6 | 0.6 | 0.02 | 10.1 | 3.7 | 43 | 67.4 | 89 | 2 | 1.5 | 0.93 | 7.3 | 3.7 | ||
| Atropine | 124 | 16.1 | 28 | 2.6 | 1.3 | 0.14 | 8.5 | 2.9 | 73 | 49.3 | 74 | 2.5 | 1.2 | 0.02 | 7.7 | 2.9 | ||
| PPADs | 70 | 11.4 | 19 | 1.4 | 0.9 | 0.01 | 10.7 | 4.9 | 57 | 24.6 | 24 | 1.6 | 0.6 | 0.1 | 8 | 2.4 | ||
| Caffeine | 142 | 22.5 | 75 | 1.9 | 0.7 | 0.05 | 9.4 | 4.8 | 32 | 62.5 | 33 | 2 | 0.9 | 0.84 | 8.8 | 3.2 | ||
| Prazosin | 111 | 13.5 | 21 | 1.3 | 0.4 | ** | 9.7 | 3.7 | 81 | 40.7 | 50 | 1.7 | 0.8 | 0.18 | 10.4 | 4.6 | ||
| Propranolol | 123 | 7.3 | 11 | 1.9 | 0.5 | 0.55 | 11.4 | 4.3 | 59 | 38.9 | 54 | 2.7 | 1.2 | 0.01 | 8.7 | 4.1 | ||
| SEA0400 | 85 | 11.7 | 12 | 2 | 1.3 | 0.65 | 8.8 | 3.1 | 18 | 44.4 | 9 | 2.6 | 1 | 0.21 | 8 | 1.4 | ||
| Bumet | 132 | 6.1 | 8 | 1.8 | 0.5 | 0.44 | 11 | 3.1 | 29 | 37.9 | 15 | 1.6 | 1.3 | 0.28 | 8.5 | 3.5 | ||
| Amiloride | 162 | 11.1 | 31 | 1.8 | 0.6 | 0.11 | 8.7 | 2.7 | 50 | 28 | 20 | 1.7 | 0.5 | 0.34 | 8.8 | 3.5 | ||
| La3+ | 144 | 5.2 | 8 | 1.8 | 0.6 | 0.43 | 11.9 | 5.3 | 84 | 40.5 | 74 | 2.0 | 1.2 | 0.71 | 8.4 | 3.4 | ||
| C57Bl/6 WT | 460 | 11.1 | 66 | 1.5 | 0.8 | 10.8 | 4.9 | 548 | 51.1 | 620 | 2.1 | 1.5 | 8.7 | 4.0 | ||||
| Cx -/- | 77 | 18.2 | 30 | 1.6 | 0.6 | 0.65 | 11.4 | 5.6 | 46 | 60.1 | 58 | 2.2 | 1.5 | 0.77 | 8.7 | 4.3 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felix, L.; Ziemens, D.; Seifert, G.; Rose, C.R. Spontaneous Ultraslow Na+ Fluctuations in the Neonatal Mouse Brain. Cells 2020, 9, 102. https://doi.org/10.3390/cells9010102
Felix L, Ziemens D, Seifert G, Rose CR. Spontaneous Ultraslow Na+ Fluctuations in the Neonatal Mouse Brain. Cells. 2020; 9(1):102. https://doi.org/10.3390/cells9010102
Chicago/Turabian StyleFelix, Lisa, Daniel Ziemens, Gerald Seifert, and Christine R. Rose. 2020. "Spontaneous Ultraslow Na+ Fluctuations in the Neonatal Mouse Brain" Cells 9, no. 1: 102. https://doi.org/10.3390/cells9010102
APA StyleFelix, L., Ziemens, D., Seifert, G., & Rose, C. R. (2020). Spontaneous Ultraslow Na+ Fluctuations in the Neonatal Mouse Brain. Cells, 9(1), 102. https://doi.org/10.3390/cells9010102






