The Singularity of the Drosophila Male Germ Cell Centriole: The Asymmetric Distribution of Sas4 and Sas6
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Strains
2.2. Reagents
2.3. Culture and Drug Treatment Experiments
2.4. Immunofluorescence Staining
2.5. Transmission Electron Microscopy
2.6. Image Acquisition and Data Analysis
3. Results
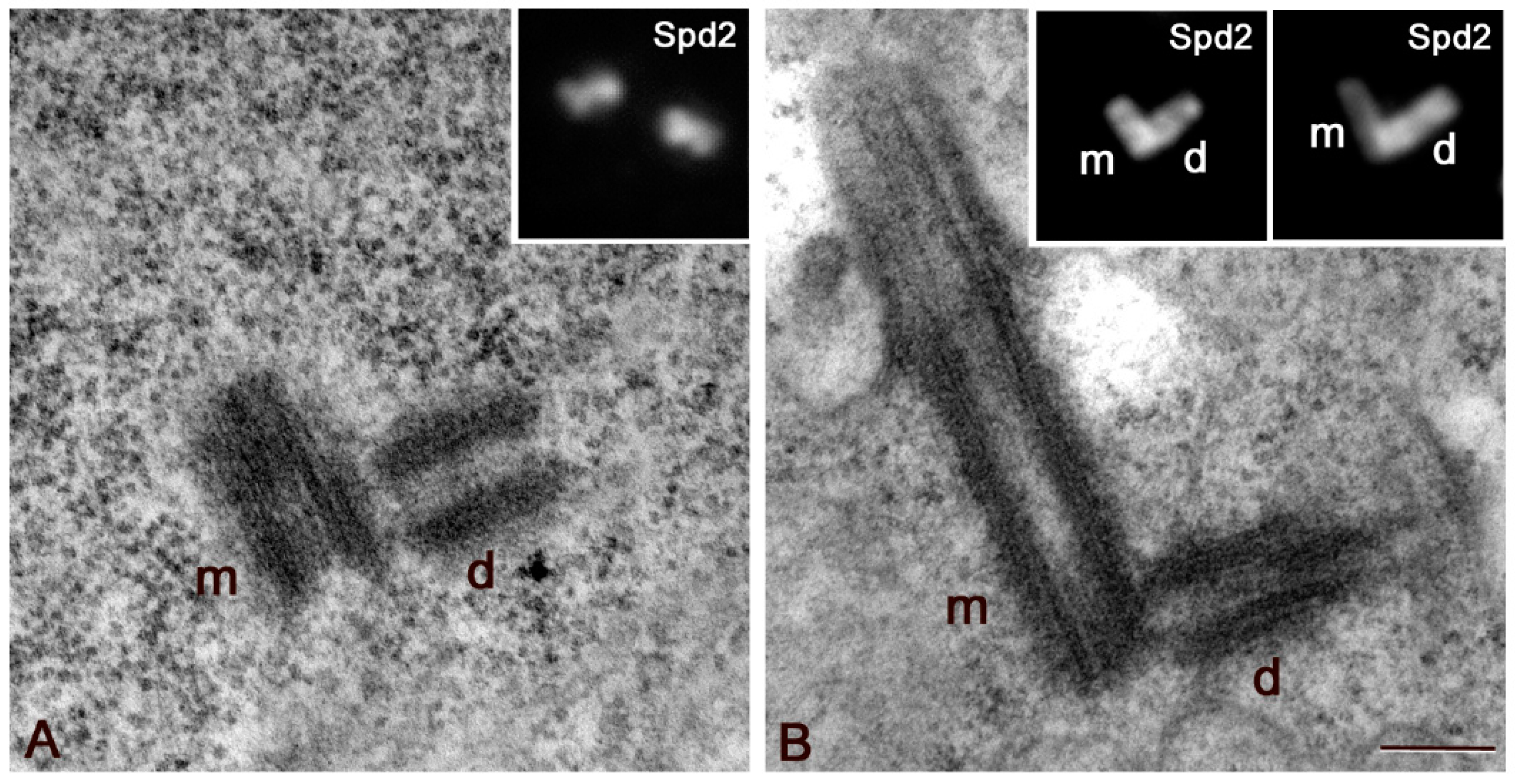
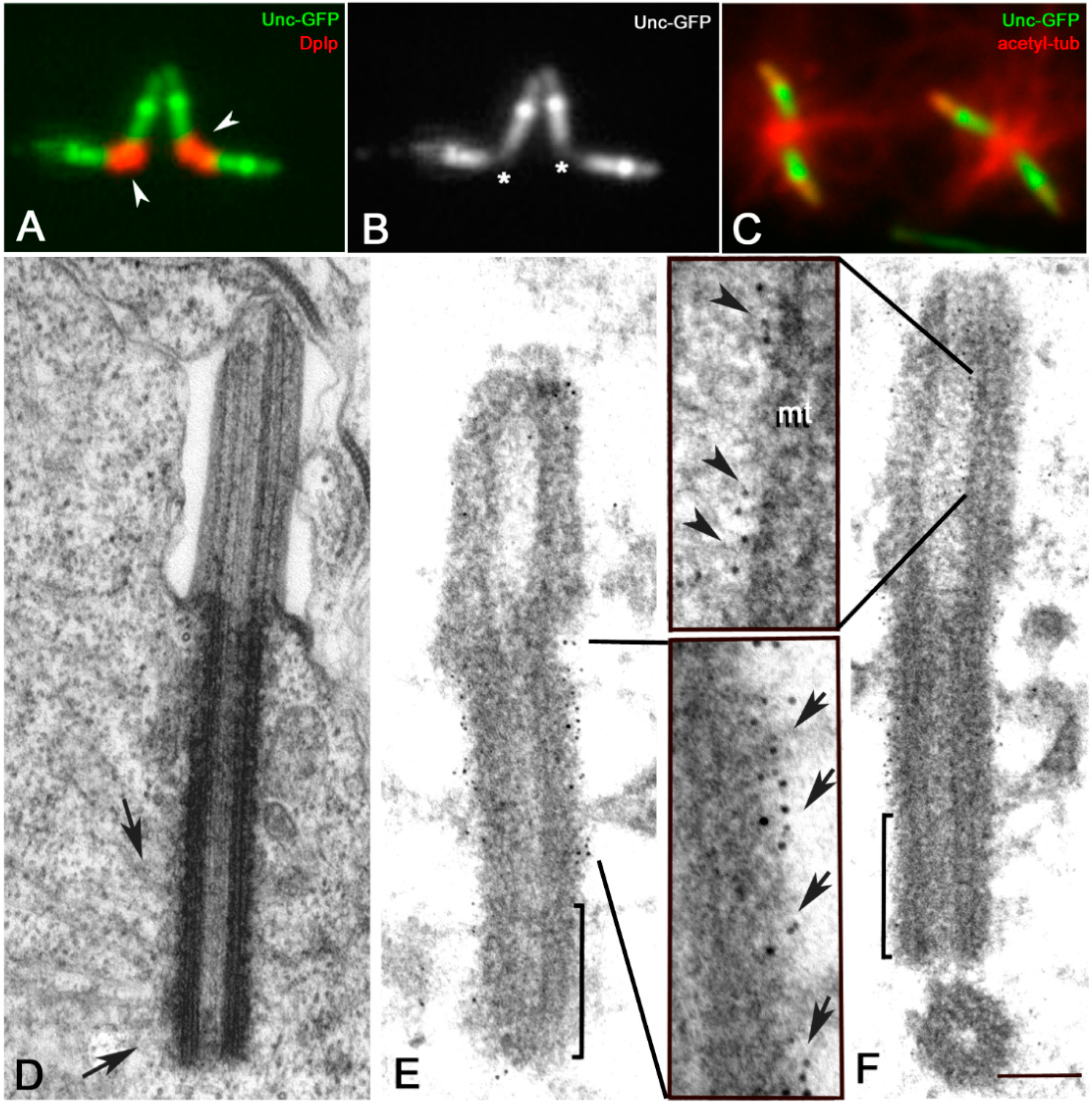
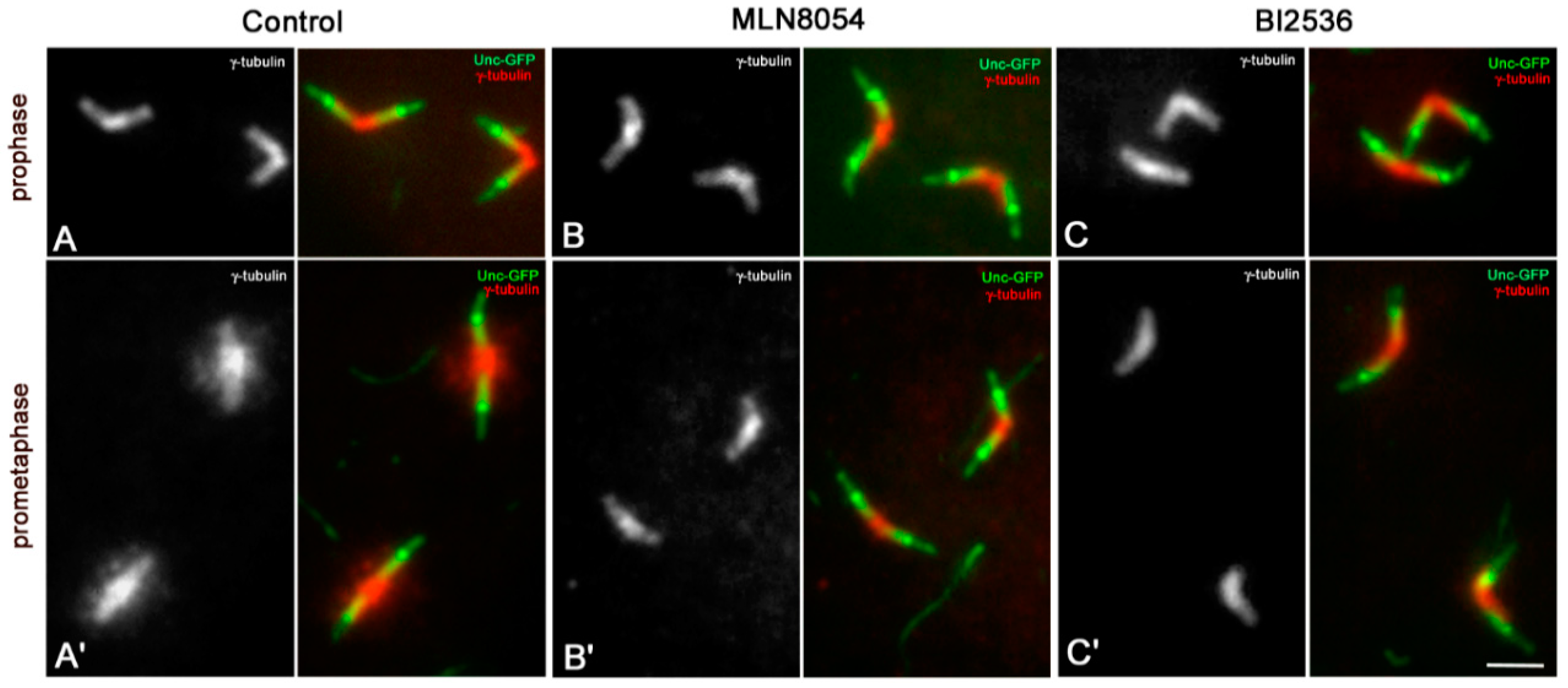
3.1. The Proximal Region of the Giant Drosophila Spermatocyte Centriole Is the Main Site for Microtubule Nucleation
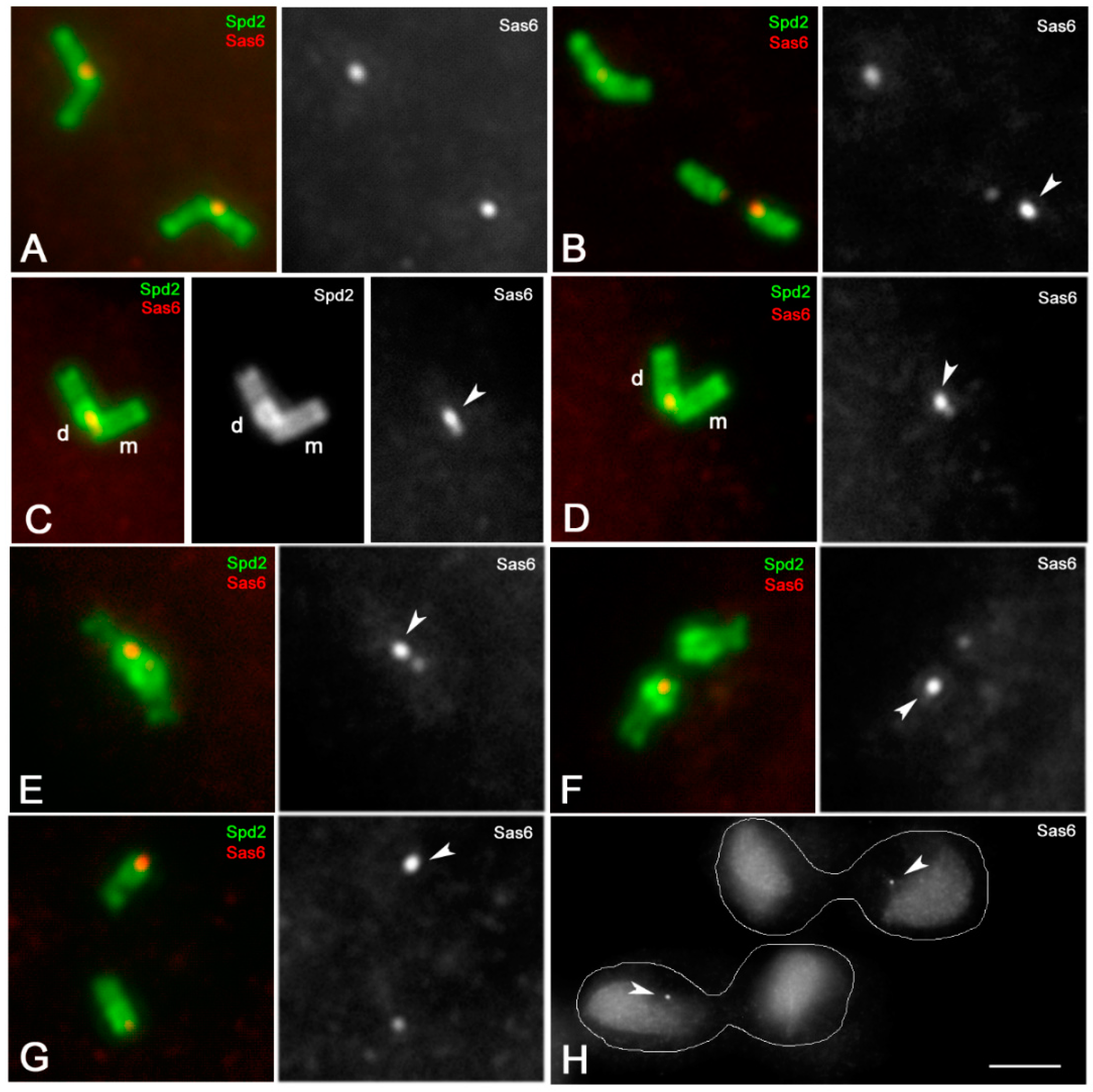
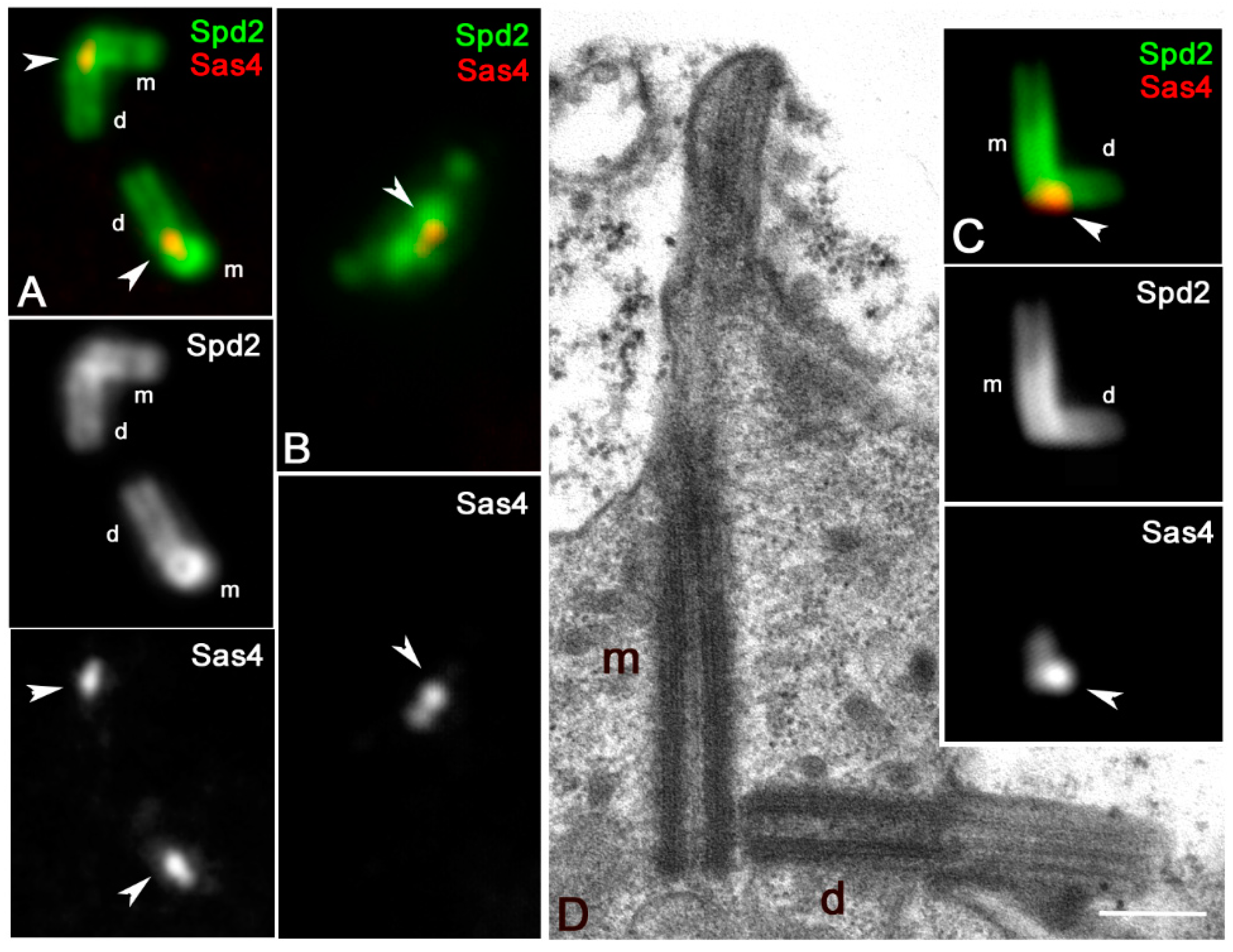
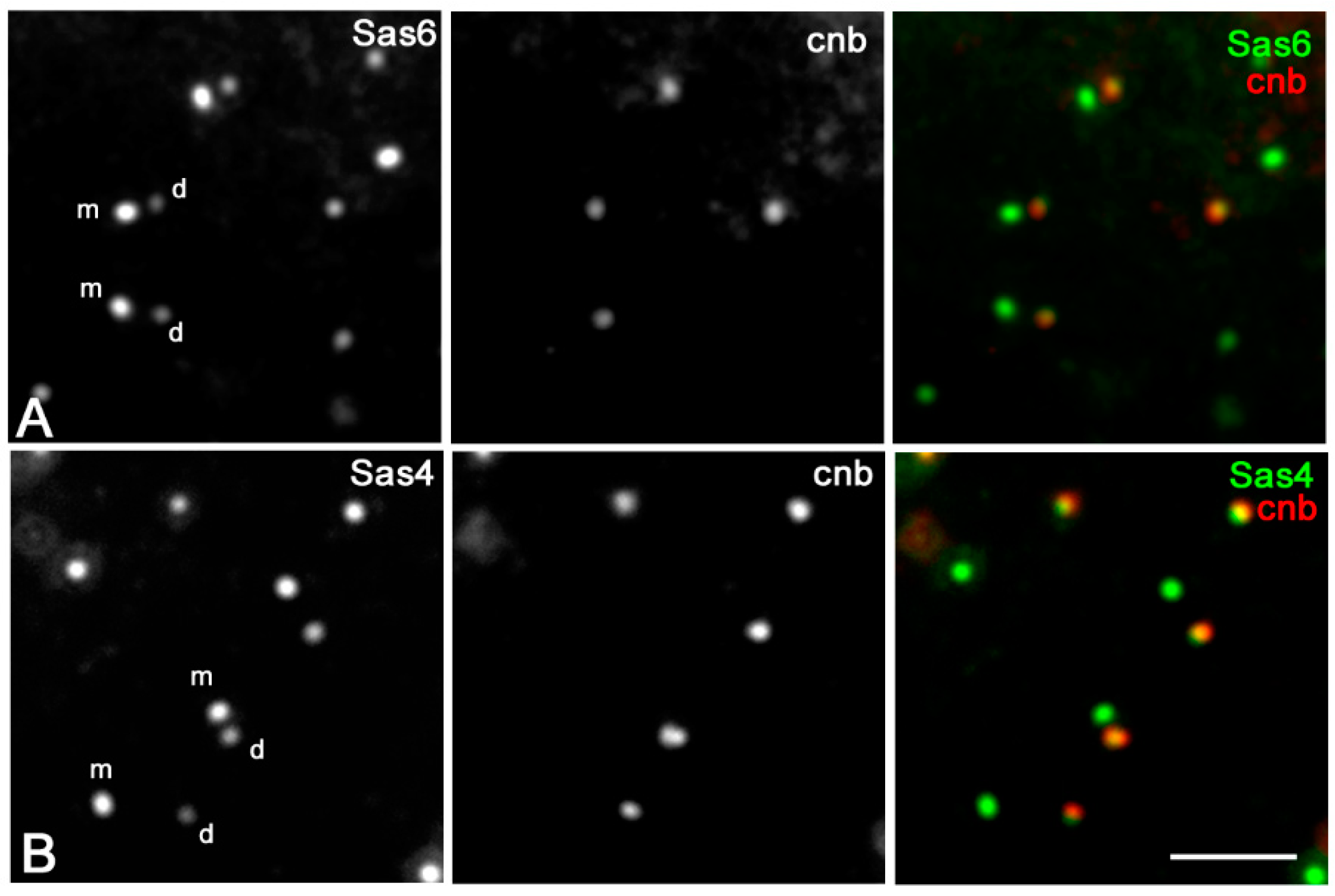
3.2. Sas6 and Sas4 Are Enriched to the Proximal Region of the Daughter Centrioles during Male Meiosis
3.3. Sas6 and Sas4 Are Enriched to the Mother Centrioles in Somatic Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bettencourt-Dias, M.; Glover, D.M. Centrosome biogenesis and function: Centrosomics brings new understanding. Nat. Rev. Mol. Cell Biol. 2007, 8, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Joukov, V.; De Nicolo, A. The Centrosome and the Primary Cilium: The Yin and Yang of a Hybrid Organelle. Cells 2019, 8, 701. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A.; Čajánek, L.; Arquint, C. The centrosome duplication cycle in health and disease. FEBS Lett. 2014, 588, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A.; Schnerch, D.; Ganier, O. Impact of Centrosome Aberrations on Chromosome Segregation and Tissue Architecture in Cancer. Cold Spring Harb. Symp. Quant. Biol. 2017, 82, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A.; Holland, A.J. Once and only once: Mechanisms of centriole duplication and their deregulation in disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Breslow, D.K.; Holland, A.J. Mechanism and regulation of centriole and cilium biogenesis. Annu. Rev. Biochem. 2019, 88, 691–724. [Google Scholar] [CrossRef]
- Reina, J.; Gonzalez, C. When fate follows age: Unequal centrosomes in asymmetric cell division. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130466. [Google Scholar] [CrossRef] [PubMed]
- Tanos, B.E.; Yang, H.-J.; Soni, R.; Wang, W.-J.; Macaluso, F.P.; Asara, J.M.; Tsou, M.-F.B. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 2013, 27, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Stinchcombe, J.C.; Randzavola, L.O.; Angus, K.L.; Mantell, J.M.; Verkade, P.; Griffiths, G.M. Mother centriole cistal appendages mediate centrosome docking at the immunological synapse and reveal mechanistic parallels with ciliogenesis. Curr. Biol. 2015, 25, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, I.; Dynlacht, B.D. Cilium assembly and disassembly. Nat. Cell Biol. 2016, 18, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dynlacht, B.D. The regulation of cilium assembly and disassembly in development and disease. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.Y.; Mack, G.J.; Zhang, M.; Rattner, J.B. CEP110 and ninein are located in a specific domain of the centrosome associated with centrosome maturation. J. Cell Sci. 2002, 115, 1825–1835. [Google Scholar] [PubMed]
- Kubo, A.; Tsukita, S.; Tsukita, S.; Ishikawa, H. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat. Cell Biol. 2005, 7, 517–524. [Google Scholar]
- Gromley, A.; Jurczyk, A.; Sillibourne, J.; Halilovic, E.; Mogensen, M.; Groisman, I.; Blomberg, M.; Doxsey, S.J. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J. Cell Biol. 2003, 161, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Graser, S.; Stierhof, Y.D.; Lavoie, S.B.; Gassner, O.S.; Lamla, S.; Le Clech, M.; Nigg, E.A. Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 2007, 179, 321–330. [Google Scholar] [CrossRef]
- Zou, C.; Li, J.; Bai, Y.; Gunning, W.T.; Wazer, D.E.; Band, V.; Gao, Q. Centrobin: A novel daughter centriole-associated protein that is required for centriole duplication. J. Cell Biol. 2005, 171, 437–445. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.; Kobayashi, T.; Liang, F.-X.; Korzeniewski, N.; Duensing, S.; Dynlacht, B.D. Neurl4, a novel daughter centriole protein, prevents formation of ectopic microtubule organizing centres. EMBO Rep. 2012, 13, 547–553. [Google Scholar] [CrossRef]
- Mahjoub, M.R.; Xie, Z.; Stearns, T. Cep120 is asymmetrically localized to the daughter centriole and is essential for centriole assembly. J. Cell Biol. 2010, 191, 331–346. [Google Scholar] [CrossRef]
- Mennella, V.; Agard, D.A.; Bo, H.; Pelletier, L. Amorphous no more: Subdiffraction view of the pericentriolar material architecture. Trends Cell Biol. 2014, 24, 188–197. [Google Scholar] [CrossRef]
- Anderson, C.T.; Stearns, T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr. Biol. 2009, 19, 1498–1502. [Google Scholar] [CrossRef]
- Wang, X.; Tsai, J.-W.; Imai, J.H.; Lian, W.-N.; Vallee, R.B.; Shi, S.-H. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 2009, 461, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Paridaen, J.T.M.L.; Wilsch-Brauninger, M.; Huttner, W.B. Asymmetric inheritance of centrosome associated primary cilium membrane directs ciliogenesis after cell division. Cell 2013, 155, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.M.; Jones, D.L.; Fuller, M.T. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 2003, 301, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Januschke, J.; Llamazares, S.; Reina, J.; Gonzalez, C. Drosophila neuroblasts retain the daughter centrosome. Nat. Commun. 2011, 2, 243. [Google Scholar] [CrossRef] [PubMed]
- Conduit, P.T.; Raff, J.W. Cnn dynamics drive centrosome size asymmetry to ensure daughter centriole retention in Drosophila neuroblasts. Curr. Biol. 2010, 20, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.M.; Mahowald, A.P.; Perlin, J.R.; Fuller, M.T. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science 2007, 315, 518–521. [Google Scholar] [CrossRef]
- Callaini, G.; Whitfield, W.G.; Riparbelli, M.G. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila. Exp. Cell Res. 1997, 234, 183–190. [Google Scholar] [CrossRef]
- Persico, V.; Callaini, G.; Riparbelli, M.G. The Microtubule-depolymerizing kinesin-13 Klp10A is enriched in the transition zone of the ciliary structures of Drosophila melanogaster. Front. Cell Dev. Biol. 2019, 7, 173. [Google Scholar] [CrossRef]
- Fu, J.; Lipinszki, Z.; Rangone, H.; Min, M.; Mykura, C.; Chao-Chu, J.; Schneider, S.; Dzhindzhev, N.S.; Gottardo, M.; Riparbelli, M.G.; et al. Conserved molecular interactions in centriole-to centrosome conversion. Nat. Cell Biol. 2016, 18, 87–99. [Google Scholar] [CrossRef]
- Lerit, D.A.; Jordan, H.A.; Poulton, J.S.; Fagerstrom, C.J.; Galletta, B.J.; Peifer, M.; Rusan, N.M. Interphase centrosome organization by the PLP-Cnn scaffold is required for centrosome function. J. Cell Biol. 2015, 210, 79–97. [Google Scholar] [CrossRef]
- Richens, J.H.; Barros, T.P.; Lucas, E.P.; Peel, N.; Pinto, D.M.; Wainman, A.; Raff, J.W. The Drosophila Pericentrin-like-protein (PLP) cooperates with Cnn to maintain the integrity of the outer PCM. Biol. Open 2015, 4, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Januschke, J.; Reina, J.; Llamazares, S.; Bertran, T.; Rossi, F.; Roig, J.; Gonzalez, C. Centrobin controls mother-daughter centriole asymmetry in Drosophila neuroblasts. Nat. Cell Biol. 2013, 15, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, M.; Pollarolo, G.; Llamazares, S.; Reina, J.; Riparbelli, M.G.; Callaini, G.; Gonzalez, C. Loss of Centrobin enables daughter centrioles to form sensory cilia in Drosophila. Curr. Biol. 2015, 25, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Riparbelli, M.G.; Persico, V.; Gottardo, M.; Callaini, G. The developing Drosophila eye—A new model to study centriole reduction. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, V.; Chen, C.; Chiang, C.-Y.A.; Tiyaboonchai, A.; Mayer, M.; Yamashita, Y.M. Centrosome-dependent asymmetric inheritance of the midbody ring in Drosophila germline stem cell division. Mol. Biol. Cell 2014, 25, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Reina, J.; Gottardo, M.; Riparbelli, M.G.; Llamazares, S.; Callaini, G.; Gonzalez, C. Centrobin is essential for C-tubule assembly and flagellum development in Drosophila melanogaster spermatogenesis. J. Cell Biol. 2018, 201, 801032. [Google Scholar] [CrossRef]
- Fu, J.; Glover, D.M. Structured illumination of the interface between centriole and peri-centriolar material. Open Biol. 2012, 2, 120104. [Google Scholar] [CrossRef]
- Riparbelli, M.G.; Callaini, G.; Megraw, T.L. Assembly and persistence of primary cilia in dividing Drosophila spermatocytes. Dev. Cell 2012, 23, 425–432. [Google Scholar] [CrossRef]
- Stevens, N.R.; Roque, H.; Raff, J.W. DSas-6 and Ana2 coassemble into tubules to promote centriole duplication and engagement. Dev. Cell 2010, 19, 913–919. [Google Scholar] [CrossRef]
- Delgehyr, N.; Rangone, H.; Fu, J.; Mao, G.; Tom, B.; Riparbelli, M.G.; Callaini, G.; Glover, D.M. Klp10A, a microtubule-depolymerizing kinesin-13, cooperates with CP110 to control Drosophila centriole length. Curr. Biol. 2012, 22, 502–509. [Google Scholar] [CrossRef]
- Chen, C.; Inaba, M.; Venkei, Z.G.; Yamashita, Y.M. Klp10A, a stem cell centrosome-enriched kinesin, balances asymmetries in Drosophila male germline stem cell division. Elife 2016, 5, e20977. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, M.; Callaini, G.; Riparbelli, M.G. Klp10A modulates the localization of centriole-associated proteins during Drosophila male gametogenesis. Cell Cycle 2016, 15, 3432–3441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baker, J.D.; Adhikarakunnathu, S.; Kernan, M.J. Mechanosensory-defective, male-sterile unc mutants identify a novel basal body protein required for ciliogenesis in Drosophila. Development 2004, 131, 3411–3422. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Martins, A.; Bettencourt-Dias, M.; Riparbelli, M.; Ferreira, C.; Ferreira, I.; Callaini, G.; Glover, D.M. DSAS-6 organizes a tube-like centriole precursor, and its absence suggests modularity in centriole assembly. Curr. Biol. 2007, 17, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Martins, A.; Riparbelli, M.; Callaini, G.; Glover, D.M.; Bettencourt-Dias, M. Revisiting the role of the mother centriole in centriole biogenesis. Science 2007, 316, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, J.; Mennella, V.; Blachon, S.; Zhai, B.; Smith, A.H.; Megraw, T.L.; Nicastro, D.; Gygi, S.P.; Agard, D.A.; Avidor-Reiss, T. Sas-4 provides a scaffold for cytoplasmic complexes and tethers them in a centrosome. Nat. Commun. 2011, 2, 359. [Google Scholar] [CrossRef]
- Blachon, S.; Cai, X.; Roberts, K.A.; Yang, K.; Polyanovsky, A.; Church, A.; Avidor-Reiss, T. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics 2009, 182, 133–144. [Google Scholar] [CrossRef]
- Martinez-Campos, M.; Basto, R.; Baker, J.; Kernan, M.; Raff, J.W. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J. Cell Biol. 2004, 165, 673–683. [Google Scholar] [CrossRef]
- Riparbelli, M.G.; Cabrera, O.A.; Callaini, G.; Megraw, T.L. Unique properties of Drosophila spermatocyte primary cilia. Biol. Open 2013, 2, 1137–1147. [Google Scholar] [CrossRef]
- Roque, H.; Saurya, S.; Pratt, M.B.; Johnson, E.; Raff, J.W. Drosophila PLP assembles pericentriolar clouds that promote centriole stability, cohesion and MT nucleation. PLoS Genet. 2018, 14, e1007198. [Google Scholar] [CrossRef]
- Blachon, S.; Gopalakrishnan, J.; Omori, Y.; Polyanovsky, A.; Church, A.; Nicastro, D.; Malicki, J.; Avidor-Reiss, T. Drosophila asterless and vertebrate Cep152 Are orthologs essential for centriole duplication. Genetics 2008, 180, 2081–2094. [Google Scholar] [CrossRef]
- Galletta, B.J.; Jacobs, K.C.; Fagerstrom, C.J.; Rusan, N.M. Asterless is required for centriole length control and sperm development. J. Cell Biol. 2016, 213, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jiang, Q.; Zhang, C. The role of mitotic kinases in coupling the centrosome cycle with the assembly of the mitotic spindle. J. Cell Sci. 2014, 127, 4111–4122. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.G.; Ecsedy, J.A.; Meetze, K.A.; Balani, S.K.; Burenkova, O.; Chen, W.; Galvin, K.M.; Hoar, K.M.; Huck, J.J.; LeRoy, P.J.; et al. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc. Natl. Acad. Sci. USA 2007, 104, 4106–4111. [Google Scholar] [CrossRef] [PubMed]
- Lénárt, P.; Petronczki, M.; Steegmaier, M.; Di Fiore, B.; Lipp, J.J.; Hoffmann, M.; Rettig, W.J.; Kraut, N.; Peters, J.M. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of Polo-like kinase 1. Curr. Biol. 2007, 17, 304–315. [Google Scholar]
- Dzhindzhev, N.S.; Tzolovsky, G.; Lipinszki, Z.; Schneider, S.; Lattao, R.; Fu, J.; Debski, J.; Dadlez, M.; Glover, D.M. Plk4 phosphorylates Ana2 to trigger Sas6 recruitment and procentriole formation. Curr. Biol. 2014, 24, 2526–2532. [Google Scholar] [CrossRef]
- Dzhindzhev, N.S.; Tzolovsky, G.; Lipinszki, Z.; Abdelaziz, M.; Debski, J.; Dadlez, M.; Glover, D.M. Two-step phosphorylation of Ana2 by Plk4 is required for the sequential loading of Ana2 and Sas6 to initiate procentriole formation. Open Biol. 2017, 7, 170247. [Google Scholar] [CrossRef]
- Gönczy, P.; Hatzopoulos, G.N. Centriole assembly at a glance. J. Cell Sci. 2019, 132, 228833. [Google Scholar] [CrossRef]
- Kratz, A.S.; Bärenz, F.; Richter, K.T.; Hoffmann, I. Plk4-dependent phosphorylation of STIL is required for centriole duplication. Biol. Open. 2015, 4, 370–377. [Google Scholar] [CrossRef]
- Moyer, T.C.; Holland, A.J. PLK4 promotes centriole duplication by phosphorylating STIL to link the procentriole cartwheel to the microtubule wall. Elife 2019. [Google Scholar] [CrossRef]
- Ohta, M.; Ashikawa, T.; Nozaki, Y.; Kozuka-Hata, H.; Goto, H.; Inagaki, M.; Oyama, M.; Kitagawa, D. Direct interaction of Plk4 with STIL ensures formation of a single procentriole per parental centriole. Nat. Commun. 2014, 5, 5267. [Google Scholar] [CrossRef] [PubMed]
- Avidor-Reiss, T. Rapid evolution of sperm produces diverse centriole structures that reveal the most rudimentary structure needed for function. Cells 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M. Spermatogenesis. In The Development of Drosophila Melanogaster; Bate, M., Martinez Arias, A., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1993; Volume 1, pp. 71–147. [Google Scholar]
- Gottardo, M.; Callaini, G.; Riparbelli, M.G. Structural characterization of procentrioles in Drosophila spermatids. Cytoskeleton 2015, 72, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Khire, A.; Jo, K.H.; Kong, D.; Akhshi, T.; Blachon, S.; Cekic, A.R.; Hynek, S.; Ha, A.; Loncarek, J.; Mennella, V.; et al. Centriole remodeling during spermiogenesis in Drosophila. Curr. Biol. 2016, 26, 3183–3189. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.; Müller-Reichert, T.; Oegema, K.; Grill, S.; Hyman, A.A. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell 2003, 112, 575–587. [Google Scholar] [CrossRef]
- Kohlmaier, G.; Loncarek, J.; Meng, X.; McEwen, B.F.; Mogensen, M.M.; Spektor, A.; Dynlacht, B.D.; Khodjakov, A.; Gönczy, P. Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr. Biol. 2009, 19, 1012–1018. [Google Scholar] [CrossRef]
- Schmidt, T.I.; Kleylein-Sohn, J.; Westendorf, J.; Le Clech, M.; Lavoie, S.B.; Stierhof, Y.D.; Nigg, E.A. Control of centriole length by CPAP and CP110. Curr. Biol. 2009, 19, 1005–1011. [Google Scholar] [CrossRef]
- Tang, C.J.; Fu, R.H.; Wu, K.S.; Hsu, W.B.; Tang, T.K. CPAP is a cell-cycle regulated protein that controls centriole length. Nat. Cell Biol. 2009, 11, 825–831. [Google Scholar] [CrossRef]
- Cottee, M.A.; Muschalik, N.; Wong, Y.L.; Johnson, C.M.; Johnson, S.; Andreeva, A.; Oegema, K.; Lea, S.M.; Raff, J.W.; van Breugel, M. Crystal structures of the CPAP/STIL complex reveal its role in centriole assembly and human microcephaly. Elife 2013. [Google Scholar] [CrossRef]
- Galletta, B.J.; Fagerstrom, C.J.; Schoborg, T.A.; McLamarrah, T.A.; Ryniawec, J.M.; Buster, D.W.; Slep, K.C.; Rogers, G.C.; Rusan, N.M. A centrosome interactome provides insight into organelle assembly and reveals a non-duplication role for Plk4. Nat. Commun. 2016, 7, 12476. [Google Scholar] [CrossRef]
- Hatzopoulos, G.N.; Erat, M.C.; Cutts, E.; Rogala, K.B.; Slater, L.M.; Stansfeld, P.J.; Vakonakis, I. Structural analysis of the G-box domain of the microcephaly protein CPAP suggests a role in centriole architecture. Structure 2013, 21, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- McLamarrah, T.A.; Buster, D.W.; Galletta, B.J.; Boese, C.J.; Ryniawec, J.M.; Hollingsworth, N.A.; Byrnes, A.E.; Brownlee, C.W.; Slep, K.C.; Rusan, N.M.; et al. An ordered pattern of Ana2 phosphorylation by Plk4 is required for centriole assembly. J. Cell Biol. 2018, 217, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, M.; Callaini, G.; Riparbelli, M.G. The cilium-like region of the Drosophila spermatocyte: An emerging flagellum? J. Cell Sci. 2013, 126, 5441–5452. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persico, V.; Migliorini, M.; Callaini, G.; Riparbelli, M.G. The Singularity of the Drosophila Male Germ Cell Centriole: The Asymmetric Distribution of Sas4 and Sas6. Cells 2020, 9, 115. https://doi.org/10.3390/cells9010115
Persico V, Migliorini M, Callaini G, Riparbelli MG. The Singularity of the Drosophila Male Germ Cell Centriole: The Asymmetric Distribution of Sas4 and Sas6. Cells. 2020; 9(1):115. https://doi.org/10.3390/cells9010115
Chicago/Turabian StylePersico, Veronica, Massimo Migliorini, Giuliano Callaini, and Maria Giovanna Riparbelli. 2020. "The Singularity of the Drosophila Male Germ Cell Centriole: The Asymmetric Distribution of Sas4 and Sas6" Cells 9, no. 1: 115. https://doi.org/10.3390/cells9010115
APA StylePersico, V., Migliorini, M., Callaini, G., & Riparbelli, M. G. (2020). The Singularity of the Drosophila Male Germ Cell Centriole: The Asymmetric Distribution of Sas4 and Sas6. Cells, 9(1), 115. https://doi.org/10.3390/cells9010115



