Establishment of A Reversibly Inducible Porcine Granulosa Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construct of the Inducible and Reversible Large T Lentiviral Plasmid
2.2. Isolation and Cultivation of Granulosa Cells
2.3. Lentivirus Packaging, Infection, and Cell Screening
2.4. Cell Cycle Analysis
2.5. Cell Proliferation Assays
2.6. Immunofluorescence
2.7. RNA Extraction and Quantitative Reverse Transcriptase (RT)-PCR
2.8. Morphology and Phentypic Analysis of CIPGCs with or without Dox
2.9. Determination of Steroid Hormones’ Secretion
2.10. Statistics Analysis
3. Results
3.1. Construction of the Inducible Large T Expressing Lentiviral Plasmid
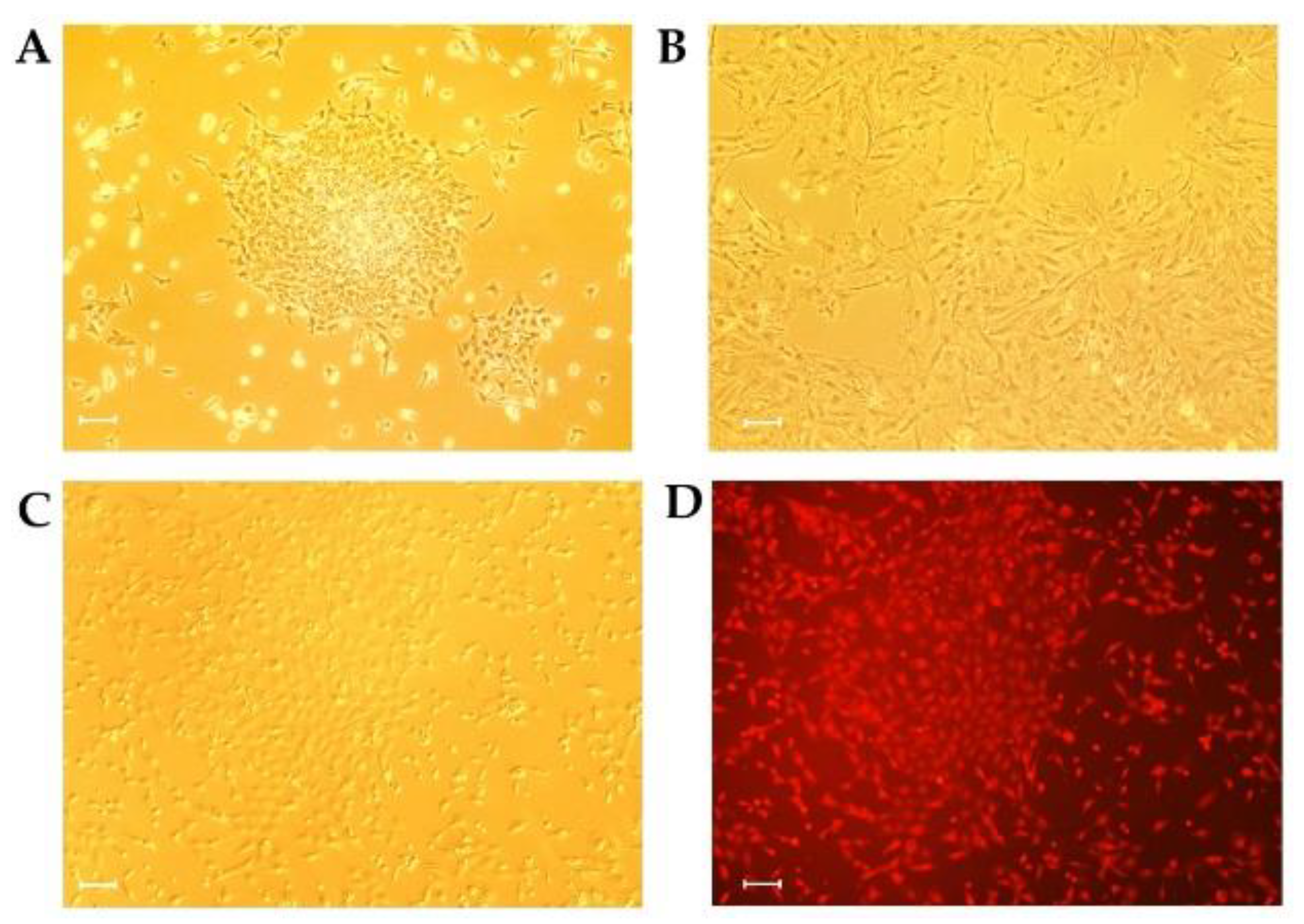
3.2. Isolation and Lentivirus Transduction of Porcine GCs
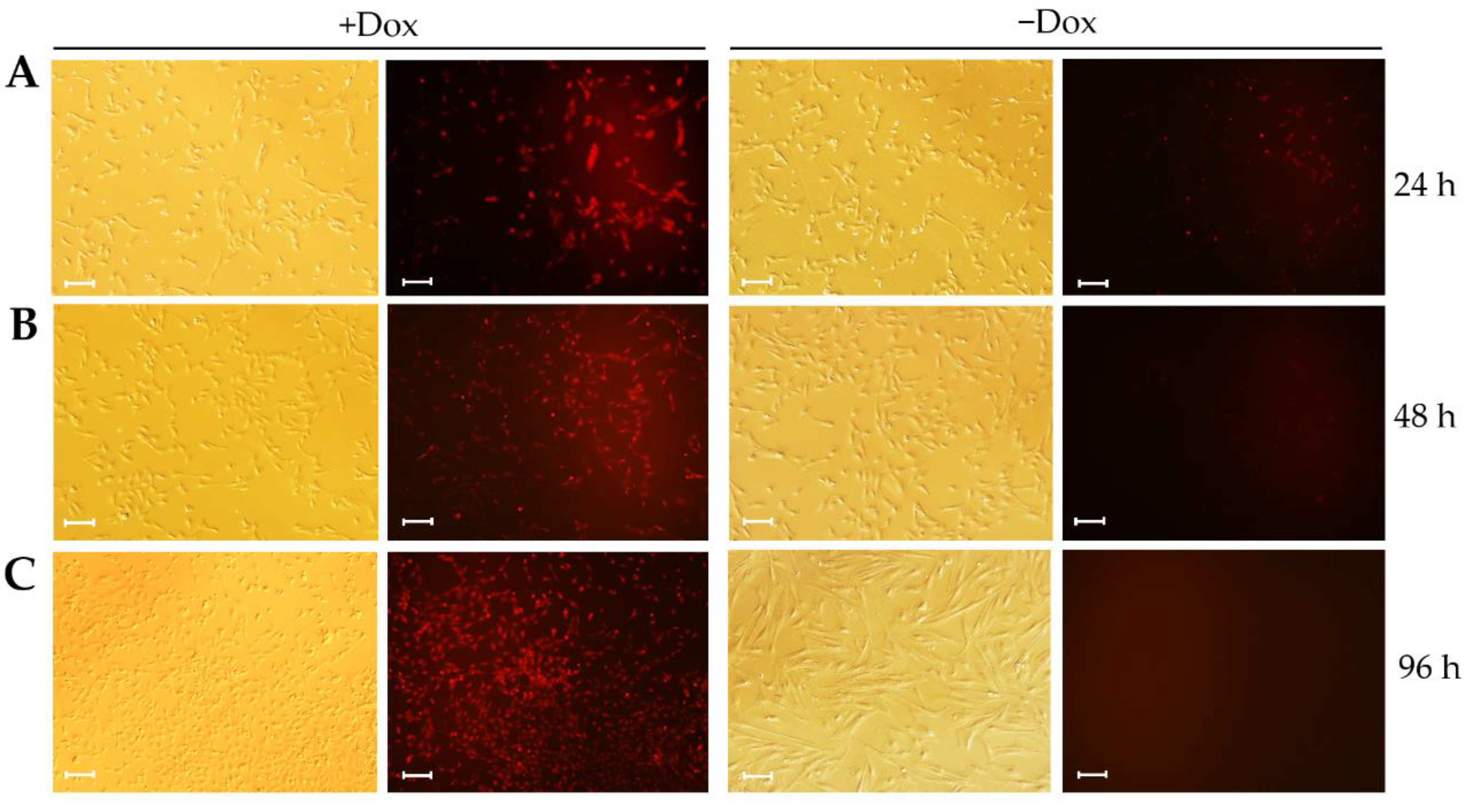
3.3. Large T-T2A-mCherry Expression Is Reversible in CIPGCs
3.4. The Proliferation of CIPGCs Is Controlled by Dox Induction
3.5. The Reversible Regulation on Proliferation State of CIPGCs by Dox
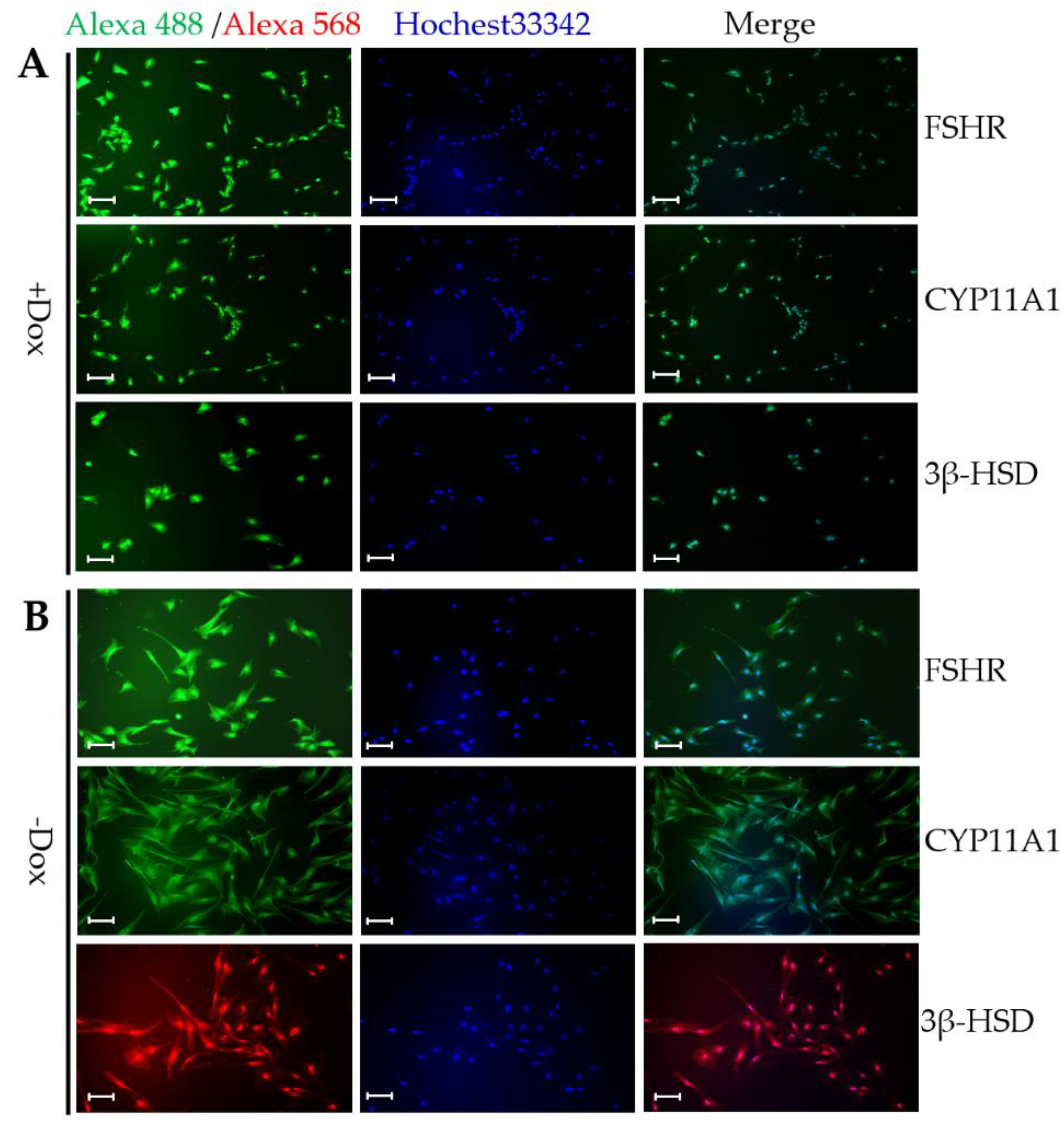
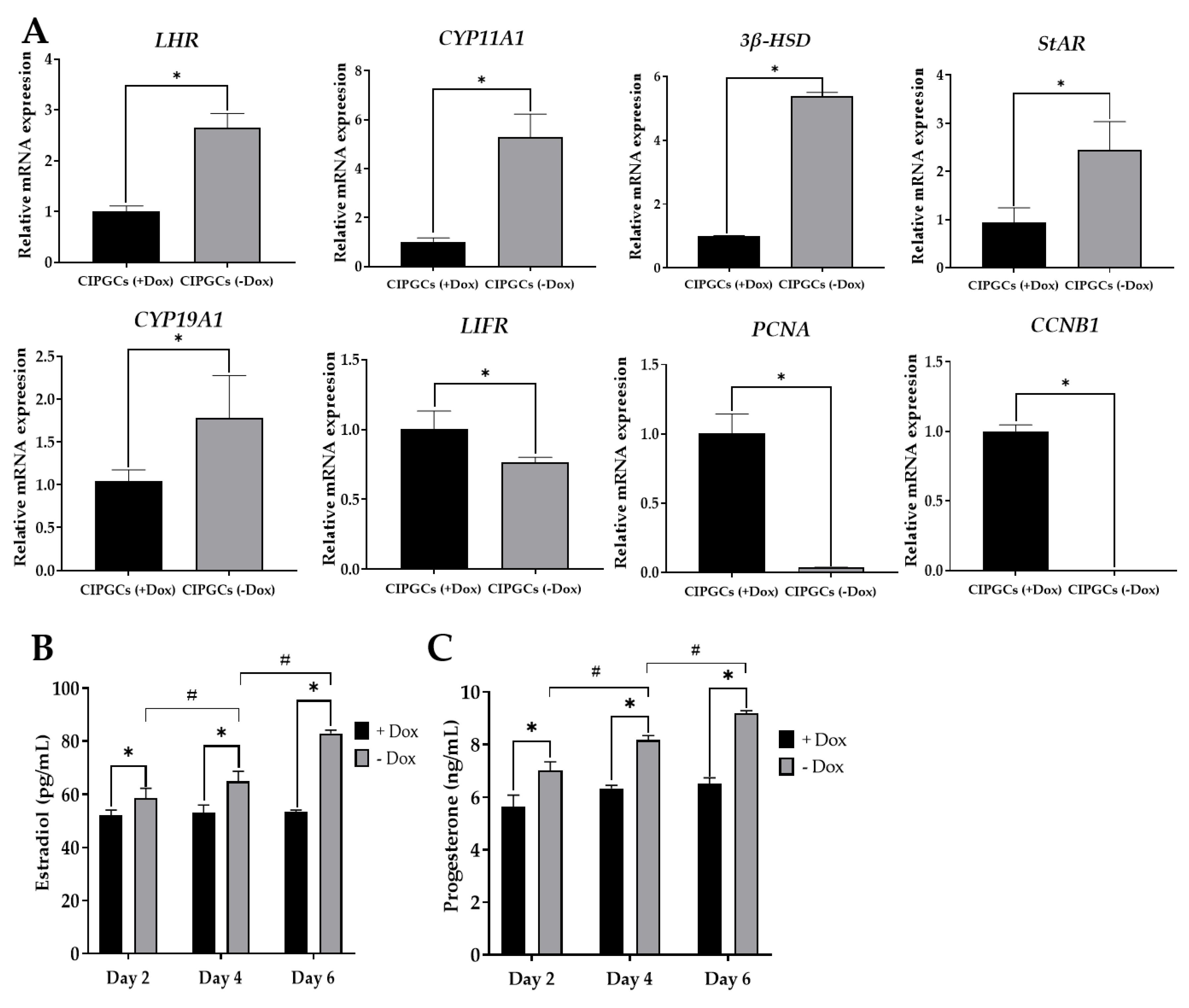
3.6. Comparison of Steroidogenesis and Gonadotropin Response of CIPGCs and Primary GCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pohler, K.G.; Geary, T.W.; Atkins, J.A.; Perry, G.A.; Jinks, E.M.; Smith, M.F. Follicular determinants of pregnancy establishment and maintenance. Cell Tissue Res. 2012, 349, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.H.; Wang, L.; Riaz, H.; Wu, J.B.; Yuan, Y.F.; Han, L.; Wang, Y.L.; Zhao, Y.; Dan, Y.; Huo, L.J. Knockdown of CEBPbeta by RNAi in porcine granulosa cells resulted in S phase cell cycle arrest and decreased progesterone and estradiol synthesis. J. Steroid Biochem. Mol. Biol. 2014, 143, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Breckwoldt, M.; Selvaraj, N.; Aharoni, D.; Barash, A.; Segal, I.; Insler, V.; Amsterdam, A. Expression of Ad4-BP/cytochrome P450 side chain cleavage enzyme and induction of cell death in long-term cultures of human granulosa cells. Mol. Hum. Reprod. 1996, 2, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.T. Establishment of an immortalized porcine granulosa cell line (PGV) and the study on the potential mechanisms of PGV cell proliferation. Keio J. Med. 2005, 54, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Rao, I.M.; Gadson, P.J.; Anderson, E.; Hornsby, P.J.; Mahesh, V.B. Characterization of progesterone biosynthesis in a transformed granulosa cell line. Mol. Cell. Endocrinol. 1993, 94, 121–128. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Lin, P.; Jiang, T.; Wang, N.; Zhao, F.; Chen, H.; Tang, K.; Zhou, D.; Wang, A.; et al. An immortalized steroidogenic goat granulosa cell line as a model system to study the effect of the endoplasmic reticulum (ER)-stress response on steroidogenesis. J. Reprod. Dev. 2017, 63, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wen, L.; Yuan, Q.; Sun, M.; Niu, M.; He, Z. Establishment and applications of male germ cell and sertoli cell lines. Reproduction 2016, 152, R31–40. [Google Scholar] [CrossRef] [Green Version]
- Kamei, Y.; Aoyama, Y.; Fujimoto, T.; Kenmotsu, N.; Kishi, C.; Koushi, M.; Sugano, S.; Morohashi, K.; Kamiyama, R.; Asakai, R. A steroidogenic cell line with differentiation potential from mouse granulosa cells, transfected with Ad4BP and SV40 large T antigen genes. J. Endocrinol. 2005, 185, 187–195. [Google Scholar] [CrossRef]
- Gillio-Meina, C.; Swan, C.L.; Crellin, N.K.; Stocco, D.M.; Chedrese, P.J. Generation of stable cell lines by spontaneous immortalization of primary cultures of porcine granulosa cells. Mol. Reprod. Dev. 2000, 57, 366–374. [Google Scholar] [CrossRef]
- Havelock, J.C.; Rainey, W.E.; Carr, B.R. Ovarian granulosa cell lines. Mol. Cell. Endocrinol. 2004, 228, 67–78. [Google Scholar] [CrossRef]
- Darimont, C.; Avanti, O.; Tromvoukis, Y.; Vautravers-Leone, P.; Kurihara, N.; Roodman, G.D.; Colgin, L.M.; Tullberg-Reinert, H.; Pfeifer, A.M.; Offord, E.A.; et al. SV40 T antigen and telomerase are required to obtain immortalized human adult bone cells without loss of the differentiated phenotype. Cell Growth. Differ. 2002, 13, 59–67. [Google Scholar] [PubMed]
- Sadowska, A.; Nynca, A.; Korzeniewska, M.; Piasecka-Srader, J.; Jablonska, M.; Orlowska, K.; Swigonska, S.; Ciereszko, R.E. Characterization of porcine granulosa cell Line AVG-16. Folia Biol. (Praha) 2015, 61, 184–194. [Google Scholar] [PubMed]
- Leighton, J.K.; Grimes, R.W.; Canning, S.; Hammond, J.M. Expression of the IGF system in primary and immortalized porcine ovarian granulosa cells. Mol. Cell. Endocrinol. 1993, 97, 29–35. [Google Scholar] [CrossRef]
- Kwan, I.; Farookhi, R.; Huynh, H.T.; Murphy, B.D.; Turner, J.D.; Downey, B.R. Steroidogenic properties of a spontaneously established porcine granulosa cell line (PGC-2). Mol. Reprod. Dev. 1996, 45, 299–307. [Google Scholar] [CrossRef]
- Rodway, M.R.; Swan, C.L.; Gillio-Meina, C.; Crellin, N.K.; Flood, P.F.; Chedrese, P.J. Regulation of steroidogenesis in jc-410, a stable cell line of porcine granulosa origin. Mol. Cell. Endocrinol. 1999, 148, 87–94. [Google Scholar] [CrossRef]
- Kowolik, C.M.; Liang, S.; Yu, Y.; Yee, J.K. Cre-mediated reversible immortalization of human renal proximal tubular epithelial cells. Oncogene 2004, 23, 5950–5957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.L.; Wang, Y.; Zhang, P.; Li, S.F.; Chen, X.; Chen, Y.K.; Li, J.G.; Yang, S.M.; Su, Y.P.; Wang, J.P.; et al. Reversible immortalization of rat pancreatic beta cells with a novel immortalizing and tamoxifen-mediated self-recombination tricistronic vector. J. Biotechnol. 2011, 151, 231–241. [Google Scholar] [CrossRef]
- He, J.; Duan, X.; Li, W.; Peng, Y.; Yu, J.; Hu, L.; Zeng, S.; Wang, Y.; Lu, G.; Lin, G.; et al. Establishment and characterization of a human embryonic stem cell line, NERCe002-A-3, with inducible 14-3-3zeta overexpression. Stem. Cell Res. 2018, 28, 11–15. [Google Scholar] [CrossRef]
- Collet, B.; Lester, K. Establishment of an Atlantic salmon kidney cell line with an inducible gene expression system. J. Biotechnol. 2011, 154, 209–211. [Google Scholar] [CrossRef]
- Ravassard, P.; Hazhouz, Y.; Pechberty, S.; Bricout-Neveu, E.; Armanet, M.; Czernichow, P.; Scharfmann, R. A genetically engineered human pancreatic beta cell line exhibiting glucose-inducible insulin secretion. J. Clin. Investig. 2011, 121, 3589–3597. [Google Scholar] [CrossRef]
- Benazra, M.; Lecomte, M.J.; Colace, C.; Muller, A.; Machado, C.; Pechberty, S.; Bricout-Neveu, E.; Grenier-Godard, M.; Solimena, M.; Scharfmann, R.; et al. A human beta cell line with drug inducible excision of immortalizing transgenes. Mol. Metab. 2015, 4, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.; Zhang, J.; Wang, Y. Drug inducible CRISPR/Cas systems. Comput. Struct. Biotechnol. J. 2019, 17, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Feng, M.; Liu, S.; Wei, H.; Li, L.; Zhang, X.; Shen, C.; Zhang, S.; Ma, N. Differential gene expression in mouse spermatogonial stem cells and embryonic stem cells. Int. J. Mol. Med. 2016, 38, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.L.; Wu, Y.; Tan, L.B.; Tian, Z.; Liu, J.H.; Zhu, D.S.; Zeng, S.M. Follicle-stimulating hormone regulates pro-apoptotic protein Bcl-2-interacting mediator of cell death-extra long (BimEL)-induced porcine granulosa cell apoptosis. J. Biol. Chem. 2012, 287, 10166–10177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.C.; Oskay-Ozcelik, G.; Buhling, K.J.; Kopstein, U.; Mentze, M.; Lichtenegger, W.; Sehouli, J. Prognostic value of serum and ascites levels of estradiol, FSH, LH and prolactin in ovarian cancer. Anticancer Res. 2009, 29, 1575–1578. [Google Scholar]
- Kaiser, B.; Bottner, M.; Wedel, T.; Brunner, R.M.; Goldammer, T.; Lesko, S.; Gabel, G.; Gleich, A.; Pfannkuche, H. Establishment and characterization of an SV40 Large T antigen-transduced porcine colonic epithelial cell line. Cells Tissues Organs 2017, 203, 267–286. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, S.; Wang, M.; Zhao, B.; Yang, N.; Li, J.; Chen, Q.; Liu, M.; Zhou, J.; Bao, G.; et al. Characterization and Establishment of an immortalized rabbit melanocyte cell Line using the SV40 Large T antigen. Int. J. Mol. Sci. 2019, 20, 4874. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Niu, M.; Liu, L.; Zhu, Z.; Wang, X.; Sun, M.; Yuan, Q.; Yang, S.; Zeng, W.; Liu, Y.; et al. Establishment and Characterization of human germline stem cell line with unlimited proliferation potentials and no tumor formation. Sci. Rep. 2015, 5, 16922. [Google Scholar] [CrossRef] [Green Version]
- Blum, W.; Pecze, L.; Felley-Bosco, E.; Worthmuller-Rodriguez, J.; Wu, L.; Vrugt, B.; de Perrot, M.; Schwaller, B. Establishment of immortalized murine mesothelial cells and a novel mesothelioma cell line. In Vitr. Cell. Dev. Biol. Anim. 2015, 51, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Daniele, N.; Halse, R.; Grinyo, E.; Yeaman, S.J.; Shepherd, P.R. Conditionally immortalized cell lines as model systems for high-throughput biology in drug discovery. Biochem. Soc. Trans. 2002, 30, 800–802. [Google Scholar] [CrossRef]
- Liu, Y.; Patel, G.C.; Mao, W.; Clark, A.F. Establishment of a conditionally immortalized mouse optic nerve astrocyte line. Exp. Eye Res. 2018, 176, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Church, C.; Brown, M.; Rodeheffer, M.S. Conditional immortalization of primary adipocyte precursor cells. Adipocyte 2015, 4, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leithner, A.; Renkawitz, J.; De Vries, I.; Hauschild, R.; Hacker, H.; Sixt, M. Fast and efficient genetic engineering of hematopoietic precursor cells for the study of dendritic cell migration. Eur. J. Immunol. 2018, 48, 1074–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Petitt, M.; Gamboa, M.; Huang, M.; Dhal, S.; Druzin, M.L.; Wu, J.C.; Chen-Tsai, Y.; Nayak, N.R. Transient, inducible, placenta-specific gene expression in mice. Endocrinology 2012, 153, 5637–5644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Zheng, T.; Lee, C.G.; Homer, R.J.; Elias, J.A. Tetracycline-controlled transcriptional regulation systems: Advances and application in transgenic animal modeling. Semin. Cell Dev. Biol. 2002, 13, 121–128. [Google Scholar] [CrossRef]
- Xu, K.; Deng, X.Y.; Yue, Y.; Guo, Z.M.; Huang, B.; Hong, X.; Xiao, D.; Chen, X.G. Generation of the regulatory protein rtTA transgenic mice. World J. Gastroenterol. 2005, 11, 2885–2891. [Google Scholar] [CrossRef]
- Iwata, H. Age-associated changes in granulosa cells and follicular fluid in cows. J. Reprod. Dev. 2017, 63, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Das, A.T.; Zhou, X.; Metz, S.W.; Vink, M.A.; Berkhout, B. Selecting the optimal Tet-On system for doxycycline-inducible gene expression in transiently transfected and stably transduced mammalian cells. Biotechnol. J. 2016, 11, 71–79. [Google Scholar] [CrossRef]
- Xu, E.Y.; Lee, D.F.; Klebes, A.; Turek, P.J.; Kornberg, T.B.; Reijo, P.R. Human BOULE gene rescues meiotic defects in infertile flies. Hum. Mol. Genet. 2003, 12, 169–175. [Google Scholar] [CrossRef]
- Xu, E.Y.; Moore, F.L.; Pera, R.A. A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proc. Natl. Acad. Sci. USA 2001, 98, 7414–7419. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Vink, M.; Klaver, B.; Berkhout, B.; Das, A.T. Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene. Ther. 2006, 13, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Rodway, M.R.; Swan, C.L.; Crellin, N.K.; Gillio-Meina, C.; Chedrese, P.J. Steroid regulation of progesterone synthesis in a stable porcine granulosa cell line: A role for progestins. J. Steroid. Biochem. Mol. Biol. 1999, 68, 173–180. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yu, C.P.; Lee, T.H.; Goh, K.S.; Chu, K.H.; Wang, P.H.; Ismail, W.; Shih, C.J.; Chiang, Y.R. Biochemical mechanisms and catabolic enzymes involved in bacterial estrogen degradation pathways. Cell Chem. Biol. 2017, 24, 712–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Zhu, C.; Feng, M.; Pan, B.; Zhang, S.; Zhan, X.; Chen, H.; Wang, B.; Li, J. Establishment of A Reversibly Inducible Porcine Granulosa Cell Line. Cells 2020, 9, 156. https://doi.org/10.3390/cells9010156
Bai Y, Zhu C, Feng M, Pan B, Zhang S, Zhan X, Chen H, Wang B, Li J. Establishment of A Reversibly Inducible Porcine Granulosa Cell Line. Cells. 2020; 9(1):156. https://doi.org/10.3390/cells9010156
Chicago/Turabian StyleBai, Yinshan, Cui Zhu, Meiying Feng, Bo Pan, Shouquan Zhang, Xiaoshu Zhan, Huifang Chen, Bingyun Wang, and Julang Li. 2020. "Establishment of A Reversibly Inducible Porcine Granulosa Cell Line" Cells 9, no. 1: 156. https://doi.org/10.3390/cells9010156




