Spectral Characterization of Stem Cell-Derived Myelination within the Injured Adult PNS Using the Solvatochromic Dye Nile Red
Abstract
:1. Introduction
2. Methods
2.1. Spectral Imaging and Data
2.2. Spectral Image Processing
2.3. Cell Culture and Histology
2.4. Experimental Designs for Analysis of Remyelination Spectra
2.4.1. Fixed Tissue Analysis of Tibial Nerve Remyelination Spectra after Doxorubicin Injury in Rats
2.4.2. In-Vitro Analysis of Myelination Spectra in Live Culture
2.4.3. Intravital Analysis of Tibial Nerve Remyelination after Doxorubicin injury
2.5. Surgery
2.5.1. Ethics Statement
2.5.2. Tibial Doxorubicin Injury and Cell Graft Injection
2.5.3. Tibial Nerve Window for Live Imaging
2.6. Imaging Techniques
2.6.1. Live Rodent Imaging
2.6.2. Imaging of Live Cultures
2.6.3. Imaging of Fixed Tissue
2.7. Cell Culture
2.7.1. Schwann Cell and SKP-SC Primary Culture
2.7.2. BFP/GFP Transduction of SKP-SCs and SCs
2.7.3. DRG Harvest/Culture
2.7.4. Co-Culture
2.8. Histology
2.8.1. Tissue Processing
2.8.2. Dye Preparation
2.8.3. Fixed Tissue Staining
2.8.4. In-Vitro Immunohistochemistry
2.9. Statistics
- Fixed Tissue SKP myelin regions (Day 21, n = 24; Day 24, n = 24; Day 27, n = 24; Day 30, n = 33; Day 33, n = 37; Adult, n = 10) vs. Schwann cell myelin regions (Day 21, n = 14; Day 24, n = 22; Day 27, n = 16; Day 30, n = 19; Day 33, n = 17; Adult, n = 10); independent replicate measurements.
- In vitro transplanted BFP-SKP-SC myelin regions (Day 13, n = 18; Day 16, n = 15; Day 19, n = 15; Day 22, n = 20.) vs. transplanted BFP-SC myelin regions (Day 13, n = 19; Day 16, n = 15; day 19, n = 29; day 22, n = 30); independent replicate measurements.
- In vitro endogenous GFP-Schwann cell myelin regions, co-cultured with SKP-SCs (Day 13, n = 19; Day 16, n = 17; Day 19, n = 31; Day 22, n = 20) vs. endogenous GFP-Schwann cell myelin regions, co-cultured with Schwann cells (Day 13, n = 11; Day 16, n = 12; Day 19, n = 30; Day 22, n = 16); independent replicate measurements.
- In vivo living SKP-SC myelin regions (Day 21, n = 7; Day 24, n = 28; Day 27, n = 5; Day 30, n = 31; Day 33, n = 25; Day 40, n = 15) vs. in vivo living Schwann cell myelin regions (Day 21, n = 8; Day 24 n = 28; Day 27 n = 27; Day 30 n = 20; day 33, n = 15; day 40, n = 17); independent replicate measurements.
3. Results
3.1. Spectral Interrogation of Cell Graft-Derived Myelination
3.1.1. Fixed Tissue Analysis
3.1.2. In Vitro Analysis of Live Myelin
3.1.3. Intravital Analysis
4. Discussion
4.1. Environmentally Sensitive and Solvatochromic Membrane Dyes
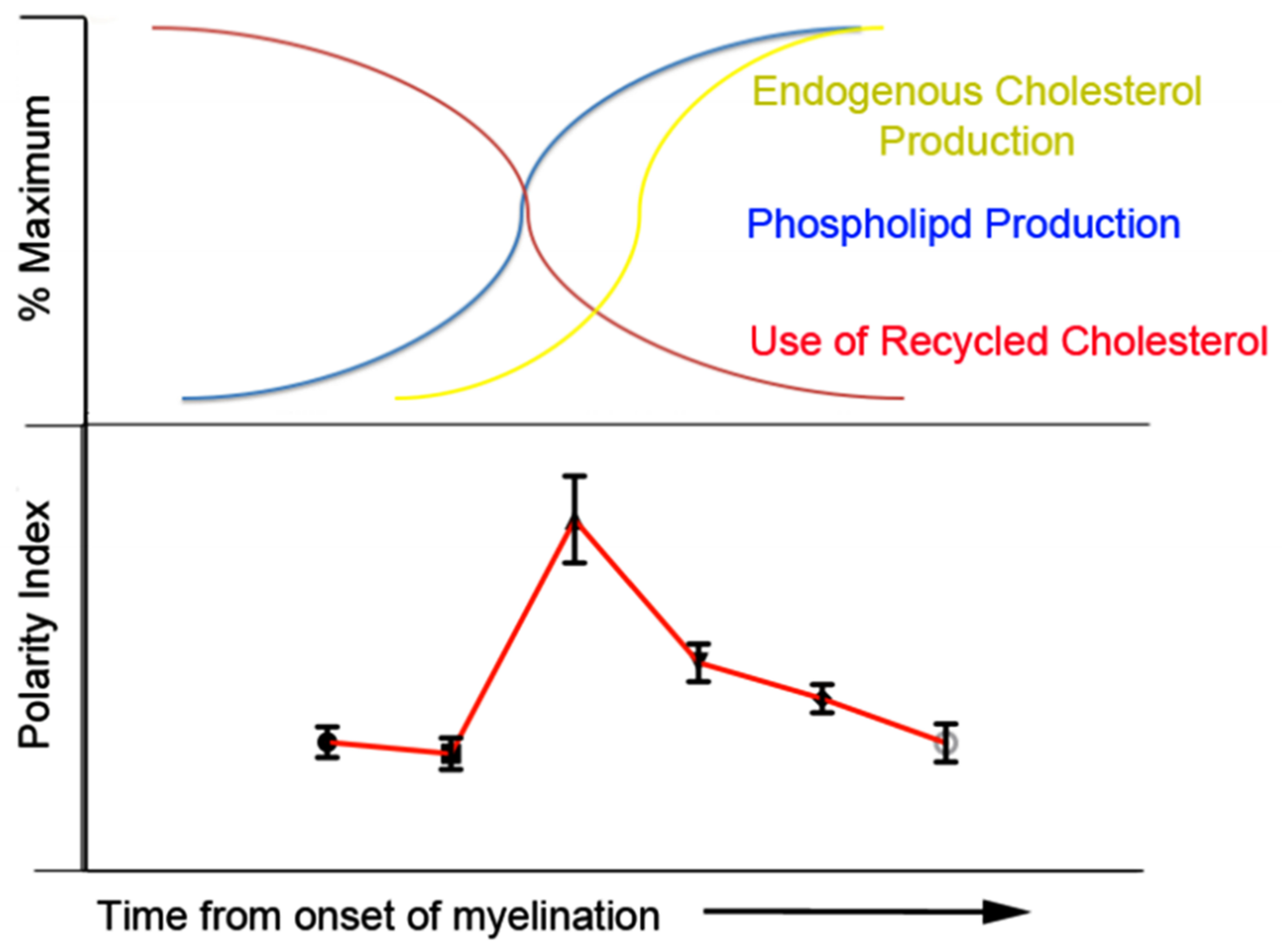
4.2. Regenerating Myelin Displays a Biphasic Progression in Polarity with Increasing Maturity
4.3. Composition of Culture Media May Influence Myelin Biochemistry
4.4. Myelination by Schwann Cells and SKP-SCs Proceeds in a Biochemically Similar Fashion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| bFGF | Beta fibroblast growth factor |
| BFP | Blue fluorescent protein |
| BSA | Bovine serum albumin |
| CMV | Cytomegalovirus |
| CNS | Central nervous system |
| CW | Continuous wavelength |
| DLPC | Dilauroylphosphatidylcholine |
| DMEM | Dubelco’s modified eagle media |
| DMSO | Dimethylsulfoxide |
| DNA | Deoxyribonucleic acid |
| DRG | Dorsal root ganglia |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| eGFP | Enhanced green fluorescent protein |
| ER | Endoplasmic reticulum |
| ESMD | Environmentally sensitive membrane dyes |
| F12 | Ham’s F-12 Nutrient Mixture |
| G | Gravity |
| GFP | Green fluorescent protein |
| HEK | Human embryonic kidney |
| HMG-CoA | 3-hydroxy-3-methylglutaryl-coenzyme |
| LV | Lentivirus |
| MBP | Myelin basic protein |
| mRNA | Messenger ribonucleic acid |
| NA | Numerical aperture |
| NF | Neurofilament |
| NGF | Nerve growth factor |
| NRSSA | Nile red solvatochromic shift assay |
| OD | Overdose |
| p# (eg. P3) | Post natal day # (eg. Post natal day 3) |
| P0 | Myelin protein zero |
| PBS | Phosphate buffered saline |
| PDL | Poly-d-lysine |
| Pen/Strep | Penicillin and streptomycin |
| PI | Polarity index |
| PLP | Proteolipid protein |
| PMP22 | Peripheral myelin protein 22 |
| PNS | Peripheral nervous system |
| SCAP | SREBP cleavage activation protein |
| Shi | Shiverer (gene) |
| SKPs | Skin-derived precursor cells |
| SKP-SCs | Skin-derived precursor Schwann cells |
| SREBP | Sterol regulatory element binding protein |
| VSV-G | Vesicular stomatitis virus glycoprotein |
References
- Dechemko, A.P.; Mély, Y.; Duportail, G.; Klymchenko, A.S. Monitoring biophysical properties of lipid membranes by environmentally-sensitive fluroescent probes. Biophys. J. 2009, 3461–3470. [Google Scholar] [CrossRef] [Green Version]
- Loving, G.S.; Sainlos, M.; Imperiali, B. Monitoring protein interactions and dynamics with solvatochromic fluorophores. Trends Biotechnol. 2010, 28, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kransnowska, E.K.; Bagatolli, L.A.; Gratton, E.; Parasassi, T. Surface properties of cholesterol-containing membranes detected by Prodan fluorescence. Biochim. Biophys. Acta (BBA) Biomembr. 2001, 1511, 330–340. [Google Scholar] [CrossRef] [Green Version]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.A.; Gratton, E. Laurdan and Prodan as polarity-sensitive fluorescent membrane probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Kusube, M.; Tamai, N.; Matsuki, H.; Kaneshina, S. Pressure-induced phase transitions of lipid bilayers observed by fluorescent probes Prodan and Laurdan. Biophys. Chem. 2005, 117, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Yablon, D.G.; Schilowitz, A.M. Solvatochromism of Nile Red in Non-Polar Solvents. Appl. Spectrosc. 2004, 58, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.K.; Raghuraman, H.; Chattopadyay, A. Membrane localization and dynamics of Nile Red: Effect of cholesterol. Biochim. Et Biophys. Acta 2007, 1768, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Greenspan, P.; Fowler, S. Spectrofluorometric studies of the lipid probe, nile red. J. Lipid Reserach 1985, 26, 781–789. [Google Scholar]
- Teo, W. Mechanisms of Central Axon and Myelin Injury Using Advanced Morphological and Biochemical Characterization. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2015. [Google Scholar]
- Grochmal, J.; Teo, W.; Ghambir, H.; Kumar, R.; Stratton, J.; Dhaliwal, R.; Brideau, C.; Biernaskie, J.; Stys, P.K.; Midha, R. A novel approach to 32-channel PNS myelin imaging in-vivo, with single axon resolution. J. Neurosurg 2018, 130, 163–171. [Google Scholar]
- Grochmal, J.; Dhaliwal, S.; Stys, P.K.; Van Minnen, J.; Midha, R. Skin derived precursor schwann cell myelination capacity in focal tibial demyelination. Muscle Nerve 2013, 50, 262–272. [Google Scholar] [CrossRef]
- He, Y.; Kim, J.Y.; Dupree, J.; Tewari, A.; Melendez-Vasquez, C.; Svaren, J.; Casaccia, P. Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat. Neurosci. 2010, 13, 1472–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, P.M.; Schachner, M.; Bunge, R.P. Inhibition of Schwann cell myelination in-vitro by antibody to the L1 adhesion molecule. J. Neurosci. 1990, 10, 3635–3645. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, A.F.; Shan, W.S.; Colman, D.R.; Pedraza, L. Rapid method for culturing embryonic neuron-glia cocultures. J. Neurosci. Res. 2003, 72, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Borchel, G.H.; Santosa, K.A.; Flagg, E.R.; Tong, A.Y.; Kasukurthi, R.; Newton, P.; Yan, Y.; Hunter, D.A.; Johnson, P.J.; et al. A transgenic rat expressing green fluorescent protein (GFP) in peripheral nerves provides a new hindlimb model for the study of nerve injury and regeneration. J. Neurosci. Methods 2012, 204, 19–27. [Google Scholar] [CrossRef]
- Toma, J.G.; McKenzie, I.A.; Bagli, D.; Miller, F.D. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells 2005, 23, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.K.; Gordon, T.; Addas, B.M.; Kemp, S.W.; Midha, R. Skin-derived precursor cells enhance peripheral nerve regeneration following chronic denervation. Exp. Neurol. 2009, 223, 221–228. [Google Scholar] [CrossRef]
- Walsh, S.; Biernaskie, J.; Kemp, S.W.; Midha, R. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience 2009, 164, 1097–1107. [Google Scholar] [CrossRef]
- Komiyama, T.; Nakao, Y.; Toyama, Y.; Asou, H.; Vacanti, C.A.; Vacanti, M.P. A novel technique to isolate adult Schwann cells for an artificial nerve conduit. J. Neurosci. Methods 2003, 122, 195–200. [Google Scholar] [CrossRef]
- Court, F.A.; Midha, R.; Cisterna, B.A.; Grochmal, J.; Shakhbazau, A.; Hendriks, W.T.; Van Minnen, J. Morphological evidence for a transport of ribosomes from schwann cells to regenerating axons. Glia 2011, 59, 1529–1539. [Google Scholar] [CrossRef]
- Vroemen, M.; Weidner, N. Purification of Schwann cells by selection of p75 low affinity nerve growth factor receptor expressing cell sfrom adult peripheral nerve. J. Neurosci. Methods 2003, 124, 135–143. [Google Scholar] [CrossRef]
- Biernaskie, J.; Sparling, J.S.; Liu, J.; Shannon, C.P.; Plemel, J.R.; Xie, Y.; Miller, F.D.; Tetzlaff, W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J. Neurosci. 2007, 27, 9545–9559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, I.A.; Biernaskie, J.; Toma, J.G.; Midha, R.; Miller, F.D. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 2006, 14, 6651–6660. [Google Scholar] [CrossRef] [PubMed]
- Kucherak, O.A.; Oncul, S.; Darwinch, Z.; Yushchenko, D.A.; Amtz, Y.; Didier, P.; Mely, Y.; Klymchenko, A.S. Switchable Nile Red-based probe for cholesterol and lipid order at the outer leaflet of biomembranes. J. Am. Chem. Soc. 2010, 132, 4907–4916. [Google Scholar] [CrossRef] [PubMed]
- Goodrum, J.F.; Weaver, J.E.; Goines, N.D.; Bouldin, T.W. Fatty acids from degenerating myelin lipids are conserved and reutilized for myelin synthesis during regeneration in peripheral nerve. J. Neurochem. 1995, 65, 1752–1759. [Google Scholar] [CrossRef]
- Goodrum, J.F.; Earnhardt, T.; Golmes, N.; Bouldin, T.W. Fate of myelin lipids during degeneration and regeneration of peripheral nerve: An autoradiographic study. J. Neurosci 1994, 14, 357–367. [Google Scholar] [CrossRef]
- Goodrum, J.F. Cholesterol synthesis is down-regulated during regeneration of periphral nerve. J. Neurochem. 1990, 54, 1709–1715. [Google Scholar] [CrossRef]
- Goodrum, J.F. Cholesterol from degenerating nerve myelin becomes associated with lipoproteins containing apolipoprotein E. J. Neurochem. 1991, 1991, 2082–2086. [Google Scholar] [CrossRef]
- Rawlins, F.A.; Hedley-Whyte, E.T.; Villegas, G.M.; Uzman, B.G. Reutilization of cholesterol-1,2-3H in the degenerating and regenerating mosue sciatic nerve. Lab. Invest. 1970, 22, 237–240. [Google Scholar]
- Rawlins, F.A.; Villegas, G.M.; Hedley-Whyte, E.T.; Uzman, B.G. Fine structural localization of cholesterol-1,2-3H in degenerating and regenerating mouse sciatic nerve. J. Cell Biol 1972, 52, 615–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harry, G.J.; Goodrum, J.F.; Bouldin, T.W.; Wagner-Recio, M.; Toews, A.D.; Morell, P. Tellurium-induced neuropathy: Metabolic alterations associated with demyelination and remyelination in rat sciatic nerve. J. Neurochem. 1989, 52, 938–945. [Google Scholar] [CrossRef]
- White, F.V.; Toews, A.D.; Goodrum, J.F.; Novicki, D.L.; Bouldin, T.W.; Morell, P. Lipid metabolism during early stages of Wallerian degeneration in the sciatic nerve of the rat. J. Neurochem. 1989, 52, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W. Cholesterol transport protein with expanding role in cell biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Kandustch, A.A. Apo B-dependent and independent cellular cholesterol homeostasis. In Biochemistry and Biology of Plasma Lipoproteins; Scanu, A.M., Spector, A.A., Eds.; Marcel Dekker: New York, NY, USA, 1986; pp. 281–300. [Google Scholar]
- Gelman, B.B.; Rifia, N.; Goodrum, J.F.; Bouldin, T.W.; Krigman, M.R. Apolipoprotein E is released by rat sciatic nerve during segmental demyelination and remyelination. J. Neuropathol. Exp. Neurol. 1987, 46, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Gelman, B.B.; Goodrum, J.F.; Bouldin, T.W. Macrophage apolipoprotein synthesis and endoneural distribution as a response to segmental demyelination. J. Neuropathol. Exp. Neurol. 1991, 50, 383–407. [Google Scholar] [CrossRef]
- Watson, A.D. Thematic review series: Systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: A global approach to lipid analysis in biological systems. J. Lipid Res. 2006, 47, 2101–2111. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochmal, J.; Teo, W.; Gambhir, H.; Kumar, R.; Stratton, J.A.; Dhaliwal, R.; Brideau, C.; Biernaskie, J.; Stys, P.; Midha, R. Spectral Characterization of Stem Cell-Derived Myelination within the Injured Adult PNS Using the Solvatochromic Dye Nile Red. Cells 2020, 9, 189. https://doi.org/10.3390/cells9010189
Grochmal J, Teo W, Gambhir H, Kumar R, Stratton JA, Dhaliwal R, Brideau C, Biernaskie J, Stys P, Midha R. Spectral Characterization of Stem Cell-Derived Myelination within the Injured Adult PNS Using the Solvatochromic Dye Nile Red. Cells. 2020; 9(1):189. https://doi.org/10.3390/cells9010189
Chicago/Turabian StyleGrochmal, Joey, Wulin Teo, Hardeep Gambhir, Ranjan Kumar, Jo Anne Stratton, Raveena Dhaliwal, Craig Brideau, Jeff Biernaskie, Peter Stys, and Rajiv Midha. 2020. "Spectral Characterization of Stem Cell-Derived Myelination within the Injured Adult PNS Using the Solvatochromic Dye Nile Red" Cells 9, no. 1: 189. https://doi.org/10.3390/cells9010189
APA StyleGrochmal, J., Teo, W., Gambhir, H., Kumar, R., Stratton, J. A., Dhaliwal, R., Brideau, C., Biernaskie, J., Stys, P., & Midha, R. (2020). Spectral Characterization of Stem Cell-Derived Myelination within the Injured Adult PNS Using the Solvatochromic Dye Nile Red. Cells, 9(1), 189. https://doi.org/10.3390/cells9010189




