Direct Activation of TRPC3 Channels by the Antimalarial Agent Artemisinin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Detection of Cell Viability
2.4. Fluorometric [Ca2+]i Imaging
2.5. Immunofluorescence Analysis
2.6. Electrophysiological Procedures
2.7. Statistical Analyses
3. Results
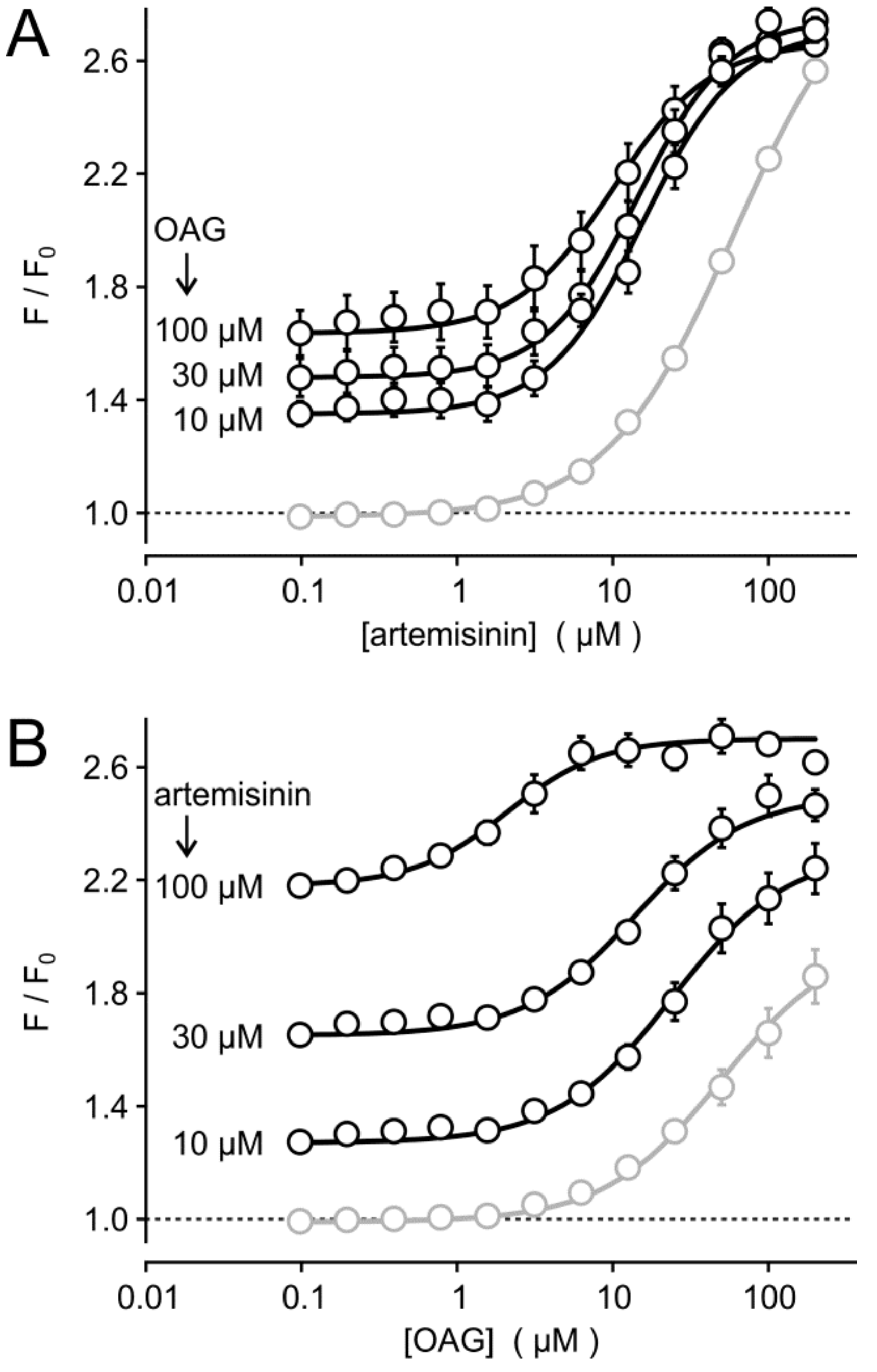
3.1. Primary Screening, Hit Validation, and Concentration–Response Analyses
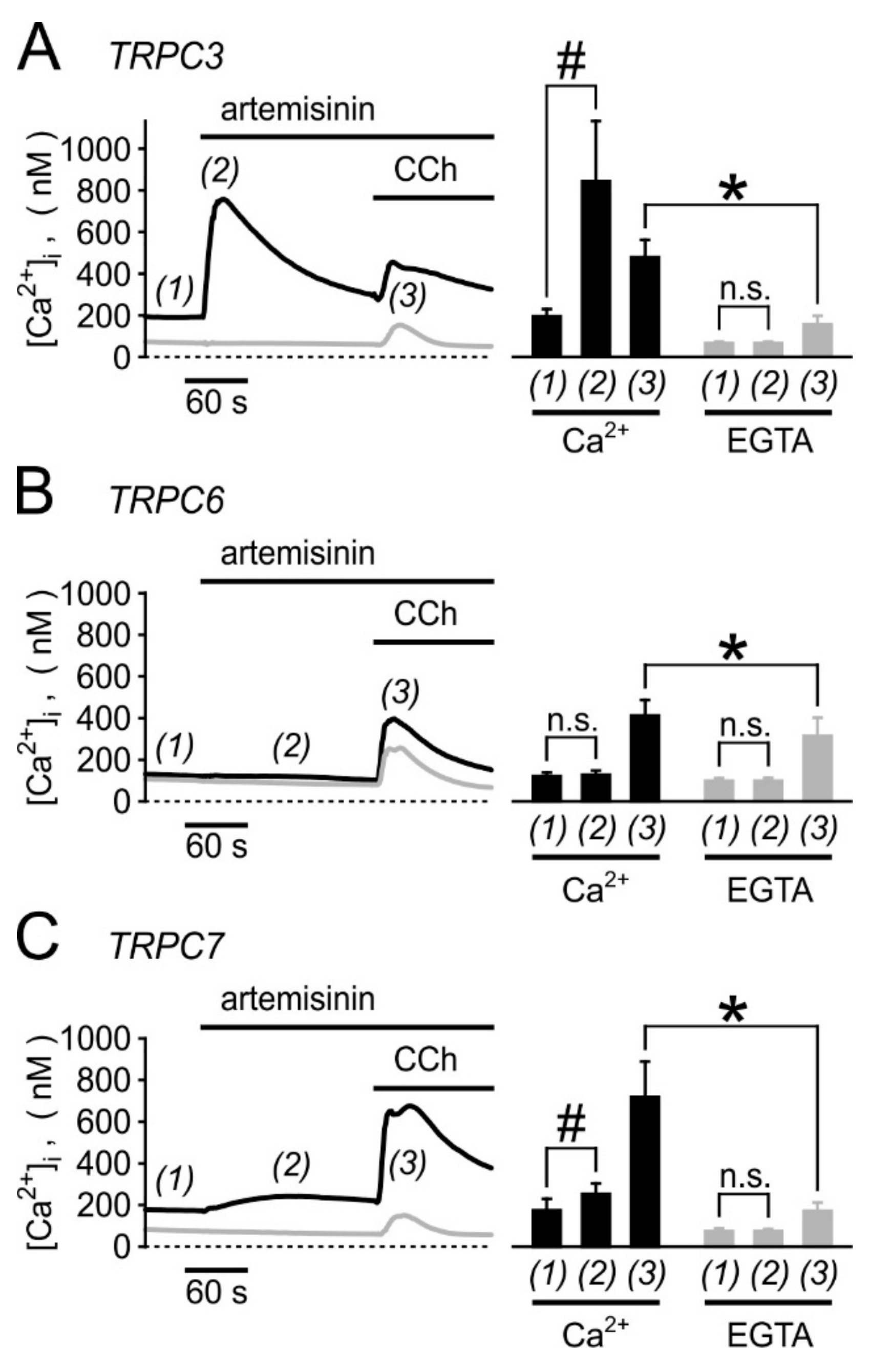
3.2. Selectivity Profiling and Analysis of Cytotoxicity
3.3. Synergism of Artemisinin and Diacylglycerol Effects on TRPC3
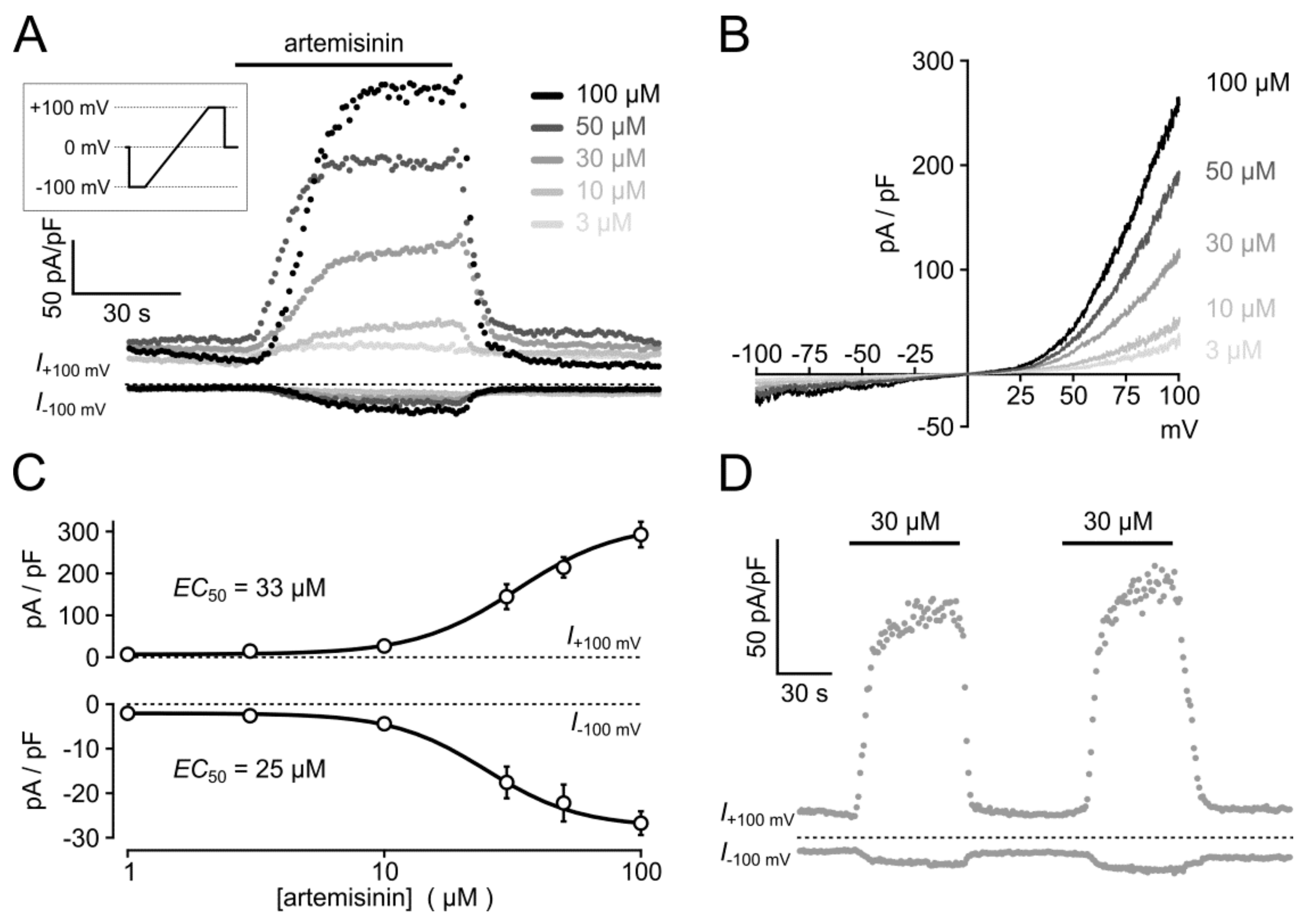
3.4. Electrophysiological Analysis
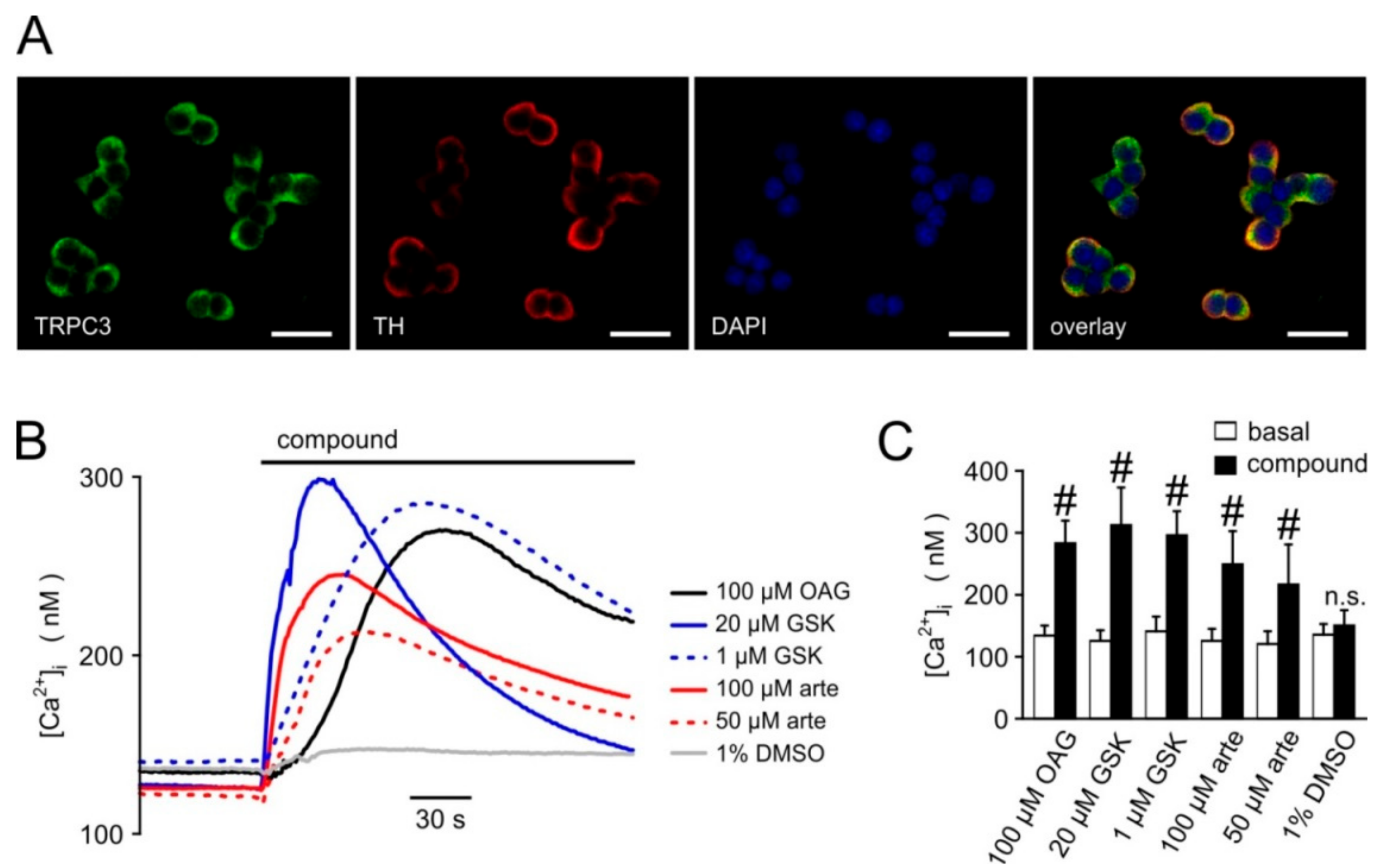
3.5. Biological Activity Towards Native TRPC3-Bearing Channel Complexes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boulay, G.; Zhu, X.; Peyton, M.; Jiang, M.; Hurst, R.; Stefani, E.; Birnbaumer, L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J. Biol. Chem. 1997, 272, 29672–29680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, T.; Obukhov, A.G.; Schaefer, M.; Harteneck, C.; Gudermann, T.; Schultz, G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999, 397, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Dragicevic, E.; Adelsberger, H.; Henning, H.A.; Sumser, M.; Abramowitz, J.; Blum, R.; Dietrich, A.; Freichel, M.; Flockerzi, V.; et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron 2008, 59, 392–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Blot, F.G.; Wong, A.B.; Osório, C.; Adolfs, Y.; Pasterkamp, R.J.; Hartmann, J.; Becker, E.B.; Boele, H.-J.; de Zeeuw, C.I.; et al. TRPC3 is a major contributor to functional heterogeneity of cerebellar Purkinje cells. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.B.E.; Oliver, P.L.; Glitsch, M.D.; Banks, G.T.; Achilli, F.; Hardy, A.; Nolan, P.M.; Fisher, E.M.C.; Davies, K.E. A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc. Natl. Acad. Sci. USA 2009, 106, 6706–6711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiapko, O.; Groschner, K. TRPC3 as a target of novel therapeutic interventions. Cells 2018, 83. [Google Scholar] [CrossRef] [Green Version]
- Doleschal, B.; Primessnig, U.; Wölkart, G.; Wolf, S.; Schernthaner, M.; Lichtenegger, M.; Glasnov, T.N.; Kappe, C.O.; Mayer, B.; Antoons, G.; et al. TRPC3 contributes to regulation of cardiac contractility and arrhythmogenesis by dynamic interaction with NCX1. Cardiovasc. Res. 2015, 106, 163–173. [Google Scholar] [CrossRef]
- Svobodova, B.; Lichtenegger, M.; Platzer, D.; Di Giuro, C.M.L.; de La Cruz, G.G.; Glasnov, T.; Schreibmayer, W.; Groschner, K. A single point mutation in the TRPC3 lipid-recognition window generates supersensitivity to benzimidazole channel activators. Cell Calcium 2019, 79, 27–34. [Google Scholar] [CrossRef]
- Xu, X.; Lozinskaya, I.; Costell, M.; Lin, Z.; Ball, J.A.; Bernard, R.; Behm, D.J.; Marino, J.P.; Schnackenberg, C.G. Characterization of small molecule TRPC3 and TRPC6 agonist and antagonists. Biophys. J. 2013, 104, 454a. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Ding, M.; Zhu, Y.; Lu, Y.; Du, J.; Miller, M.; Tian, J.; Zhu, J.; Xu, J.; Wen, M.; et al. Pyrazolopyrimidines as potent stimulators for transient receptor potential canonical 3/6/7 channels. J. Med. Chem. 2017, 60, 4680–4692. [Google Scholar] [CrossRef]
- Tesfai, Y.; Brereton, H.M.; Barritt, G.J. A diacylglycerol-activated Ca2+ channel in PC12 cells (an adrenal chromaffin cell line) correlates with expression of the TRP-6 (transient receptor potential) protein. Biochem. J. 2001, 358, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.M.; Schaefer, M.; Hill, K. Riluzole activates TRPC5 channels independently of PLC activity. Br. J. Pharmacol. 2014, 171, 158–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, M.; Albrecht, N.; Hofmann, T.; Gudermann, T.; Schultz, G. Diffusion-limited translocation mechanism of protein kinase C isotypes. FASEB J. 2001, 15, 1634–1636. [Google Scholar] [CrossRef] [PubMed]
- Urban, N.; Wang, L.; Kwiek, S.; Rademann, J.; Kuebler, W.M.; Schaefer, M. Identification and validation of larixyl acetate as a potent TRPC6 inhibitor. Mol. Pharmacol. 2016, 89, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Edelstein, A.; Amodaj, N.; Hoover, K.; Vale, R.; Stuurman, N. Computer control of microscopes using µManager. Curr. Protoc. Mol. Biol. 2010, 92, 14.20.1–14.20.17. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lenz, J.C.; Reusch, H.P.; Albrecht, N.; Schultz, G.; Schaefer, M. Ca2+-controlled competitive diacylglycerol binding of protein kinase C isoenzymes in living cells. J. Cell Biol. 2002, 159, 291–302. [Google Scholar] [CrossRef]
- Frank, J.A.; Yushchenko, D.A.; Hodson, D.J.; Lipstein, N.; Nagpal, J.; Rutter, G.A.; Rhee, J.-S.; Gottschalk, A.; Brose, N.; Schultz, C.; et al. Photoswitchable diacylglycerols enable optical control of protein kinase C. Nat. Chem. Biol. 2016, 12, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Leinders-Zufall, T.; Storch, U.; Bleymehl, K.; Mederos Y Schnitzler, M.; Frank, J.A.; Konrad, D.B.; Trauner, D.; Gudermann, T.; Zufall, F. PhoDAGs enable optical control of diacylglycerol-sensitive transient receptor potential channels. Cell Chem. Biol. 2018, 25, 215–223.e3. [Google Scholar] [CrossRef] [Green Version]
- Tiapko, O.; Shrestha, N.; Lindinger, S.; La Guedes de Cruz, G.; Graziani, A.; Klec, C.; Butorac, C.; Graier, W.F.; Kubista, H.; Freichel, M.; et al. Lipid-independent control of endothelial and neuronal TRPC3 channels by light. Chem. Sci. 2019, 10, 2837–2842. [Google Scholar] [CrossRef] [Green Version]
- Leuner, K.; Kazanski, V.; Müller, M.; Essin, K.; Henke, B.; Gollasch, M.; Harteneck, C.; Müller, W.E. Hyperforin - a key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB J. 2007, 21, 4101–4111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sell, T.S.; Belkacemi, T.; Flockerzi, V.; Beck, A. Protonophore properties of hyperforin are essential for its pharmacological activity. Sci. Rep. 2014, 4, 7500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, R.; Okada, T.; Onoue, H.; Hara, Y.; Shimizu, S.; Naitoh, S.; Ito, Y.; Mori, Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ. Res. 2001, 88, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häfner, S.; Urban, N.; Schaefer, M. Discovery and characterization of a positive allosteric modulator of transient receptor potential canonical 6 (TRPC6) channels. Cell Calcium 2019, 78, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbulut, Y.; Gaunt, H.J.; Muraki, K.; Ludlow, M.J.; Amer, M.S.; Bruns, A.; Vasudev, N.S.; Radtke, L.; Willot, M.; Hahn, S.; et al. (-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew. Chem. Int. Ed Engl. 2015, 54, 3787–3791. [Google Scholar] [CrossRef]
- Klambauer, G.; Wischenbart, M.; Mahr, M.; Unterthiner, T.; Mayr, A.; Hochreiter, S. Rchemcpp: A web service for structural analoging in ChEMBL, Drugbank and the Connectivity Map. Bioinformatics 2015, 31, 3392–3394. [Google Scholar] [CrossRef] [Green Version]
- Batty, K.T.; Salman, S.; Moore, B.R.; Benjamin, J.; Lee, S.T.; Page-Sharp, M.; Pitus, N.; Ilett, K.F.; Mueller, I.; Hombhanje, F.W.; et al. Artemisinin-naphthoquine combination therapy for uncomplicated pediatric malaria: A pharmacokinetic study. Antimicrob. Agents Chemother. 2012, 56, 2472–2484. [Google Scholar] [CrossRef] [Green Version]
- Delves, M.; Plouffe, D.; Scheurer, C.; Meister, S.; Wittlin, S.; Winzeler, E.A.; Sinden, R.E.; Leroy, D. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: A comparative study with human and rodent parasites. PLoS Med. 2012, 9, e1001169. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, P.M.; Barton, V.E.; Ward, S.A. The molecular mechanism of action of artemisinin - the debate continues. Molecules 2010, 15, 1705–1721. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.-J.; Chia, W.N.; Loh, C.C.Y.; Li, Z.; Lee, Y.M.; He, Y.; Yuan, L.-X.; Lim, T.K.; Liu, M.; et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015, 6, 10111. [Google Scholar] [CrossRef]
- Lam, N.S.; Long, X.; Wong, J.W.; Griffin, R.C.; Doery, J.C.G. Artemisinin and its derivatives: A potential treatment for leukemia. Anticancer. Drugs 2019, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, W.; Wang, D.; Ma, X. Chinese medicine in the battle against obesity and metabolic diseases. Front. Physiol. 2018, 9, 850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, T.; Morishita, M.; Mori, Y.; Ito, S. Ca2+ store-independent augmentation of Ca2+i responses to G-protein coupled receptor activation in recombinantly TRPC5-expressed rat pheochromocytoma (PC12) cells. Neurosci. Lett. 2004, 358, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jiang, M.; Peyton, M.; Boulay, G.; Hurst, R.; Stefani, E.; Birnbaumer, L. Trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell 1996, 85, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Oboukhova, E.A.; Kumar, S.; Sturek, M.; Obukhov, A.G. Canonical transient receptor potential channels expression is elevated in a porcine model of metabolic syndrome. Mol. Endocrinol. 2009, 23, 689–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Antalffy, B.; Kang, D.; Orr, H.T.; Zoghbi, H.Y. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat. Neurosci. 2000, 3, 157–163. [Google Scholar] [CrossRef]
- Han, L.; Li, J. Canonical transient receptor potential 3 channels in atrial fibrillation. Eur. J. Pharmacol. 2018, 837, 1–7. [Google Scholar] [CrossRef]
- Seo, K.; Rainer, P.P.; Shalkey Hahn, V.; Lee, D.-I.; Jo, S.-H.; Andersen, A.; Liu, T.; Xu, X.; Willette, R.N.; Lepore, J.J.; et al. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2014, 111, 1551–1556. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Si, B.; Ju, J.-F.; Zhu, M.; You, F.; Wang, D.; Ren, J.; Ning, Y.-S.; Zhang, F.-Q.; Dong, K.; et al. Nicotine induces cardiomyocyte hypertrophy through TRPC3-mediated Ca2+/NFAT signalling pathway. Can. J. Cardiol. 2016, 32, 1260.e1–1260.e10. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Iribe, G.; Nishida, M.; Naruse, K. Role of TRPC3 and TRPC6 channels in the myocardial response to stretch: Linking physiology and pathophysiology. Prog. Biophys. Mol. Biol. 2017, 130, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Domes, K.; Patrucco, E.; Loga, F.; Dietrich, A.; Birnbaumer, L.; Wegener, J.W.; Hofmann, F. Murine cardiac growth, TRPC channels, and cGMP kinase I. Pflugers Arch. 2015, 467, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Saliba, Y.; Karam, R.; Smayra, V.; Aftimos, G.; Abramowitz, J.; Birnbaumer, L.; Farès, N. Evidence of a role for fibroblast transient receptor potential canonical 3 Ca2+ channel in renal fibrosis. J. Am. Soc. Nephrol. 2015, 26, 1855–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.A.; Xue, A.; Chini, E.N.; Thompson, M.; Sieck, G.C.; Wylam, M.E. Role of transient receptor potential C3 in TNF-α-enhanced calcium influx in human airway myocytes. Am. J. Respir. Cell Mol. Biol. 2006, 35, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, T.; Zheng, Y.-M.; Vincent, P.A.; Cai, D.; Rosenberg, P.; Wang, Y.-X. Canonical transient receptor potential 3 channels activate NF-κB to mediate allergic airway disease via PKC-α/IκB-α and calcineurin/IκB-β pathways. FASEB J. 2016, 30, 214–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhani, H.; Ase, A.R.; Grant, R.; O’Donnell, D.; Groschner, K.; Séguéla, P. Contribution of TRPC3 to store-operated calcium entry and inflammatory transductions in primary nociceptors. Mol. Pain 2014, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urban, N.; Schaefer, M. Direct Activation of TRPC3 Channels by the Antimalarial Agent Artemisinin. Cells 2020, 9, 202. https://doi.org/10.3390/cells9010202
Urban N, Schaefer M. Direct Activation of TRPC3 Channels by the Antimalarial Agent Artemisinin. Cells. 2020; 9(1):202. https://doi.org/10.3390/cells9010202
Chicago/Turabian StyleUrban, Nicole, and Michael Schaefer. 2020. "Direct Activation of TRPC3 Channels by the Antimalarial Agent Artemisinin" Cells 9, no. 1: 202. https://doi.org/10.3390/cells9010202
APA StyleUrban, N., & Schaefer, M. (2020). Direct Activation of TRPC3 Channels by the Antimalarial Agent Artemisinin. Cells, 9(1), 202. https://doi.org/10.3390/cells9010202




