RhoA-GTPase Modulates Neurite Outgrowth by Regulating the Expression of Spastin and p60-Katanin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. Culture of Primary DRG Neurons
2.1.2. Culture and Neuronal Induction of PC12 Cells
2.2. Pharmacological Treatment
2.3. Cell Transfection and Lentivirus Infection
2.4. Immunocytochemistry
2.5. Measurement of Neurite Outgrowth
2.6. Western Blotting
2.7. RNA Extraction and Quantitative Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. The RhoA Signaling Pathway Negatively Modulates Neurite Outgrowth in DRG Neurons
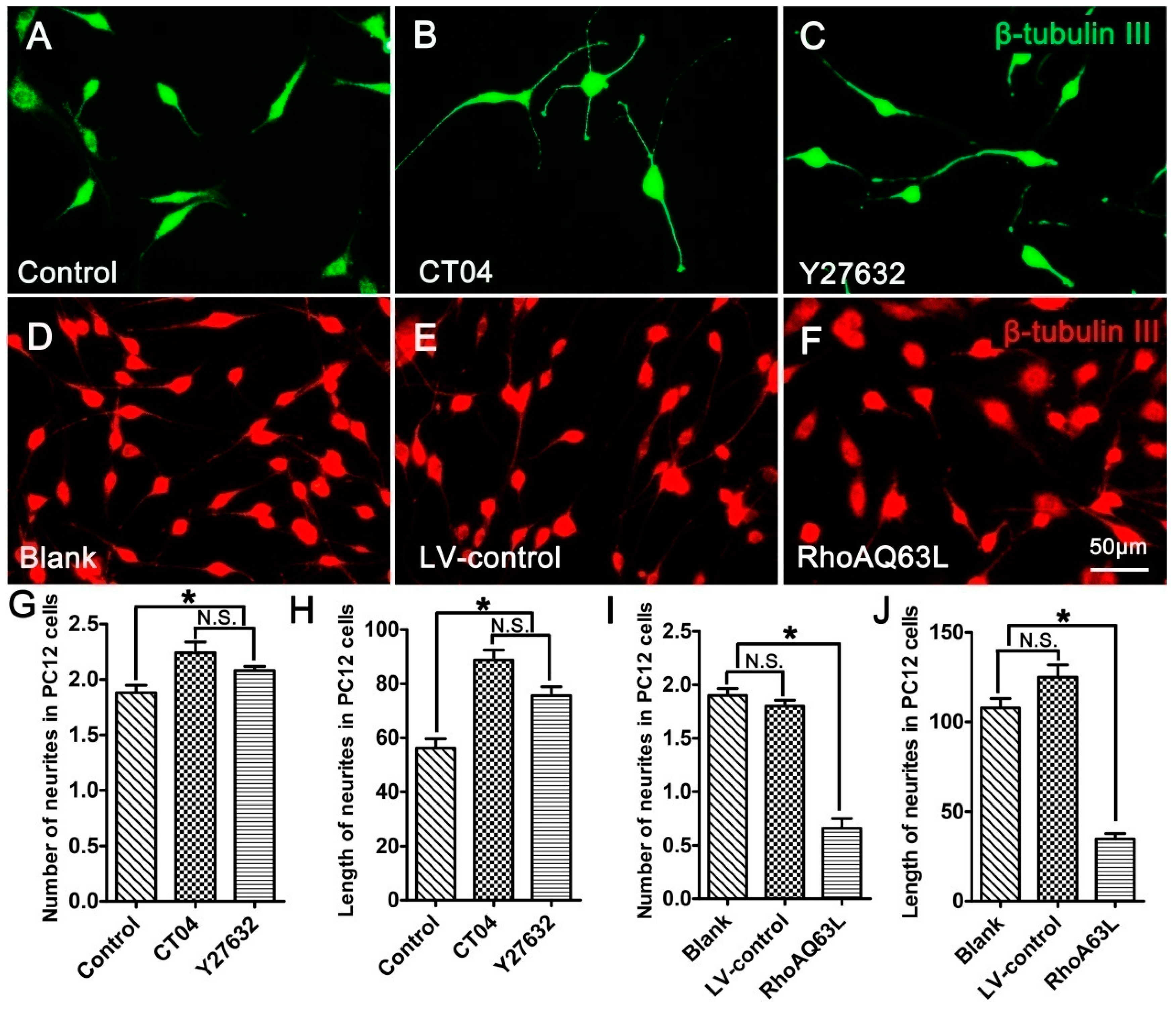
3.2. The RhoA Pathway Negatively Regulates Neurite Outgrowth in Neuronally Differentiated PC12 Cells
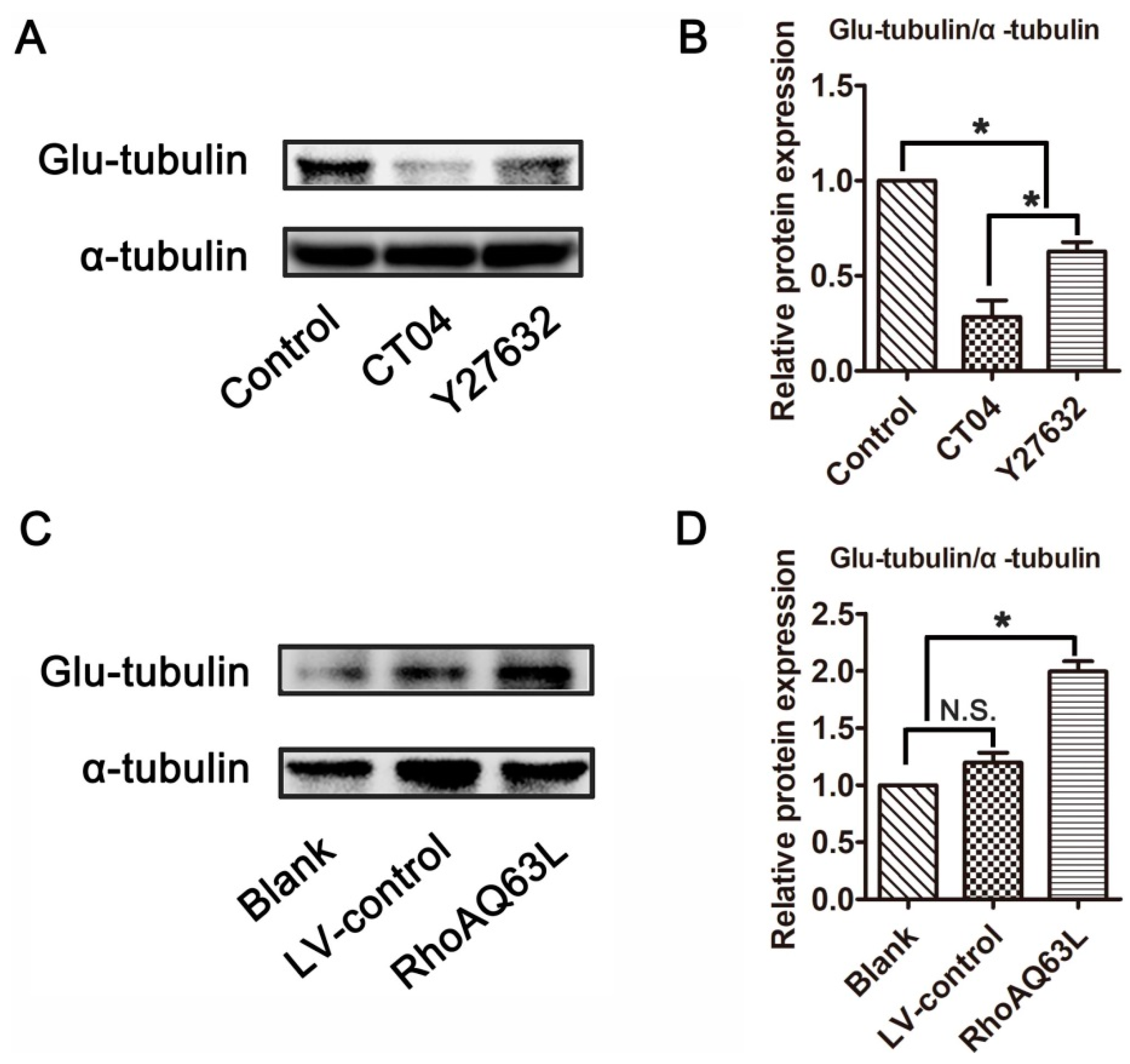
3.3. The RhoA Signaling Pathway Increases Glu-Tubulin Expression in Neuronally Differentiated PC12 Cells
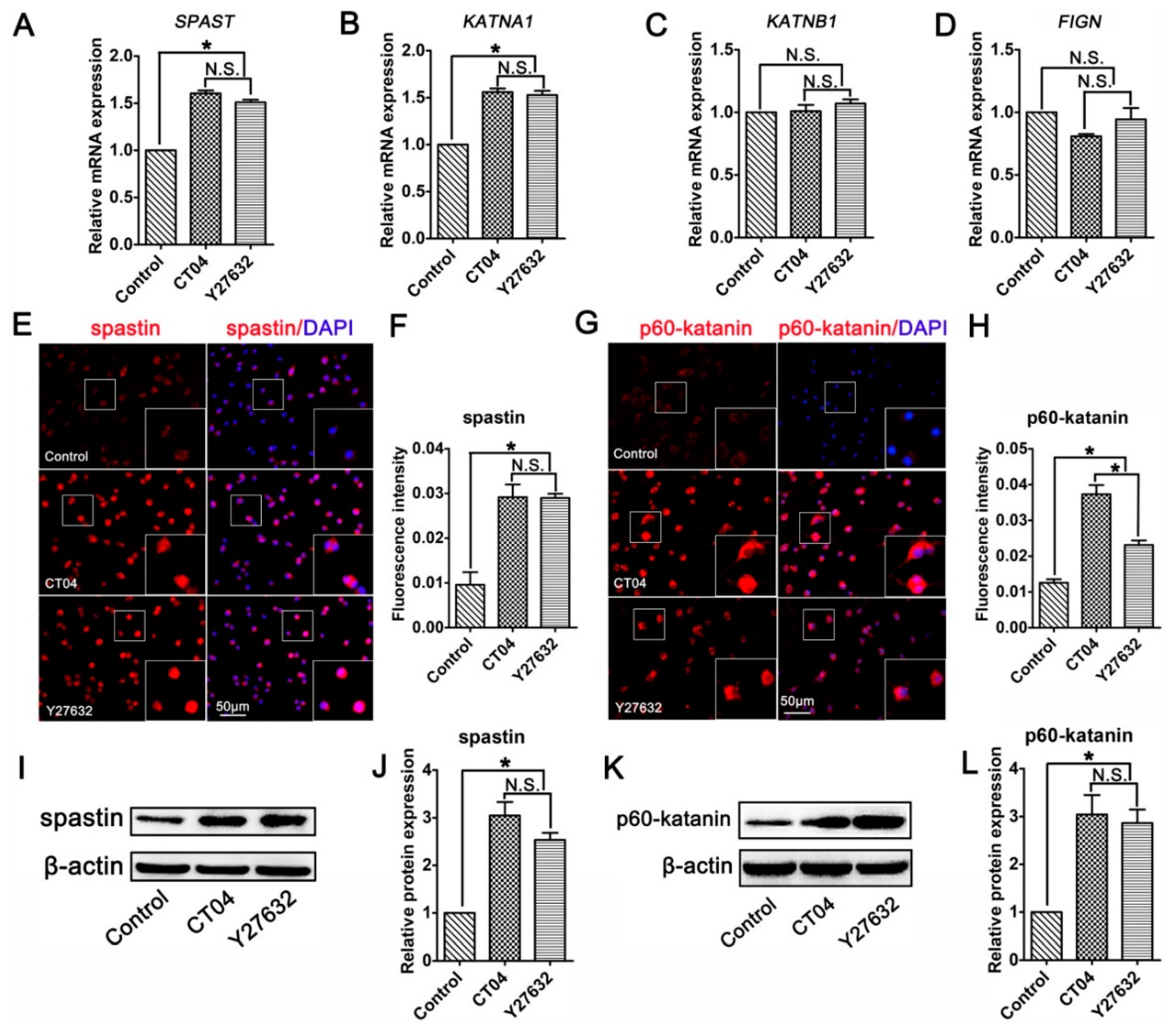
3.4. The RhoA Signaling Pathway Inhibits Spastin and p60-Katanin Expression
3.5. Spastin or p60-Katanin Knockdown Reverses the Positive Effect of CT04 on Neurite Outgrowth
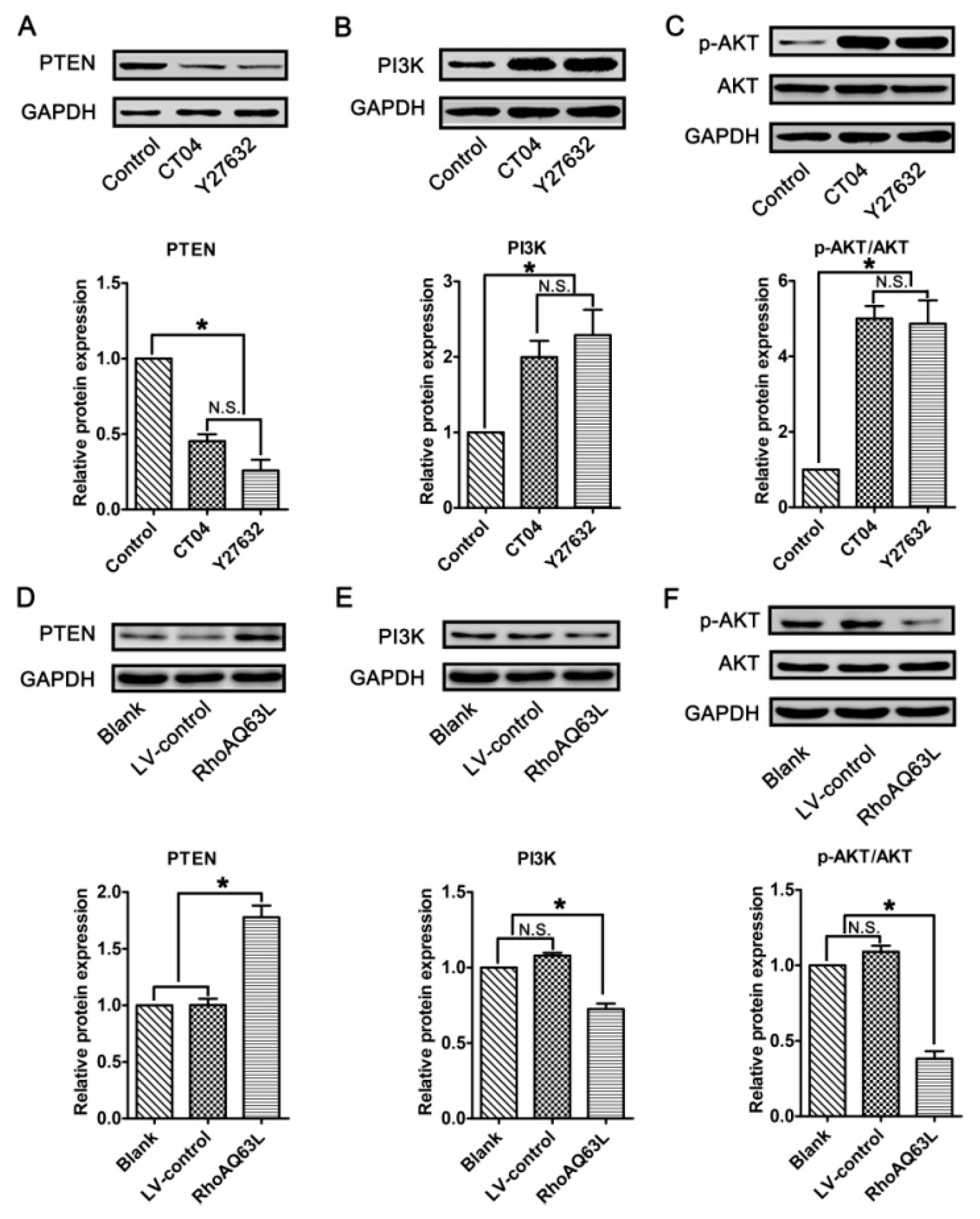
3.6. The Regulation of Spastin and p60-Katanin Expression by the RhoA Signaling Pathway is Independent of p38 or AKT
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Miller, K.E.; Suter, D.M. An Integrated Cytoskeletal Model of Neurite Outgrowth. Front. Cell. Neurosci. 2018, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Yadaw, A.S.; Siddiq, M.M.; Rabinovich, V.; Tolentino, R.; Hansen, J.; Iyengar, R. Dynamic balance between vesicle transport and microtubule growth enables neurite outgrowth. PLoS Comput. Biol. 2019, 15, e1006877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, A.W.; Schoonderwoert, V.T.; Ji, L.; Mederios, N.; Danuser, G.; Forscher, P. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev. Cell 2008, 15, 146–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omotade, O.F.; Pollitt, S.L.; Zheng, J.Q. Actin-based growth cone motility and guidance. Mol. Cell. Neurosci. 2017, 84, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, M.J.; Leterrier, C. The functional architecture of axonal actin. Mol. Cell. Neurosci. 2018, 91, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.W.; Gupton, S.L.; Gertler, F.B. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb. Perspect. Biol. 2011, 3, a001800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, O.I.; Baas, P.W. Microtubules and Growth Cones: Motors Drive the Turn. Trends Neurosci. 2016, 39, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foldi, I.; Szikora, S.; Mihaly, J. Formin’ bridges between microtubules and actin filaments in axonal growth cones. Neural Regen. Res. 2017, 12, 1971–1973. [Google Scholar]
- Baas, P.W.; Rao, A.N.; Matamoros, A.J.; Leo, L. Stability properties of neuronal microtubules. Cytoskeleton 2016, 73, 442–460. [Google Scholar] [CrossRef] [Green Version]
- Menon, S.; Gupton, S.L. Building Blocks of Functioning Brain: Cytoskeletal Dynamics in Neuronal Development. Int. Rev. Cell Mol. Biol. 2016, 322, 183–245. [Google Scholar] [PubMed] [Green Version]
- Mi, S.; Blake Pepinsky, R.; Cadavid, D. Blocking LINGO-1 as a Therapy to Promote CNS Repair: From Concept to the Clinic. CNS Drugs 2013, 27, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, G.; Rodemer, W.; Jin, L.-Q.; Shifman, M.; Selzer, M.E. The role of RhoA in retrograde neuronal death and axon regeneration after spinal cord injury. Neurobiol. Dis. 2017, 98, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-Q.; Liu, F.; Gao, M.-L.; Bai, J.; Wang, Y.-F. Inhibition of neurite outgrowth using commercial myelin associated glycoprotein-Fc in neuro-2a cells. Neural Regen. Res. 2018, 13, 1893. [Google Scholar] [CrossRef] [PubMed]
- Mi, S. Troy/Taj and its role in CNS axon regeneration. Cytokine Growth Factor Rev. 2008, 19, 245–251. [Google Scholar] [CrossRef]
- Blanquie, O.; Bradke, F. Cytoskeleton dynamics in axon regeneration. Curr. Opin. Neurobiol. 2018, 51, 60–69. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, J.; Rodemer, W.; Li, S.; Selzer, M.E. RhoA activation in axotomy-induced neuronal death. Exp. Neurol. 2018, 306, 76–91. [Google Scholar] [CrossRef]
- Hu, J.; Selzer, M.E. RhoA as a target to promote neuronal survival and axon regeneration. Neural Regen. Res. 2017, 12, 525–528. [Google Scholar]
- Joshi, A.R.; Bobylev, I.; Zhang, G.; Sheikh, K.A.; Lehmann, H.C. Inhibition of Rho-kinase differentially affects axon regeneration of peripheral motor and sensory nerves. Exp. Neurol. 2015, 263, 28–38. [Google Scholar] [CrossRef]
- Cen, L.-P.; Liang, J.-J.; Chen, J.-H.; Harvey, A.R.; Ng, T.K.; Zhang, M.; Pang, C.P.; Cui, Q.; Fan, Y.-M. AAV-mediated transfer of RhoA shRNA and CNTF promotes retinal ganglion cell survival and axon regeneration. Neuroscience 2017, 343, 472–482. [Google Scholar] [CrossRef]
- Joshi, A.R.; Muke, I.; Bobylev, I.; Lehmann, H.C. ROCK inhibition improves axonal regeneration in a preclinical model of amyotrophic lateral sclerosis. J. Comp. Neurol. 2019, 527, 2334–2340. [Google Scholar] [CrossRef]
- Cook, T.A.; Nagasaki, T.; Gundersen, G.G. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J. Cell Biol. 1998, 141, 175–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovelace, M.D.; Powter, E.E.; Coleman, P.R.; Zhao, Y.; Parker, A.; Chang, G.H.; Lay, A.J.; Hunter, J.; McGrath, A.P.; Jormakka, M.; et al. The RhoGAP protein ARHGAP18/SENEX localizes to microtubules and regulates their stability in endothelial cells. Mol. Biol. Cell 2017, 28, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, W.; Chen, J.; Li, S.; Guo, G. Rho-associated protein kinase modulates neurite extension by regulating microtubule remodeling and vinculin distribution. Neural Regen. Res. 2013, 8, 3027–3035. [Google Scholar] [PubMed]
- Rozés Salvador, V.; Heredia, F.; Berardo, A.; Palandri, A.; Wojnacki, J.; Vivinetto, A.L.; Sheikh, K.A.; Caceres, A.; Lopez, P.H.H. Anti-glycan antibodies halt axon regeneration in a model of Guillain Barrè Syndrome axonal neuropathy by inducing microtubule disorganization via RhoA–ROCK-dependent inactivation of CRMP-2. Exp. Neurol. 2016, 278, 42–53. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Fan, Z.; Wang, X.; Li, Z.; Wen, J.; Deng, J.; Tan, D.; Pan, M.; Hu, X.; et al. Ascorbic Acid Facilitates Neural Regeneration After Sciatic Nerve Crush Injury. Front. Cell. Neurosci. 2019, 13, 108. [Google Scholar] [CrossRef]
- Shen, L.-M.; Song, Z.-W.; Hua, Y.; Chao, X.; Liu, J.-B. miR-181d-5p promotes neurite outgrowth in PC12 Cells via PI3K/Akt pathway. CNS Neurosci. Ther. 2017, 23, 894–906. [Google Scholar] [CrossRef]
- Feng, X.; Lu, J.; He, Z.; Wang, Y.; Qi, F.; Pi, R.; Zhang, G. Mycobacterium smegmatis Induces Neurite Outgrowth and Differentiation in an Autophagy-Independent Manner in PC12 and C17.2 Cells. Front. Cell. Infect. Microbiol. 2018, 8, 201. [Google Scholar] [CrossRef]
- Csanaky, K.; Hess, M.W.; Klimaschewski, L. Membrane-Associated, Not Cytoplasmic or Nuclear, FGFR1 Induces Neuronal Differentiation. Cells 2019, 8, 243. [Google Scholar] [CrossRef] [Green Version]
- Terada, K.; Migita, K.; Matsushima, Y.; Kamei, C. Sigma-2 receptor as a potential therapeutic target for treating central nervous system disorders. Neural Regen. Res. 2019, 14, 1893–1894. [Google Scholar] [CrossRef]
- Pianu, B.; Lefort, R.; Thuiliere, L.; Tabourier, E.; Bartolini, F. The A 1–42 peptide regulates microtubule stability independently of tau. J. Cell Sci. 2014, 127, 1117–1127. [Google Scholar] [CrossRef] [Green Version]
- Tsushima, H.; Emanuele, M.; Polenghi, A.; Esposito, A.; Vassalli, M.; Barberis, A.; Difato, F.; Chieregatti, E. HDAC6 and RhoA are novel players in Abeta-driven disruption of neuronal polarity. Nat. Commun. 2015, 6, 7781. [Google Scholar] [CrossRef] [Green Version]
- McNally, F.J.; Roll-Mecak, A. Microtubule-severing enzymes: From cellular functions to molecular mechanism. J. Cell Biol. 2018, 217, 4057–4069. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-W.; Trottier, O.; Mahamdeh, M.; Howard, J. Spastin is a dual-function enzyme that severs microtubules and promotes their regrowth to increase the number and mass of microtubules. Proc. Natl. Acad. Sci. USA 2019, 116, 5533–5541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riano, E.; Martignoni, M.; Mancuso, G.; Cartelli, D.; Crippa, F.; Toldo, I.; Siciliano, G.; Di Bella, D.; Taroni, F.; Bassi, M.T.; et al. Pleiotropic effects of spastin on neurite growth depending on expression levels. J. Neurochem. 2009, 108, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Yu, W.; Liu, M.; Solowska, J.M.; Baas, P.W.; Forscher, P. Basic Fibroblast Growth Factor Elicits Formation of Interstitial Axonal Branches via Enhanced Severing of Microtubules. Mol. Biol. Cell 2010, 21, 334–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.W.; Oh, K.H.; Park, E.; Chang, H.M.; Park, J.M.; Seong, M.W.; Ka, S.H.; Song, W.K.; Park, D.E.; Baas, P.W.; et al. USP47 and C terminus of Hsp70-interacting protein (CHIP) antagonistically regulate katanin-p60-mediated axonal growth. J. Neurosci. 2013, 33, 12728–12738. [Google Scholar] [CrossRef] [Green Version]
- Matamoros, A.J.; Tom, V.J.; Wu, D.; Rao, Y.; Sharp, D.J.; Baas, P.W. Knockdown of Fidgetin Improves Regeneration of Injured Axons by a Microtubule-Based Mechanism. J. Neurosci. 2019, 39, 2011–2024. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, E.; Yamanaka, H.; Kobayashi, K.; Yagi, H.; Sakagami, M.; Noguchi, K. RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain. Glia 2015, 63, 216–228. [Google Scholar] [CrossRef]
- Stankiewicz, T.R.; Linseman, D.A. Rho family GTPases: Key players in neuronal development, neuronal survival, and neurodegeneration. Front. Cell. Neurosci. 2014, 8, 314. [Google Scholar] [CrossRef] [Green Version]
- Nikolaeva, I.; Kazdoba, T.M.; Crowell, B.; D’Arcangelo, G. Differential roles for Akt and mTORC1 in the hypertrophy of Pten mutant neurons, a cellular model of brain overgrowth disorders. Neuroscience 2017, 354, 196–207. [Google Scholar] [CrossRef]
- Zhu, J.L.; Wu, Y.Y.; Wu, D.; Luo, W.F.; Zhang, Z.Q.; Liu, C.F. SC79, a novel Akt activator, protects dopaminergic neuronal cells from MPP(+) and rotenone. Mol. Cell. Biochem. 2019, 461, 81–89. [Google Scholar] [CrossRef]
- Jeon, C.-Y.; Moon, M.-Y.; Kim, J.-H.; Kim, H.-J.; Kim, J.-G.; Li, Y.; Jin, J.-K.; Kim, P.-H.; Kim, H.-C.; Meier, K.E.; et al. Control of neurite outgrowth by RhoA inactivation. J. Neurochem. 2012, 120, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, Y.; Chen, Y. Spatiotemporal Regulation of Rho GTPases in Neuronal Migration. Cells 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.; Webber, C.A.; Wang, J.; Xu, Y.; Martinez, J.A.; Liu, W.Q.; McDonald, D.; Guo, G.F.; Nguyen, M.D.; Zochodne, D.W. Activated RHOA and peripheral axon regeneration. Exp. Neurol. 2008, 212, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yamashita, T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front. Neurosci. 2014, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, J.; Liu, X.; Cheng, Y.U.; Deng, L.; Zhong, Y. Y-39983 downregulates RhoA/Rho-associated kinase expression during its promotion of axonal regeneration. Oncol. Rep. 2013, 29, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.H.K. Myelin-Associated Inhibitors Regulate Cofilin Phosphorylation and Neuronal Inhibition through LIM Kinase and Slingshot Phosphatase. J. Neurosci. 2006, 26, 1006–1015. [Google Scholar] [CrossRef] [Green Version]
- Kalpachidou, T.; Spiecker, L.; Kress, M.; Quarta, S. Rho GTPases in the Physiology and Pathophysiology of Peripheral Sensory Neurons. Cells 2019, 8, 591. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Ye, Y.; Ji, Z.; Tan, M.; Li, S.; Zhang, J.; Guo, G.; Lin, H. Katanin p60 promotes neurite growth and collateral formation in the hippocampus. Int. J. Clin. Exp. Med. 2014, 7, 2463–2470. [Google Scholar]
- Ji, Z.; Zhang, G.; Chen, L.; Li, J.; Yang, Y.; Cha, C.; Zhang, J.; Lin, H.; Guo, G. Spastin Interacts with CRMP5 to Promote Neurite Outgrowth by Controlling the Microtubule Dynamics. Dev. Neurobiol. 2018, 78, 1191–1205. [Google Scholar] [CrossRef]
- Bailey, M.E.; Sackett, D.L.; Ross, J.L. Katanin Severing and Binding Microtubules are Inhibited by Tubulin Carboxy Tails. Biophys. J. 2015, 109, 2546–2561. [Google Scholar] [CrossRef] [Green Version]
- Matamoros, A.J.; Baas, P.W. Microtubules in health and degenerative disease of the nervous system. Brain Res. Bull. 2016, 126, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde, C.; Cáceres, A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009, 10, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.C.; Rao, K.; Gheres, K.W.; Kim, S.; Tao, J.; La Rochelle, C.; Folker, C.T.; Sherwood, N.T.; Rolls, M.M. Normal spastin gene dosage is specifically required for axon regeneration. Cell Rep. 2012, 2, 1340–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leo, L.; Yu, W.; D’Rozario, M.; Waddell, E.A.; Marenda, D.R.; Baird, M.A.; Davidson, M.W.; Zhou, B.; Wu, B.; Baker, L.; et al. Vertebrate Fidgetin Restrains Axonal Growth by Severing Labile Domains of Microtubules. Cell Rep. 2015, 12, 1723–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, D.; Zhang, H.; Deng, J.; Liu, J.; Wen, J.; Li, L.; Wang, X.; Pan, M.; Hu, X.; Guo, J. RhoA-GTPase Modulates Neurite Outgrowth by Regulating the Expression of Spastin and p60-Katanin. Cells 2020, 9, 230. https://doi.org/10.3390/cells9010230
Tan D, Zhang H, Deng J, Liu J, Wen J, Li L, Wang X, Pan M, Hu X, Guo J. RhoA-GTPase Modulates Neurite Outgrowth by Regulating the Expression of Spastin and p60-Katanin. Cells. 2020; 9(1):230. https://doi.org/10.3390/cells9010230
Chicago/Turabian StyleTan, Dandan, Haowen Zhang, Junyao Deng, Jingmin Liu, Jinkun Wen, Lixia Li, Xianghai Wang, Mengjie Pan, Xiaofang Hu, and Jiasong Guo. 2020. "RhoA-GTPase Modulates Neurite Outgrowth by Regulating the Expression of Spastin and p60-Katanin" Cells 9, no. 1: 230. https://doi.org/10.3390/cells9010230
APA StyleTan, D., Zhang, H., Deng, J., Liu, J., Wen, J., Li, L., Wang, X., Pan, M., Hu, X., & Guo, J. (2020). RhoA-GTPase Modulates Neurite Outgrowth by Regulating the Expression of Spastin and p60-Katanin. Cells, 9(1), 230. https://doi.org/10.3390/cells9010230



