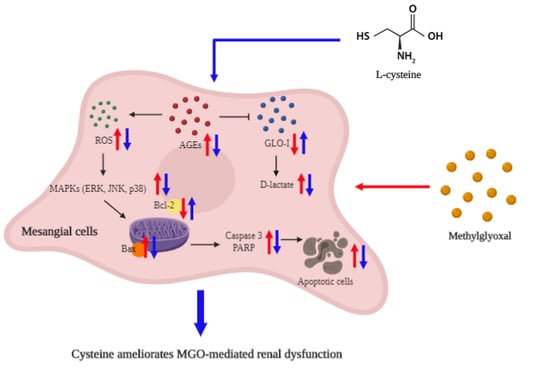
Effect of Cysteine on Methylglyoxal-Induced Renal Damage in Mesangial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Treatment
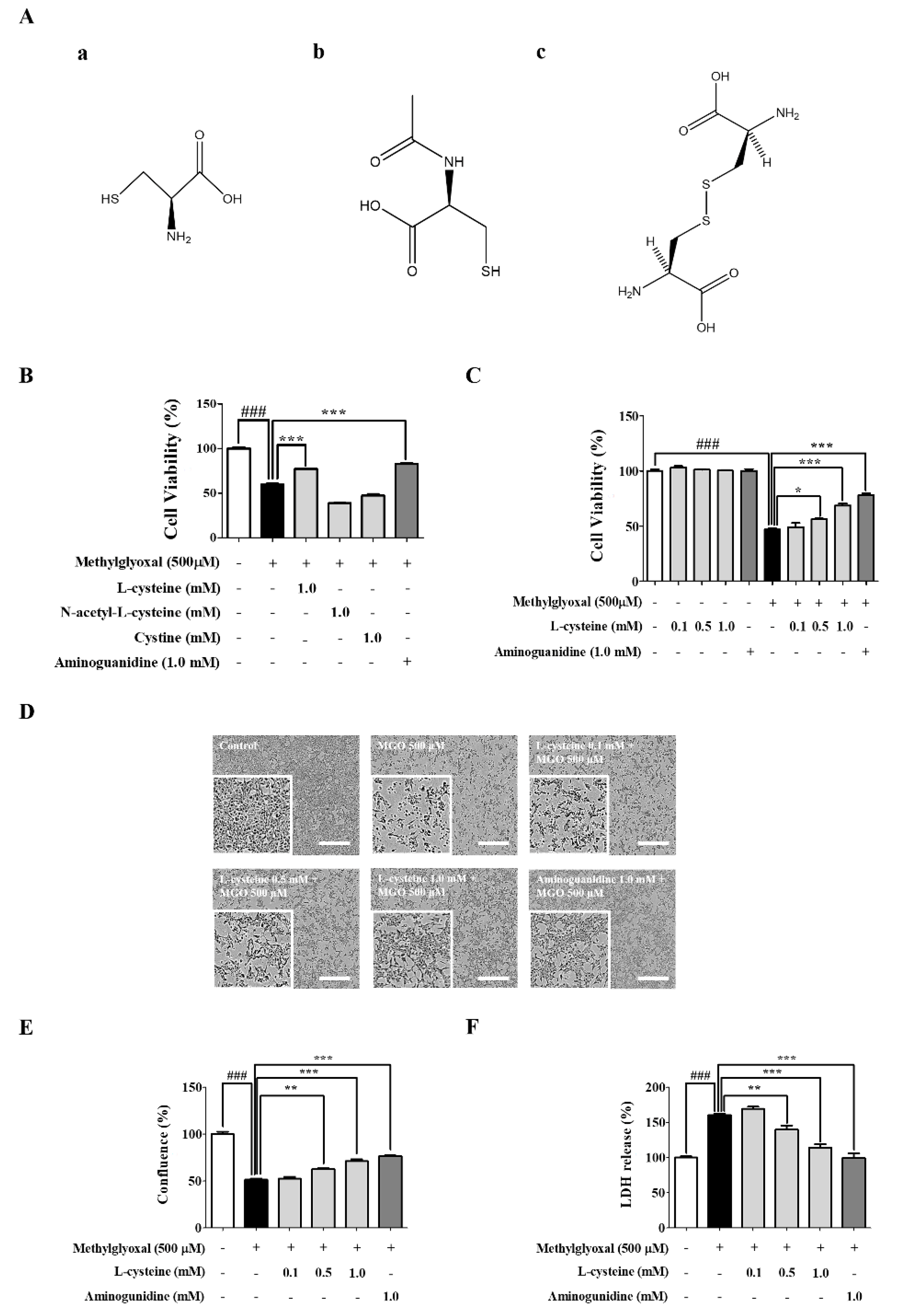
2.3. Measurement of Cell Viability and Morphological Changes
2.4. Measurement of LDH Production
2.5. Measurement of Cell Apoptosis by Flow Cytometry
2.6. Measurement of Intracellular ROS
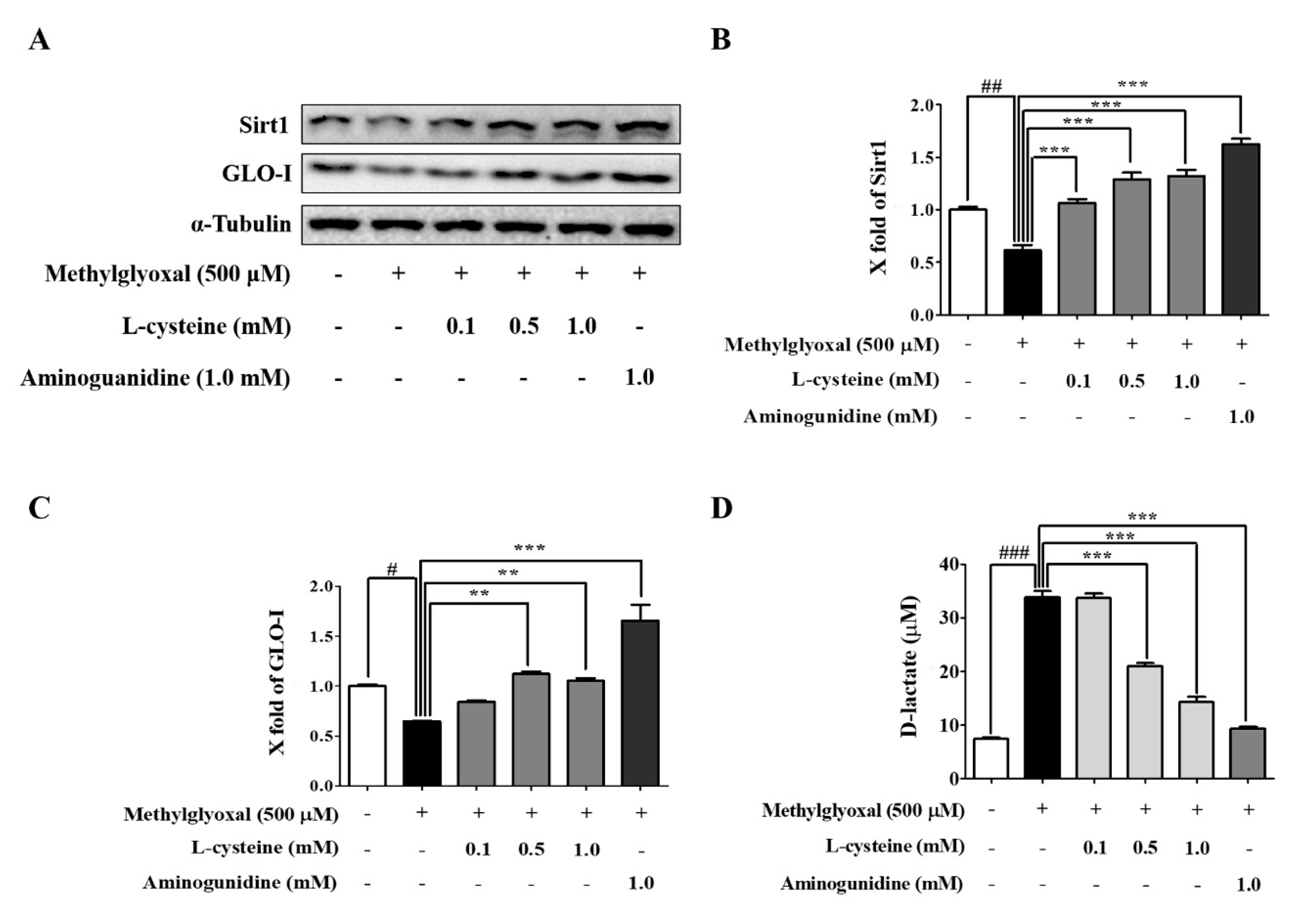
2.7. Measurement of D-lactate Production
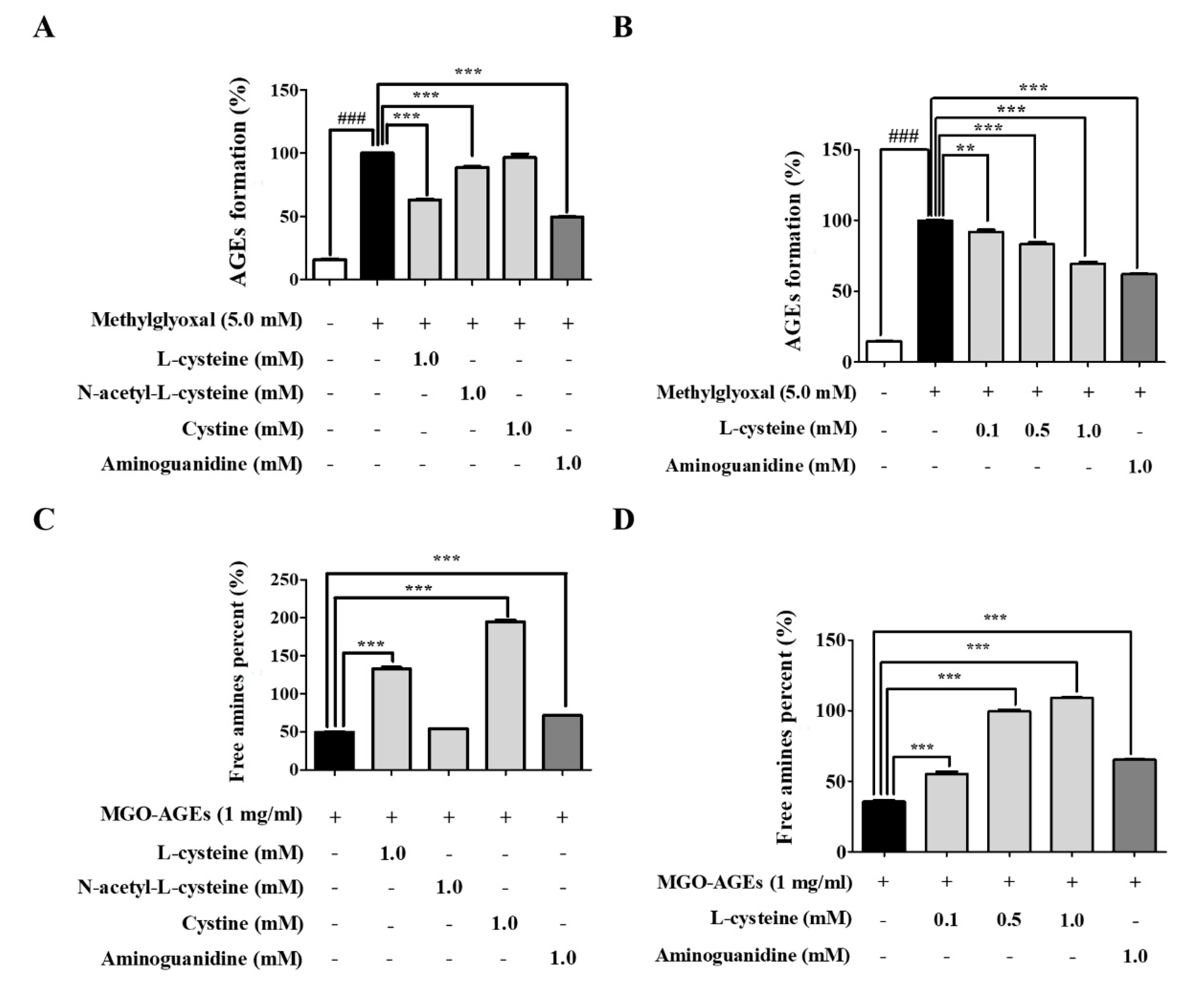
2.8. Measurement of MGO-Derived AGE Formation
2.9. Measurement of MGO-AGE Breakdown
2.10. Western Blot Analysis
2.11. Immunofluorescence Analysis
2.12. Statistical Analysis
3. Results
3.1. l-Cysteine Shows a Cytoprotective Effect against MGO-Induced MES13 Cell Toxicity
3.2. l-Cysteine Reduces MGO-Induced Intracellular ROS Generation
3.3. l-Cysteine Downregulates MGO-Induced Cell Death and Its MAPKs Signaling Pathway
3.4. l-Cysteine Regulates AGE Formation and Breakdown
3.5. l-Cysteine Prevents MGO-Induced Sirt1 and GLO-I Expression
3.6. l-Cysteine Accelerates MGO Catabolic Process to D-lactate
3.7. l-Cysteine Shows Cytoprotective Effects against MGO-Induced HEK 293 Cell Toxicity
3.8. l-Cysteine Reduces MGO-Induced Intracellular ROS Generation in HEK 293 Cells
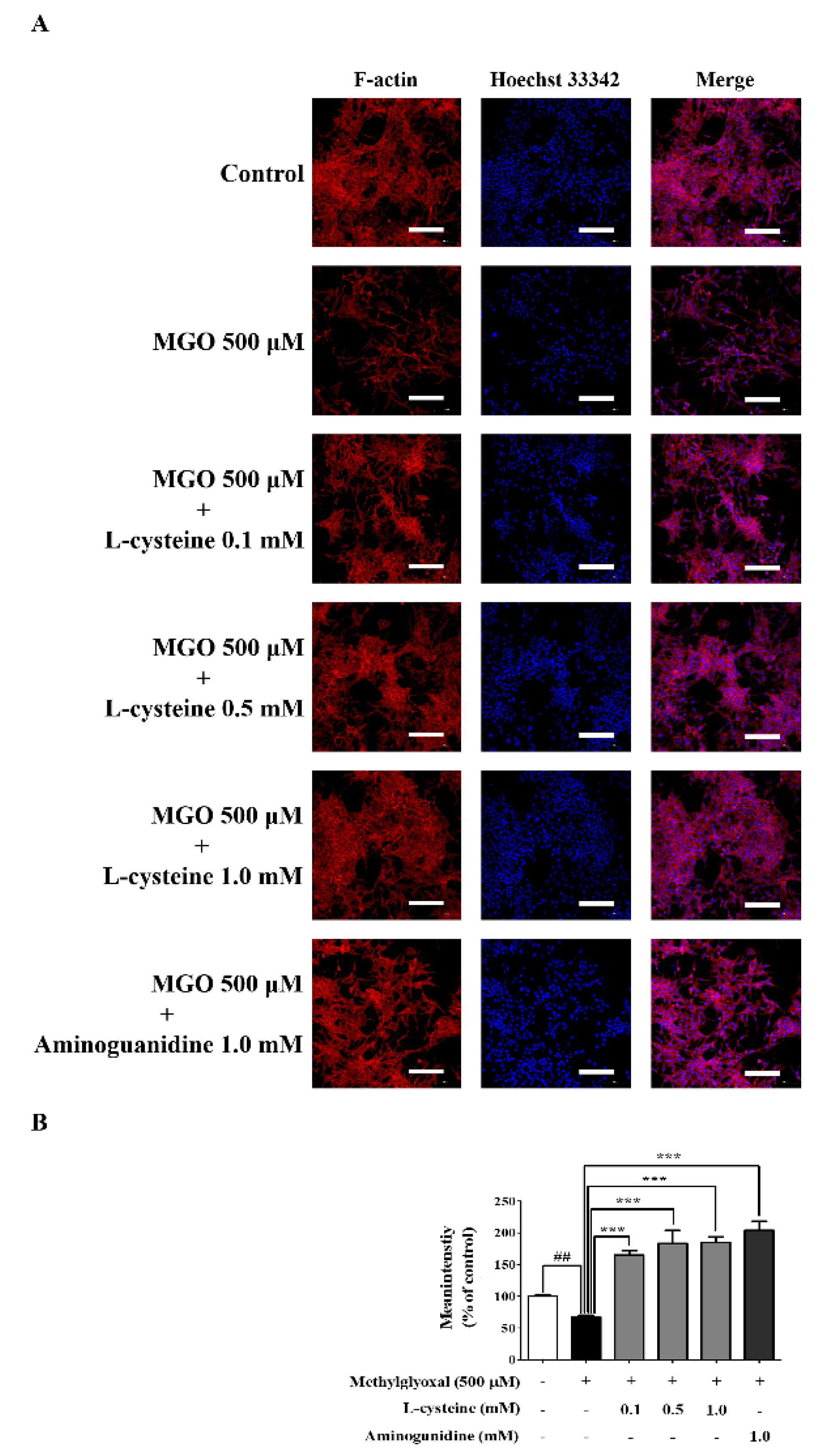
3.9. l-Cysteine Restores MGO-Induced Cytoskeletal Protein Damage in MES13 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wetzels, S.; Wouters, K.; Schalkwijk, C.G.; Vanmierlo, T.; Hendriks, J.J. Methylglyoxal-Derived Advanced Glycation Endproducts in Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Li, H.Y.; Lee, J.H.; Oh, Y.S.; Jun, H.S. Lysophosphatidic acid increases mesangial cell proliferation in models of diabetic nephropathy via Rac1/MAPK/KLF5 signaling. Exp. Mol. Med. 2019, 51, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaralingam, S.; Ibrahim, A.; Rahman, M.D.M.; Eid, A.H.; Munusamy, S. Role of Methylglyoxal in Diabetic Cardiovascular and Kidney Diseases: Insights from Basic Science for Application into Clinical Practice. Curr. Pharm. Des. 2018, 24, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Hanssen, N.M.J.; Stehouwer, C.D.A.; Schalkwijk, C.G. Methylglyoxal stress, the glyoxalase system, and diabetic chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 26–33. [Google Scholar] [CrossRef]
- Nigro, C.; Leone, A.; Fiory, F.; Prevenzano, I.; Nicolo, A.; Mirra, P.; Beguinot, F.; Miele, C. Dicarbonyl Stress at the Crossroads of Healthy and Unhealthy Aging. Cells 2019, 8, 749. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.M.; Vistisen, D.; Fleming, T.; Nawroth, P.P.; Rossing, P.; Jorgensen, M.E.; Lauritzen, T.; Sandbaek, A.; Witte, D.R. Methylglyoxal is associated with changes in kidney function among individuals with screen-detected Type 2 diabetes mellitus. Diabet. Med. J. Br. Diabet. Assoc. 2016, 33, 1625–1631. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, J.H.; Cho, K.; Kang, M.C.; Subedi, L.; Parveen, A.; Kim, S.Y. Therapeutic Potential of Lespedeza bicolor to Prevent Methylglyoxal-Induced Glucotoxicity in Familiar Diabetic Nephropathy. J. Clin. Med. 2019, 8, 1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.F.; Miyata, S.; Hirota, Y.; Higo, S.; Miyazaki, H.; Fukunaga, M.; Hamada, Y.; Ueyama, S.; Muramoto, O.; Uriuhara, A.; et al. Methylglyoxal induces apoptosis through activation of p38 mitogen-activated protein kinase in rat mesangial cells. Kidney Int. 2003, 63, 947–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, S.H.; Hwang, Y.; Heo, S.J.; Jun, H.S. Diphlorethohydroxycarmalol Attenuates Methylglyoxal-Induced Oxidative Stress and Advanced Glycation End Product Formation in Human Kidney Cells. Oxidative Med. Cell. Longev. 2018, 2018, 3654095. [Google Scholar] [CrossRef] [PubMed]
- Nakajou, K.; Horiuchi, S.; Sakai, M.; Haraguchi, N.; Tanaka, M.; Takeya, M.; Otagiri, M. Renal clearance of glycolaldehyde- and methylglyoxal-modified proteins in mice is mediated by mesangial cells through a class A scavenger receptor (SR-A). Diabetologia 2005, 48, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brings, S.; Fleming, T.; De Buhr, S.; Beijer, B.; Lindner, T.; Wischnjow, A.; Kender, Z.; Peters, V.; Kopf, S.; Haberkorn, U.; et al. A scavenger peptide prevents methylglyoxal induced pain in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 654–662. [Google Scholar] [CrossRef]
- Furlani, R.E.; Richardson, M.A.; Podell, B.K.; Ackart, D.F.; Haugen, J.D.; Melander, R.J.; Basaraba, R.J.; Melander, C. Second generation 2-aminoimidazole based advanced glycation end product inhibitors and breakers. Bioorganic Med. Chem. Lett. 2015, 25, 4820–4823. [Google Scholar] [CrossRef] [Green Version]
- Nagai, R.; Murray, D.B.; Metz, T.O.; Baynes, J.W. Chelation: A fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 2012, 61, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Dhar, A.; Dhar, I.; Desai, K.M.; Wu, L. Methylglyoxal scavengers attenuate endothelial dysfunction induced by methylglyoxal and high concentrations of glucose. Br. J. Pharmacol. 2010, 161, 1843–1856. [Google Scholar] [CrossRef] [Green Version]
- Allaman, I.; Belanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11–17. [Google Scholar]
- Echeverri-Ruiz, N.; Haynes, T.; Landers, J.; Woods, J.; Gemma, M.J.; Hughes, M.; Del Rio-Tsonis, K. A biochemical basis for induction of retina regeneration by antioxidants. Dev. Biol. 2018, 433, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H. N-acetylcysteine for Prevention of Contrast-Induced Nephropathy: A Narrative Review. Korean Circl. J. 2011, 41, 695–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.T.; Meng, F.H.; Chen, L.; Li, X.; Cen, L.J.; Wen, Y.H.; Li, C.C.; Zhang, H. Inhibition of Methylglyoxal-Induced AGEs/RAGE Expression Contributes to Dermal Protection by N-Acetyl-L-Cysteine. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 41, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zheng, X.Q.; Zhang, Z.J.; Teng, G.J. Effects of N-acetylcysteine upon methylglyoxal-induced damage in hippocampal neuronal cells. Zhonghua Yi Xue Za Zhi 2009, 89, 2789–2792. [Google Scholar] [PubMed]
- Schmidt, L.E.; Dalhoff, K. Risk factors in the development of adverse reactions to N-acetylcysteine in patients with paracetamol poisoning. Br. J. Clin. Pharmacol. 2001, 51, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, D.; Arik, M.; Cakir, Y.; Civi, Z. Comparison of N-acetyl-L-cysteine and L-cysteine in respect to their transmembrane fluxes. Biochem. Suppl. Ser. Membr. Cell Biol. 2009, 3, 157–162. [Google Scholar] [CrossRef]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxidative Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef]
- Kiho, T.; Kato, M.; Usui, S.; Hirano, K. Effect of buformin and metformin on formation of advanced glycation end products by methylglyoxal. Clin. Chim. Acta Int. J. Clin. Chem. 2005, 358, 139–145. [Google Scholar] [CrossRef]
- Weigand, T.; Singler, B.; Fleming, T.; Nawroth, P.; Klika, K.D.; Thiel, C.; Baelde, H.; Garbade, S.F.; Wagner, A.H.; Hecker, M.; et al. Carnosine Catalyzes the Formation of the Oligo/Polymeric Products of Methylglyoxal. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 713–726. [Google Scholar] [CrossRef]
- Szwergold, B.S.; Miller, C.B. Potential of birds to serve as pathology-free models of type 2 diabetes, part 2: Do high levels of carbonyl-scavenging amino acids (e.g., taurine) and low concentrations of methylglyoxal limit the production of advanced glycation end-products? Rejuvenation Res. 2014, 17, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Bollong, M.J.; Lee, G.; Coukos, J.S.; Yun, H.; Zambaldo, C.; Chang, J.W.; Chin, E.N.; Ahmad, I.; Chatterjee, A.K.; Lairson, L.L.; et al. A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signalling. Nature 2018, 562, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Kanade, S.R.; Pasupuleti, N.; Nagaraj, R.H. Role of cysteine residues in the enhancement of chaperone function in methylglyoxal-modified human alpha A-crystallin. Mol. Cell. Biochem. 2009, 322, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chumsae, C.; Gifford, K.; Lian, W.; Liu, H.; Radziejewski, C.H.; Zhou, Z.S. Arginine modifications by methylglyoxal: Discovery in a recombinant monoclonal antibody and contribution to acidic species. Anal. Chem. 2013, 85, 11401–11409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Methylglyoxal-induced dicarbonyl stress in aging and disease: First steps towards glyoxalase 1-based treatments. Clin. Sci. 2016, 130, 1677–1696. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Kim, S.Y. Hypericin, a Naphthodianthrone Derivative, Prevents Methylglyoxal-Induced Human Endothelial Cell Dysfunction. Biomol. Ther. 2017, 25, 158–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richarme, G.; Mihoub, M.; Dairou, J.; Bui, L.C.; Leger, T.; Lamouri, A. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 2015, 290, 1885–1897. [Google Scholar] [CrossRef] [Green Version]
- Freund, M.A.; Chen, B.; Decker, E.A. The Inhibition of Advanced Glycation End Products by Carnosine and Other Natural Dipeptides to Reduce Diabetic and Age-Related Complications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1367–1378. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, S.; Figarola, J.L. Novel inhibitors of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 63–79. [Google Scholar] [CrossRef]
- Stewart, B.J.; Navid, A.; Kulp, K.S.; Knaack, J.L.; Bench, G. D-Lactate production as a function of glucose metabolism in Saccharomyces cerevisiae. Yeast 2013, 30, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.K.; Lee, Y.T.; Chen, S.M.; Hsieh, C.W.; Huang, T.C.; Li, Y.C.; Lee, J.A. Elevated urinary D-lactate levels in patients with diabetes and microalbuminuria. J. Pharm. Biomed. Anal. 2015, 116, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, L.; Zhou, C.; Lin, N.; Liu, A. Sirt 1 activator inhibits the AGE-induced apoptosis and p53 acetylation in human vascular endothelial cells. J. Toxicol. Sci. 2015, 40, 615–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, K.S.; Chon, S.; Jung, W.W.; Choi, E.M. Magnolol protects pancreatic beta-cells against methylglyoxal-induced cellular dysfunction. Chem. Biol. Interact. 2017, 277, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Di Emidio, G.; Santini, S.J.; D’Alessandro, A.M.; Vetuschi, A.; Sferra, R.; Artini, P.G.; Carta, G.; Falone, S.; Amicarelli, F.; Tatone, C. SIRT1 participates in the response to methylglyoxal-dependent glycative stress in mouse oocytes and ovary. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Jian, J.H.; Liu, Y.C.; Juang, S.J.; Shyu, K.G.; Lai, L.P.; Wang, B.W.; Leu, J.G. Advanced glycation end products-induced apoptosis attenuated by PPARdelta activation and epigallocatechin gallate through NF-kappaB pathway in human embryonic kidney cells and human mesangial cells. Diabetes/Metab. Res. Rev. 2010, 26, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Jian, J.H.; Chen, C.Y.; Hsu, C.Y.; Shih, C.Y.; Leu, J.G. L-165,041, troglitazone and their combination treatment to attenuate high glucose-induced receptor for advanced glycation end products (RAGE) expression. Eur. J. Pharmacol. 2013, 715, 33–38. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Subedi, L.; Kim, S.Y. Effect of Cysteine on Methylglyoxal-Induced Renal Damage in Mesangial Cells. Cells 2020, 9, 234. https://doi.org/10.3390/cells9010234
Lee JH, Subedi L, Kim SY. Effect of Cysteine on Methylglyoxal-Induced Renal Damage in Mesangial Cells. Cells. 2020; 9(1):234. https://doi.org/10.3390/cells9010234
Chicago/Turabian StyleLee, Jae Hyuk, Lalita Subedi, and Sun Yeou Kim. 2020. "Effect of Cysteine on Methylglyoxal-Induced Renal Damage in Mesangial Cells" Cells 9, no. 1: 234. https://doi.org/10.3390/cells9010234
APA StyleLee, J. H., Subedi, L., & Kim, S. Y. (2020). Effect of Cysteine on Methylglyoxal-Induced Renal Damage in Mesangial Cells. Cells, 9(1), 234. https://doi.org/10.3390/cells9010234