Ca2+ Channels Mediate Bidirectional Signaling between Sarcolemma and Sarcoplasmic Reticulum in Muscle Cells
Abstract
:1. Introduction
2. Voltage-Gated Ca2+ Channels (VGCCs)
3. Ryanodine Receptors (RYRs)
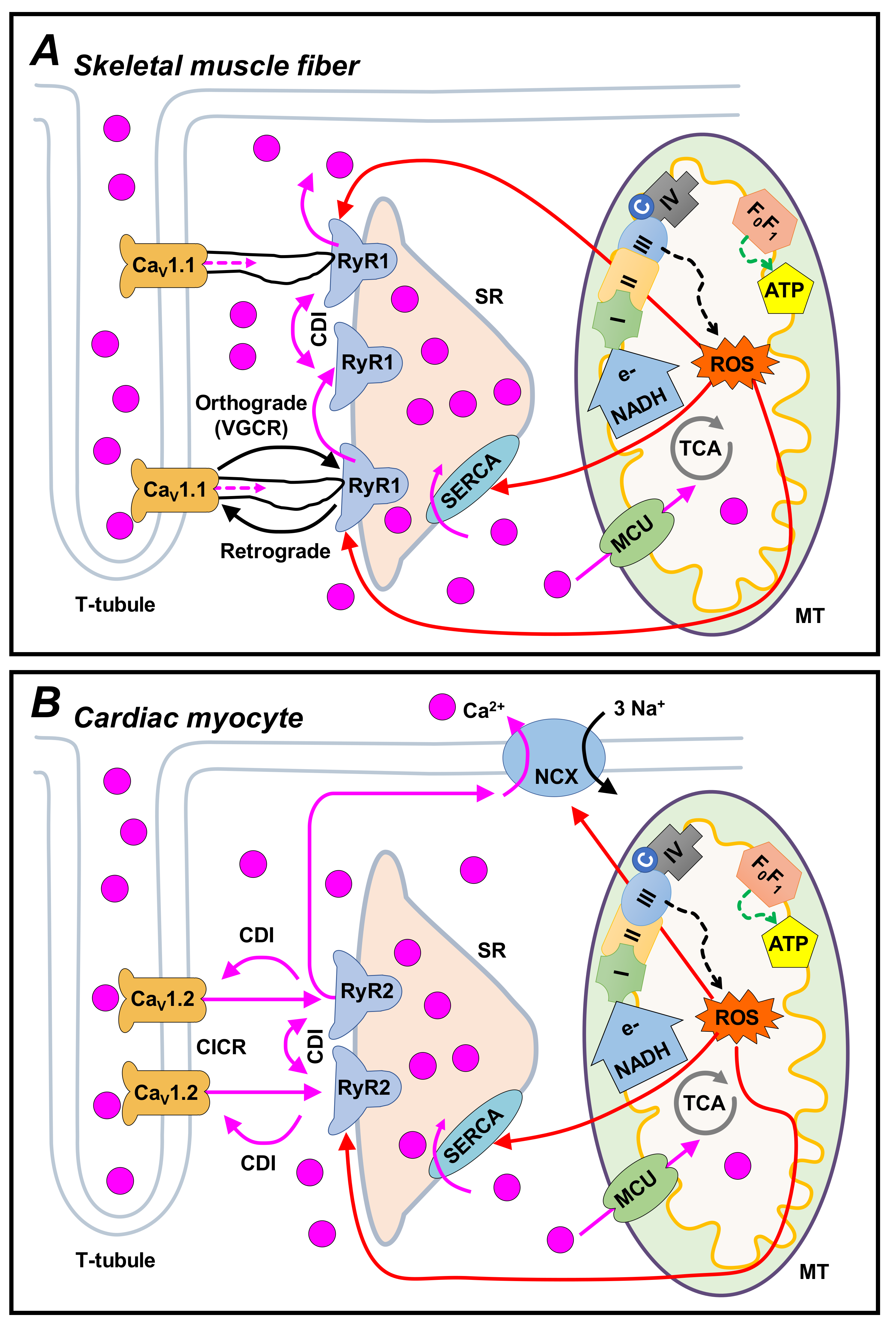
4. Skeletal Muscle EC Coupling
4.1. The Skeletal-Type EC Coupling Depends on a CaV1.1-RyR1 Physical Interaction
4.2. Role of Other Triad Proteins
4.3. CaV1.1 Contributes to Keeping the RyR1s Closed
4.4. Excitation-Coupled Calcium Entry (ECCE)
5. Retrograde Signaling in Skeletal Muscle
6. Cardiac EC Coupling
7. Retrograde Signaling in Cardiac Muscle
Ca2+-Dependent Inactivation of CaV1.2
8. Auxiliary Ca2+ Signaling
8.1. SERCA
8.2. NCX
8.3. Excitation–Metabolism Coupling
9. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | action potential |
| AT | axial tubes |
| [Ca2+]i | intracellular calcium concentration |
| CaM | calmodulin |
| CaMKII | calcium/calmodulin-dependent kinase II |
| CDI | Ca2+-dependent inactivation |
| CICR | Ca2+-induced Ca2+ release |
| PLB | phospholamban |
| cMyBP-C | cardiac myosin-binding protein-C |
| DHPR | dihydropyridine receptor |
| EC | excitation-contraction |
| ECCE | excitation-coupled calcium entry |
| ER | endoplasmic reticulum |
| FDUF | fire-diffuse-uptake-fire |
| HF | heart failure |
| ICa | calcium current |
| TCA | tricarboxylic acid |
| JP | junctophilins |
| MCU | mitochondrial calcium uniporter |
| NCX | Na+-Ca2+ exchanger |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| MT | mitochondrion |
| PKA | cAMP-dependent protein kinase |
| PKG | cGMP-dependent protein kinase |
| Po | open probability |
| ROS | reactive oxygen species |
| RyR | ryanodine receptor |
| SERCA | sarcoplasmic reticulum Ca2+-ATPase |
| SLN | sarcolipin |
| SOICR | store-overload-induced Ca2+ release |
| SR | sarcoplasmic reticulum |
| TATS | transverse-axial tubular system |
| T-tubules | transverse tubules |
| VGCC | voltage-gated Ca2+ channel |
| VGCR | voltage-gated SR Ca2+ release |
References
- Bers, D.M. Excitation-contraction coupling and cardiac contractile force; Springer: Dordrecht, Netherlands, 2001; ISBN 978-0-7923-7158-8. [Google Scholar]
- Rossi, A.E.; Dirksen, R.T. Sarcoplasmic reticulum: The dynamic calcium governor of muscle. Muscle Nerve. 2006, 33, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Ríos, E.; Figueroa, L.; Manno, C.; Kraeva, N.; Riazi, S. The couplonopathies: A comparative approach to a class of diseases of skeletal and cardiac muscle. J. Gen. Physiol. 2015, 145, 459–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protasi, F. Structural interaction between RYRs and DHPRs in calcium release units of cardiac and skeletal muscle cells. Front. Biosci. 2002, 7, d650–d658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, B.M.; Catterall, W.A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry 1984, 23, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Seagar, M.J.; Jones, J.F.; Reber, B.F.; Catterall, W.A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc. Natl. Acad. Sci. USA 1987, 84, 5478–5482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, T.; Takeshima, H.; Mikami, A.; Flockerzi, V.; Takahashi, H.; Kangawa, K.; Kojima, M.; Matsuo, H.; Hirose, T.; Numa, S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature 1987, 328, 313–318. [Google Scholar] [CrossRef]
- Ertel, E.A.; Campbell, K.P.; Harpold, M.M.; Hofmann, F.; Mori, Y.; Perez-Reyes, E.; Schwartz, A.; Snutch, T.P.; Tanabe, T.; Birnbaumer, L.; et al. Nomenclature of voltage-gated calcium channels. Neuron 2000, 25, 533–535. [Google Scholar] [CrossRef] [Green Version]
- Catterall, W.A.; Perez-Reyes, E.; Snutch, T.P.; Striessnig, J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005, 57, 411–425. [Google Scholar] [CrossRef]
- Campiglio, M.; Flucher, B.E. The role of auxiliary subunits for the functional diversity of voltage-gated calcium channels. J. Cell. Physiol. 2015, 230, 2019–2031. [Google Scholar] [CrossRef]
- Zalk, R.; Lehnart, S.E.; Marks, A.R. Modulation of the ryanodine receptor and intracellular calcium. Annu. Rev. Biochem. 2007, 76, 367–385. [Google Scholar] [CrossRef]
- Zhao, M.; Li, P.; Li, X.; Zhang, L.; Winkfein, R.J.; Chen, S.R. Molecular identification of the ryanodine receptor pore-forming segment. J. Biol. Chem. 1999, 274, 25971–25974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, G.G.; Sandhu, B.; Khanna, V.K.; Guo, X.H.; MacLennan, D.H. Topology of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (RyR1). Proc. Natl. Acad. Sci. USA 2002, 99, 16725–16730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, G.G.; Avila, G.; Sharma, P.; Khanna, V.K.; Dirksen, R.T.; MacLennan, D.H. Role of the sequence surrounding predicted transmembrane helix M4 in membrane association and function of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (ryanodine receptor isoform 1). J. Biol. Chem. 2004, 279, 37566–37574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ching, L.L.; Williams, A.J.; Sitsapesan, R. Evidence for Ca2+ activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ. Res. 2000, 87, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Györke, I.; Hester, N.; Jones, L.R.; Györke, S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 2004, 86, 2121–2128. [Google Scholar] [CrossRef] [Green Version]
- Beard, N.A.; Wei, L.; Dulhunty, A.F. Ca2+ signaling in striated muscle: The elusive roles of triadin, junctin, and calsequestrin. Eur. Biophys. J. 2009, 39, 27–36. [Google Scholar] [CrossRef]
- Goonasekera, S.A.; Beard, N.A.; Groom, L.; Kimura, T.; Lyfenko, A.D.; Rosenfeld, A.; Marty, I.; Dulhunty, A.F.; Dirksen, R.T. Triadin binding to the C-terminal luminal loop of the ryanodine receptor is important for skeletal muscle excitation contraction coupling. J. Gen. Physiol. 2007, 130, 365–378. [Google Scholar] [CrossRef] [Green Version]
- Altschafl, B.A.; Arvanitis, D.A.; Fuentes, O.; Yuan, Q.; Kranias, E.G.; Valdivia, H.H. Dual role of junctin in the regulation of ryanodine receptors and calcium release in cardiac ventricular myocytes. J. Physiol. 2011, 589, 6063–6080. [Google Scholar] [CrossRef] [Green Version]
- Samsó, M. A guide to the 3D structure of the ryanodine receptor type 1 by cryoEM. Protein Sci. 2017, 26, 52–68. [Google Scholar] [CrossRef]
- Yan, Z.; Bai, X.; Yan, C.; Wu, J.; Li, Z.; Xie, T.; Peng, W.; Yin, C.; Li, X.; Scheres, S.H.W.; et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 2015, 517, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Zalk, R.; Clarke, O.B.; des Georges, A.; Grassucci, R.A.; Reiken, S.; Mancia, F.; Hendrickson, W.A.; Frank, J.; Marks, A.R. Structure of a mammalian ryanodine receptor. Nature 2015, 517, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efremov, R.G.; Leitner, A.; Aebersold, R.; Raunser, S. Architecture and conformational switch mechanism of the ryanodine receptor. Nature 2015, 517, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meissner, G. The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 2017, 149, 1065–1089. [Google Scholar] [CrossRef] [PubMed]
- Ríos, E. Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus. J. Gen. Physiol. 2018, 150, 521–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, C.M.; Bezanilla, F.M.; Horowicz, P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N′-tetracetic acid. Biochim. Biophys. Acta 1972, 267, 605–608. [Google Scholar] [CrossRef]
- Gonzalez-Serratos, H.; Valle-Aguilera, R.; Lathrop, D.A.; Garcia, M.C. Slow inward calcium currents have no obvious role in muscle excitation-contraction coupling. Nature 1982, 298, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Nakai, J.; Dirksen, R.T.; Nguyen, H.T.; Pessah, I.N.; Beam, K.G.; Allen, P.D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 1996, 380, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, R.T.; Beam, K.G. Role of calcium permeation in dihydropyridine receptor function. Insights into channel gating and excitation-contraction coupling. J. Gen. Physiol. 1999, 114, 393–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayal, A.; Schrötter, K.; Pan, Y.; Föhr, K.; Melzer, W.; Grabner, M. The Ca2+ influx through the mammalian skeletal muscle dihydropyridine receptor is irrelevant for muscle performance. Nat. Commun 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miledi, R.; Parker, I.; Schalow, G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc. R. Soc. Lond. B Biol. Sci. 1977, 198, 201–210. [Google Scholar] [PubMed]
- Caputo, C.; Bezanilla, F.; Horowicz, P. Depolarization-contraction coupling in short frog muscle fibers. A voltage clamp study. J. Gen. Physiol. 1984, 84, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M.F.; Chandler, W.K. Voltage dependent charge movement of skeletal muscle: A possible step in excitation-contraction coupling. Nature 1973, 242, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Rios, E.; Brum, G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 1987, 325, 717–720. [Google Scholar] [CrossRef]
- Tanabe, T.; Beam, K.G.; Powell, J.A.; Numa, S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 1988, 336, 134–139. [Google Scholar] [CrossRef]
- Inui, M.; Saito, A.; Fleischer, S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J. Biol. Chem. 1987, 262, 1740–1747. [Google Scholar]
- Takekura, H.; Nishi, M.; Noda, T.; Takeshima, H.; Franzini-Armstrong, C. Abnormal junctions between surface membrane and sarcoplasmic reticulum in skeletal muscle with a mutation targeted to the ryanodine receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 3381–3385. [Google Scholar] [CrossRef] [Green Version]
- Bijlenga, P.; Liu, J.H.; Espinos, E.; Haenggeli, C.A.; Fischer-Lougheed, J.; Bader, C.R.; Bernheim, L. T-type alpha 1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc. Natl. Acad. Sci. USA 2000, 97, 7627–7632. [Google Scholar] [CrossRef] [Green Version]
- Berthier, C.; Monteil, A.; Lory, P.; Strube, C. Alpha(1H) mRNA in single skeletal muscle fibres accounts for T-type calcium current transient expression during fetal development in mice. J. Physiol. 2002, 539, 681–691. [Google Scholar] [CrossRef]
- Block, B.A.; Imagawa, T.; Campbell, K.P.; Franzini-Armstrong, C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 1988, 107, 2587–2600. [Google Scholar] [CrossRef]
- Ríos, E.; Gillespie, D.; Franzini-Armstrong, C. The binding interactions that maintain excitation-contraction coupling junctions in skeletal muscle. J. Gen. Physiol. 2019, 151, 593–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, M.D.; Pizarro, G.; Ríos, E. Local control model of excitation-contraction coupling in skeletal muscle. J. Gen. Physiol. 1997, 110, 415–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, M.D.; Song, L.S.; Cheng, H.; Sham, J.S.; Yang, H.T.; Boheler, K.R.; Ríos, E. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J. Gen. Physiol. 1999, 113, 469–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Lederer, W.J.; Cannell, M.B. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science 1993, 262, 740–744. [Google Scholar] [CrossRef]
- Cannell, M.B.; Cheng, H.; Lederer, W.J. The control of calcium release in heart muscle. Science 1995, 268, 1045–1049. [Google Scholar] [CrossRef]
- Sorrentino, V. The ryanodine receptor family of intracellular calcium release channels. Adv. Pharmacol. 1995, 33, 67–90. [Google Scholar]
- Otsu, K.; Willard, H.F.; Khanna, V.K.; Zorzato, F.; Green, N.M.; MacLennan, D.H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 13472–13483. [Google Scholar]
- Eckhardt, J.; Bachmann, C.; Sekulic-Jablanovic, M.; Enzmann, V.; Park, K.H.; Ma, J.; Takeshima, H.; Zorzato, F.; Treves, S. Extraocular muscle function is impaired in ryr3-/- mice. J. Gen. Physiol. 2019, 151, 929–943. [Google Scholar] [CrossRef] [Green Version]
- Adams, B.A.; Beam, K.G. Muscular dysgenesis in mice: A model system for studying excitation-contraction coupling. FASEB J. 1990, 4, 2809–2816. [Google Scholar] [CrossRef] [Green Version]
- Grabner, M.; Dirksen, R.T.; Beam, K.G. Tagging with green fluorescent protein reveals a distinct subcellular distribution of L-type and non-L-type Ca2+ channels expressed in dysgenic myotubes. Proc. Natl. Acad. Sci. USA 1998, 95, 1903–1908. [Google Scholar] [CrossRef] [Green Version]
- Takekura, H.; Paolini, C.; Franzini-Armstrong, C.; Kugler, G.; Grabner, M.; Flucher, B.E. Differential contribution of skeletal and cardiac II-III loop sequences to the assembly of dihydropyridine-receptor arrays in skeletal muscle. Mol. Biol. Cell 2004, 15, 5408–5419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, T.; Beam, K.G.; Adams, B.A.; Niidome, T.; Numa, S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature 1990, 346, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Nakai, J.; Tanabe, T.; Konno, T.; Adams, B.; Beam, K.G. Localization in the II-III loop of the dihydropyridine receptor of a sequence critical for excitation-contraction coupling. J. Biol. Chem. 1998, 273, 24983–24986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Hayek, R.; Antoniu, B.; Wang, J.; Hamilton, S.L.; Ikemoto, N. Identification of calcium release-triggering and blocking regions of the II-III loop of the skeletal muscle dihydropyridine receptor. J. Biol. Chem. 1995, 270, 22116–22118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Xu, L.; Meissner, G. Phosphorylation of dihydropyridine receptor II-III loop peptide regulates skeletal muscle calcium release channel function. Evidence for an essential role of the beta-OH group of Ser687. J. Biol. Chem. 1995, 270, 18459–18464. [Google Scholar] [CrossRef] [Green Version]
- El-Hayek, R.; Ikemoto, N. Identification of the minimum essential region in the II-III loop of the dihydropyridine receptor alpha 1 subunit required for activation of skeletal muscle-type excitation-contraction coupling. Biochemistry 1998, 37, 7015–7020. [Google Scholar] [CrossRef]
- Coronado, R.; Ahern, C.A.; Sheridan, D.C.; Cheng, W.; Carbonneau, L.; Bhattacharya, D. Functional equivalence of dihydropyridine receptor alpha1S and beta1a subunits in triggering excitation-contraction coupling in skeletal muscle. Biol. Res. 2004, 37, 565–575. [Google Scholar] [CrossRef]
- Strube, C.; Beurg, M.; Powers, P.A.; Gregg, R.G.; Coronado, R. Reduced Ca2+ current, charge movement, and absence of Ca2+ transients in skeletal muscle deficient in dihydropyridine receptor beta 1 subunit. Biophys. J. 1996, 71, 2531–2543. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, D.C.; Carbonneau, L.; Ahern, C.A.; Nataraj, P.; Coronado, R. Ca2+-dependent excitation-contraction coupling triggered by the heterologous cardiac/brain DHPR beta2a-subunit in skeletal myotubes. Biophys. J. 2003, 85, 3739–3757. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, D.C.; Cheng, W.; Carbonneau, L.; Ahern, C.A.; Coronado, R. Involvement of a heptad repeat in the carboxyl terminus of the dihydropyridine receptor beta1a subunit in the mechanism of excitation-contraction coupling in skeletal muscle. Biophys. J. 2004, 87, 929–942. [Google Scholar] [CrossRef] [Green Version]
- Rebbeck, R.T.; Karunasekara, Y.; Gallant, E.M.; Board, P.G.; Beard, N.A.; Casarotto, M.G.; Dulhunty, A.F. The β(1a) subunit of the skeletal DHPR binds to skeletal RyR1 and activates the channel via its 35-residue C-terminal tail. Biophys. J. 2011, 100, 922–930. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ochoa, E.O.; Olojo, R.O.; Rebbeck, R.T.; Dulhunty, A.F.; Schneider, M.F. β1a490-508, a 19-residue peptide from C-terminal tail of Cav1.1 β1a subunit, potentiates voltage-dependent calcium release in adult skeletal muscle fibers. Biophys. J. 2014, 106, 535–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.C.; Carrillo, E.; Galindo, J.M.; Hernández, A.; Copello, J.A.; Fill, M.; Sánchez, J.A. Short-term regulation of excitation-contraction coupling by the beta1a subunit in adult mouse skeletal muscle. Biophys. J. 2005, 89, 3976–3984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beqollari, D.; Romberg, C.F.; Filipova, D.; Meza, U.; Papadopoulos, S.; Bannister, R.A. Rem uncouples excitation-contraction coupling in adult skeletal muscle fibers. J. Gen. Physiol. 2015, 146, 97–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong King Yuen, S.M.; Campiglio, M.; Tung, C.-C.; Flucher, B.E.; Van Petegem, F. Structural insights into binding of STAC proteins to voltage-gated calcium channels. Proc. Natl. Acad. Sci. USA 2017, 114, E9520–E9528. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.R.; Wu, F.; Liu, Y.; Anderson, D.M.; McAnally, J.; Lin, W.; Cannon, S.C.; Bassel-Duby, R.; Olson, E.N. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA 2013, 110, 11881–11886. [Google Scholar] [CrossRef] [Green Version]
- Polster, A.; Perni, S.; Bichraoui, H.; Beam, K.G. Stac adaptor proteins regulate trafficking and function of muscle and neuronal L-type Ca2+ channels. Proc. Natl. Acad. Sci. USA 2015, 112, 602–606. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Quinonez, M.; DiFranco, M.; Cannon, S.C. Stac3 enhances expression of human CaV1.1 in Xenopus oocytes and reveals gating pore currents in HypoPP mutant channels. J. Gen. Physiol. 2018, 150, 475–489. [Google Scholar] [CrossRef] [Green Version]
- Horstick, E.J.; Linsley, J.W.; Dowling, J.J.; Hauser, M.A.; McDonald, K.K.; Ashley-Koch, A.; Saint-Amant, L.; Satish, A.; Cui, W.W.; Zhou, W.; et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat. Commun. 2013, 4, 1952. [Google Scholar] [CrossRef] [Green Version]
- Flucher, B.E.; Campiglio, M. STAC proteins: The missing link in skeletal muscle EC coupling and new regulators of calcium channel function. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1101–1110. [Google Scholar] [CrossRef]
- Nishi, M.; Mizushima, A.; Nakagawara, K.I.; Takeshima, H. Characterization of human junctophilin subtype genes. Biochem. Biophys. Res. Commun. 2000, 273, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Phimister, A.J.; Lango, J.; Lee, E.H.; Ernst-Russell, M.A.; Takeshima, H.; Ma, J.; Allen, P.D.; Pessah, I.N. Conformation-dependent stability of junctophilin 1 (JP1) and ryanodine receptor type 1 (RyR1) channel complex is mediated by their hyper-reactive thiols. J. Biol. Chem. 2007, 282, 8667–8677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golini, L.; Chouabe, C.; Berthier, C.; Cusimano, V.; Fornaro, M.; Bonvallet, R.; Formoso, L.; Giacomello, E.; Jacquemond, V.; Sorrentino, V. Junctophilin 1 and 2 proteins interact with the L-type Ca2+ channel dihydropyridine receptors (DHPRs) in skeletal muscle. J. Biol. Chem. 2011, 286, 43717–43725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakada, T.; Kashihara, T.; Komatsu, M.; Kojima, K.; Takeshita, T.; Yamada, M. Physical interaction of junctophilin and the CaV1.1 C terminus is crucial for skeletal muscle contraction. Proc. Natl. Acad. Sci. USA 2018, 115, 4507–4512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perni, S.; Lavorato, M.; Beam, K.G. De novo reconstitution reveals the proteins required for skeletal muscle voltage-induced Ca2+ release. Proc. Natl. Acad. Sci. USA 2017, 114, 13822–13827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melzer, W.; Ríos, E.; Schneider, M.F. The removal of myoplasmic free calcium following calcium release in frog skeletal muscle. J. Physiol. 1986, 372, 261–292. [Google Scholar] [CrossRef] [PubMed]
- Gouadon, E.; Schuhmeier, R.P.; Ursu, D.; Anderson, A.A.; Treves, S.; Zorzato, F.; Lehmann-Horn, F.; Melzer, W. A possible role of the junctional face protein JP-45 in modulating Ca2+ release in skeletal muscle. J. Physiol. 2006, 572, 269–280. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, J.; Royer, L.; Launikonis, B.S.; González, A.; García, J.; Ríos, E. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am. J. Physiol. Cell Physiol. 2006, 290, C539–C553. [Google Scholar] [CrossRef] [Green Version]
- Eltit, J.M.; Li, H.; Ward, C.W.; Molinski, T.; Pessah, I.N.; Allen, P.D.; Lopez, J.R. Orthograde dihydropyridine receptor signal regulates ryanodine receptor passive leak. Proc. Natl. Acad. Sci. USA 2011, 108, 7046–7051. [Google Scholar] [CrossRef] [Green Version]
- Cherednichenko, G.; Hurne, A.M.; Fessenden, J.D.; Lee, E.H.; Allen, P.D.; Beam, K.G.; Pessah, I.N. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc. Natl. Acad. Sci. USA 2004, 101, 15793–15798. [Google Scholar] [CrossRef] [Green Version]
- Robin, G.; Allard, B. Voltage-gated Ca2+ influx through L-type channels contributes to sarcoplasmic reticulum Ca2+loading in skeletal muscle. J. Physiol. 2015, 593, 4781–4797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherednichenko, G.; Ward, C.W.; Feng, W.; Cabrales, E.; Michaelson, L.; Samso, M.; López, J.R.; Allen, P.D.; Pessah, I.N. Enhanced excitation-coupled calcium entry in myotubes expressing malignant hyperthermia mutation R163C is attenuated by dantrolene. Mol. Pharmacol. 2008, 73, 1203–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, G.; Dirksen, R.T. Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca2+channel. J. Gen. Physiol. 2000, 115, 467–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, G.; O’Connell, K.M.; Groom, L.A.; Dirksen, R.T. Ca2+ release through ryanodine receptors regulates skeletal muscle L-type Ca2+ channel expression. J. Biol. Chem. 2001, 276, 17732–17738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabner, M.; Dirksen, R.T.; Suda, N.; Beam, K.G. The II-III loop of the skeletal muscle dihydropyridine receptor is responsible for the Bi-directional coupling with the ryanodine receptor. J. Biol. Chem. 1999, 274, 21913–21919. [Google Scholar] [CrossRef] [Green Version]
- Rebbeck, R.T.; Karunasekara, Y.; Board, P.G.; Beard, N.A.; Casarotto, M.G.; Dulhunty, A.F. Skeletal muscle excitation-contraction coupling: Who are the dancing partners? Int. J. Biochem. Cell Biol. 2014, 48, 28–38. [Google Scholar] [CrossRef]
- Bannister, R.A. Bridging the myoplasmic gap II: More recent advances in skeletal muscle excitation-contraction coupling. J. Exp. Biol. 2016, 219, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Fauré, J.; Lunardi, J.; Monnier, N.; Marty, I. Ryanodine Receptor 1 and Associated Pathologies. In Pathologies of Calcium Channels; Springer: Berlin/Heidelberg, Germany, 2014; pp. 167–187. ISBN 978-3-642-40281-4. [Google Scholar]
- Marty, I.; Fauré, J. Excitation-Contraction Coupling Alterations in Myopathies. J. Neuromuscul Dis. 2016, 3, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Andronache, Z.; Hamilton, S.L.; Dirksen, R.T.; Melzer, W. A retrograde signal from RyR1 alters DHP receptor inactivation and limits window Ca2+ release in muscle fibers of Y522S RyR1 knock-in mice. Proc. Natl. Acad. Sci. USA 2009, 106, 4531–4536. [Google Scholar] [CrossRef] [Green Version]
- Vega, A.V.; Ramos-Mondragón, R.; Calderón-Rivera, A.; Zarain-Herzberg, A.; Avila, G. Calcitonin gene-related peptide restores disrupted excitation-contraction coupling in myotubes expressing central core disease mutations in RyR1. J. Physiol. 2011, 589, 4649–4669. [Google Scholar] [CrossRef]
- Endo, M.; Tanaka, M.; Ogawa, Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature 1970, 228, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.E.; Podolsky, R.J. Regenerative calcium release within muscle cells. Science 1970, 167, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Fabiato, A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983, 245, C1–C14. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.E.; Cook, S.J.; Chamunorwa, J.P.; Trafford, A.W.; Lancaster, M.K.; O’Neill, S.C.; Eisner, D.A. Variability of spontaneous Ca2+ release between different rat ventricular myocytes is correlated with Na+-Ca2+ exchange and [Na+]i. Circ. Res. 1996, 78, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Xiao, B.; Yang, D.; Wang, R.; Choi, P.; Zhang, L.; Cheng, H.; Chen, S.R.W. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc. Natl. Acad. Sci. USA 2004, 101, 13062–13067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitsapesan, R.; Williams, A.J. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel by luminal Ca2+. J. Membr. Biol. 1994, 137, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Meissner, G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys. J. 1996, 70, 2600–2615. [Google Scholar] [CrossRef] [Green Version]
- Györke, I.; Györke, S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 1998, 75, 2801–2810. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Koop, A.; Liu, Y.; Guo, W.; Wei, J.; Wang, R.; MacLennan, D.H.; Dirksen, R.T.; Chen, S.R.W. Reduced threshold for store overload-induced Ca2+release is a common defect of RyR1 mutations associated with malignant hyperthermia and central core disease. Biochem. J. 2017, 474, 2749–2761. [Google Scholar] [CrossRef] [Green Version]
- Ríos, E. Perspectives on “Control of Ca release from within the cardiac sarcoplasmic reticulum”. J. Gen. Physiol. 2017, 149, 833–836. [Google Scholar]
- Ter Keurs, H.E.D.J.; Wakayama, Y.; Sugai, Y.; Price, G.; Kagaya, Y.; Boyden, P.A.; Miura, M.; Stuyvers, B.D.M. Role of sarcomere mechanics and Ca2+ overload in Ca2+ waves and arrhythmias in rat cardiac muscle. Ann. N. Y. Acad. Sci. 2006, 1080, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Shannon, T.R.; Ginsburg, K.S.; Bers, D.M. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys. J. 2000, 78, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Sperelakis, N.; Rubio, R. An orderly lattice of axial tubules which interconnect adjacent transverse tubules in guinea-pig ventricular myocardium. J. Mol. Cell. Cardiol. 1971, 2, 211–220. [Google Scholar] [CrossRef]
- Forbes, M.S.; Hawkey, L.A.; Sperelakis, N. The transverse-axial tubular system (TATS) of mouse myocardium: Its morphology in the developing and adult animal. Am. J. Anat. 1984, 170, 143–162. [Google Scholar] [CrossRef]
- Keizer, J.; Smith, G.D.; Ponce-Dawson, S.; Pearson, J.E. Saltatory propagation of Ca2+ waves by Ca2+ sparks. Biophys. J. 1998, 75, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, S.; Kohl, T.; Williams, G.S.B.; Gusev, K.; Wagner, E.; Rog-Zielinska, E.A.; Hebisch, E.; Dura, M.; Didié, M.; Gotthardt, M.; et al. Axial tubule junctions control rapid calcium signaling in atria. J. Clin. Invest. 2016, 126, 3999–4015. [Google Scholar] [CrossRef] [Green Version]
- Blatter, L.A. The intricacies of atrial calcium cycling during excitation-contraction coupling. J. Gen. Physiol. 2017, 149, 857–865. [Google Scholar] [CrossRef]
- Moss, R.L.; Fitzsimons, D.P.; Ralphe, J.C. Cardiac MyBP-C regulates the rate and force of contraction in mammalian myocardium. Circ. Res. 2015, 116, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Stanczyk, P.J.; Seidel, M.; White, J.; Viero, C.; George, C.H.; Zissimopoulos, S.; Lai, F.A. Association of cardiac myosin-binding protein-C with the ryanodine receptor channel - putative retrograde regulation? J. Cell. Sci. 2018, 131. [Google Scholar] [CrossRef] [Green Version]
- Brehm, P.; Eckert, R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science 1978, 202, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Cota, G.; Nicola Siri, L.; Stefani, E. Calcium channel inactivation in frog (Rana pipiens and Rana moctezuma) skeletal muscle fibres. J. Physiol. 1984, 354, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejía-Alvarez, R.; Fill, M.; Stefani, E. Voltage-dependent inactivation of T-tubular skeletal calcium channels in planar lipid bilayers. J. Gen. Physiol. 1991, 97, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Cens, T.; Rousset, M.; Leyris, J.-P.; Fesquet, P.; Charnet, P. Voltage- and calcium-dependent inactivation in high voltage-gated Ca2+channels. Prog. Biophys. Mol. Biol. 2006, 90, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.T.; Backx, P.H.; Imredy, J.P. Calcium-sensitive inactivation in the gating of single calcium channels. Science 1990, 250, 1735–1738. [Google Scholar] [CrossRef]
- Peterson, B.Z.; DeMaria, C.D.; Adelman, J.P.; Yue, D.T. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron 1999, 22, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Qin, N.; Olcese, R.; Bransby, M.; Lin, T.; Birnbaumer, L. Ca2+-induced inhibition of the cardiac Ca2+ channel depends on calmodulin. Proc. Natl. Acad. Sci. USA 1999, 96, 2435–2438. [Google Scholar] [CrossRef] [Green Version]
- Zühlke, R.D.; Pitt, G.S.; Deisseroth, K.; Tsien, R.W.; Reuter, H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 1999, 399, 159–162. [Google Scholar] [CrossRef]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Abderemane-Ali, F.; Findeisen, F.; Rossen, N.D.; Minor, D.L. A Selectivity Filter Gate Controls Voltage-Gated Calcium Channel Calcium-Dependent Inactivation. Neuron 2019, 101, 1134–1149.e3. [Google Scholar] [CrossRef] [Green Version]
- Takeshima, H.; Komazaki, S.; Nishi, M.; Iino, M.; Kangawa, K. Junctophilins: A novel family of junctional membrane complex proteins. Mol. Cell 2000, 6, 11–22. [Google Scholar] [PubMed]
- Periasamy, M.; Bhupathy, P.; Babu, G.J. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc. Res. 2008, 77, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadini-Buoninsegni, F.; Smeazzetto, S.; Gualdani, R.; Moncelli, M.R. Drug Interactions With the Ca2+-ATPase From Sarco(Endo)Plasmic Reticulum (SERCA). Front. Mol. Biosci. 2018, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.C.; Sahoo, S.K.; Maurya, S.K.; Periasamy, M. The Role of Sarcolipin in Muscle Non-shivering Thermogenesis. Front. Physiol 2018, 9, 1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, J.S.; Shanmugam, M.; Gonzalez, J.P.; Lopez, H.; Gordan, R.; Fraidenraich, D.; Babu, G.J. Increased sarcolipin expression and decreased sarco(endo)plasmic reticulum Ca2+ uptake in skeletal muscles of mouse models of Duchenne muscular dystrophy. J. Muscle Res. Cell. Motil. 2013, 34, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Voit, A.; Patel, V.; Pachon, R.; Shah, V.; Bakhutma, M.; Kohlbrenner, E.; McArdle, J.J.; Dell’Italia, L.J.; Mendell, J.R.; Xie, L.-H.; et al. Reducing sarcolipin expression mitigates Duchenne muscular dystrophy and associated cardiomyopathy in mice. Nat. Commun. 2017, 8, 1068. [Google Scholar] [CrossRef] [Green Version]
- Niranjan, N.; Mareedu, S.; Tian, Y.; Kodippili, K.; Fefelova, N.; Voit, A.; Xie, L.-H.; Duan, D.; Babu, G.J. Sarcolipin overexpression impairs myogenic differentiation in Duchenne muscular dystrophy. Am. J. Physiol. Cell Physiol. 2019, 317, C813–C824. [Google Scholar] [CrossRef]
- Fajardo, V.A.; Chambers, P.J.; Juracic, E.S.; Rietze, B.A.; Gamu, D.; Bellissimo, C.; Kwon, F.; Quadrilatero, J.; Russell Tupling, A. Sarcolipin deletion in mdx mice impairs calcineurin signalling and worsens dystrophic pathology. Hum. Mol. Genet. 2018, 27, 4094–4102. [Google Scholar] [CrossRef]
- Avila, G. Disturbed Ca2+ Homeostasis in Muscle-Wasting Disorders. Adv. Exp. Med. Biol. 2018, 1088, 307–326. [Google Scholar]
- Nicoll, D.A.; Longoni, S.; Philipson, K.D. Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+ exchanger. Science 1990, 250, 562–565. [Google Scholar] [CrossRef]
- Nicoll, D.A.; Quednau, B.D.; Qui, Z.; Xia, Y.R.; Lusis, A.J.; Philipson, K.D. Cloning of a third mammalian Na+-Ca2+ exchanger, NCX3. J. Biol. Chem. 1996, 271, 24914–24921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quednau, B.D.; Nicoll, D.A.; Philipson, K.D. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am. J. Physiol. 1997, 272, C1250–C1261. [Google Scholar] [CrossRef] [PubMed]
- Khananshvili, D. The SLC8 gene family of sodium-calcium exchangers (NCX) - structure, function, and regulation in health and disease. Mol. Aspects Med. 2013, 34, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Burr, A.R.; Millay, D.P.; Goonasekera, S.A.; Park, K.H.; Sargent, M.A.; Collins, J.; Altamirano, F.; Philipson, K.D.; Allen, P.D.; Ma, J.; et al. Na+ dysregulation coupled with Ca2+ entry through NCX1 promotes muscular dystrophy in mice. Mol. Cell. Biol. 2014, 34, 1991–2002. [Google Scholar] [CrossRef] [Green Version]
- Shattock, M.J.; Ottolia, M.; Bers, D.M.; Blaustein, M.P.; Boguslavskyi, A.; Bossuyt, J.; Bridge, J.H.B.; Chen-Izu, Y.; Clancy, C.E.; Edwards, A.; et al. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J. Physiol. 2015, 593, 1361–1382. [Google Scholar] [CrossRef] [Green Version]
- Bassani, J.W.; Bassani, R.A.; Bers, D.M. Relaxation in rabbit and rat cardiac cells: Species-dependent differences in cellular mechanisms. J. Physiol. 1994, 476, 279–293. [Google Scholar] [CrossRef]
- Hohendanner, F.; Walther, S.; Maxwell, J.T.; Kettlewell, S.; Awad, S.; Smith, G.L.; Lonchyna, V.A.; Blatter, L.A. Inositol-1,4,5-trisphosphate induced Ca2+ release and excitation-contraction coupling in atrial myocytes from normal and failing hearts. J. Physiol. 2015, 593, 1459–1477. [Google Scholar] [CrossRef] [Green Version]
- Pogwizd, S.M.; Qi, M.; Yuan, W.; Samarel, A.M.; Bers, D.M. Upregulation of Na+/Ca2+exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ. Res. 1999, 85, 1009–1019. [Google Scholar] [CrossRef] [Green Version]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.E.; Boncompagni, S.; Dirksen, R.T. Sarcoplasmic reticulum-mitochondrial symbiosis: Bidirectional signaling in skeletal muscle. Exerc. Sport Sci. Rev. 2009, 37, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Vegas, A.; Eisner, V.; Jaimovich, E. Skeletal muscle excitation-metabolism coupling. Arch. Biochem. Biophys. 2019, 664, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Zima, A.V.; Blatter, L.A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006, 71, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, S.R.; Cumming, D.V.; Kusama, Y.; Hearse, D.J.; Poole-Wilson, P.A.; Shattock, M.J.; Williams, A.J. Reactive oxygen species modify the structure and function of the cardiac sarcoplasmic reticulum calcium-release channel. Cardioscience 1991, 2, 19–25. [Google Scholar] [PubMed]
- Kuster, G.M.; Lancel, S.; Zhang, J.; Communal, C.; Trucillo, M.P.; Lim, C.C.; Pfister, O.; Weinberg, E.O.; Cohen, R.A.; Liao, R.; et al. Redox-mediated reciprocal regulation of SERCA and Na+-Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic. Biol. Med. 2010, 48, 1182–1187. [Google Scholar] [CrossRef] [Green Version]
- Chacon, E.; Ohata, H.; Harper, I.S.; Trollinger, D.R.; Herman, B.; Lemasters, J.J. Mitochondrial free calcium transients during excitation-contraction coupling in rabbit cardiac myocytes. FEBS Lett. 1996, 382, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Rudolf, R.; Mongillo, M.; Magalhães, P.J.; Pozzan, T. In vivo monitoring of Ca2+uptake into mitochondria of mouse skeletal muscle during contraction. J. Cell Biol. 2004, 166, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Franzini-Armstrong, C. ER-mitochondria communication. How privileged? Physiology 2007, 22, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, E.J.; Balaska, D.; Cheng, W.H.Y. The ups and downs of mitochondrial calcium signalling in the heart. Biochim. Biophys. Acta 2010, 1797, 856–864. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, J.A.; García, M.C.; Sharma, V.K.; Young, K.C.; Matlib, M.A.; Sheu, S.S. Mitochondria regulate inactivation of L-type Ca2+ channels in rat heart. J. Physiol. 2001, 536, 387–396. [Google Scholar] [CrossRef]
- Maack, C.; Cortassa, S.; Aon, M.A.; Ganesan, A.N.; Liu, T.; O’Rourke, B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ. Res. 2006, 99, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.-S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Shkryl, V.M.; Martins, A.S.; Ullrich, N.D.; Nowycky, M.C.; Niggli, E.; Shirokova, N. Reciprocal amplification of ROS and Ca2+signals in stressed mdx dystrophic skeletal muscle fibers. Pflugers Arch. 2009, 458, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.C.; Betzenhauser, M.J.; Reiken, S.; Meli, A.C.; Umanskaya, A.; Xie, W.; Shiomi, T.; Zalk, R.; Lacampagne, A.; Marks, A.R. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011, 14, 196–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamargo, J.; Caballero, R.; Gómez, R.; Delpón, E. Cardiac electrophysiological effects of nitric oxide. Cardiovasc. Res. 2010, 87, 593–600. [Google Scholar] [CrossRef] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, G.; de la Rosa, J.A.; Monsalvo-Villegas, A.; Montiel-Jaen, M.G. Ca2+ Channels Mediate Bidirectional Signaling between Sarcolemma and Sarcoplasmic Reticulum in Muscle Cells. Cells 2020, 9, 55. https://doi.org/10.3390/cells9010055
Avila G, de la Rosa JA, Monsalvo-Villegas A, Montiel-Jaen MG. Ca2+ Channels Mediate Bidirectional Signaling between Sarcolemma and Sarcoplasmic Reticulum in Muscle Cells. Cells. 2020; 9(1):55. https://doi.org/10.3390/cells9010055
Chicago/Turabian StyleAvila, Guillermo, Juan A. de la Rosa, Adrián Monsalvo-Villegas, and María G. Montiel-Jaen. 2020. "Ca2+ Channels Mediate Bidirectional Signaling between Sarcolemma and Sarcoplasmic Reticulum in Muscle Cells" Cells 9, no. 1: 55. https://doi.org/10.3390/cells9010055
APA StyleAvila, G., de la Rosa, J. A., Monsalvo-Villegas, A., & Montiel-Jaen, M. G. (2020). Ca2+ Channels Mediate Bidirectional Signaling between Sarcolemma and Sarcoplasmic Reticulum in Muscle Cells. Cells, 9(1), 55. https://doi.org/10.3390/cells9010055





