CD38, CD157, and RAGE as Molecular Determinants for Social Behavior
Abstract
:1. Introduction
2. Genes and Single-Nucleotide Polymorphisms
3. Messenger RNA (mRNA) Expression Patterns during Development
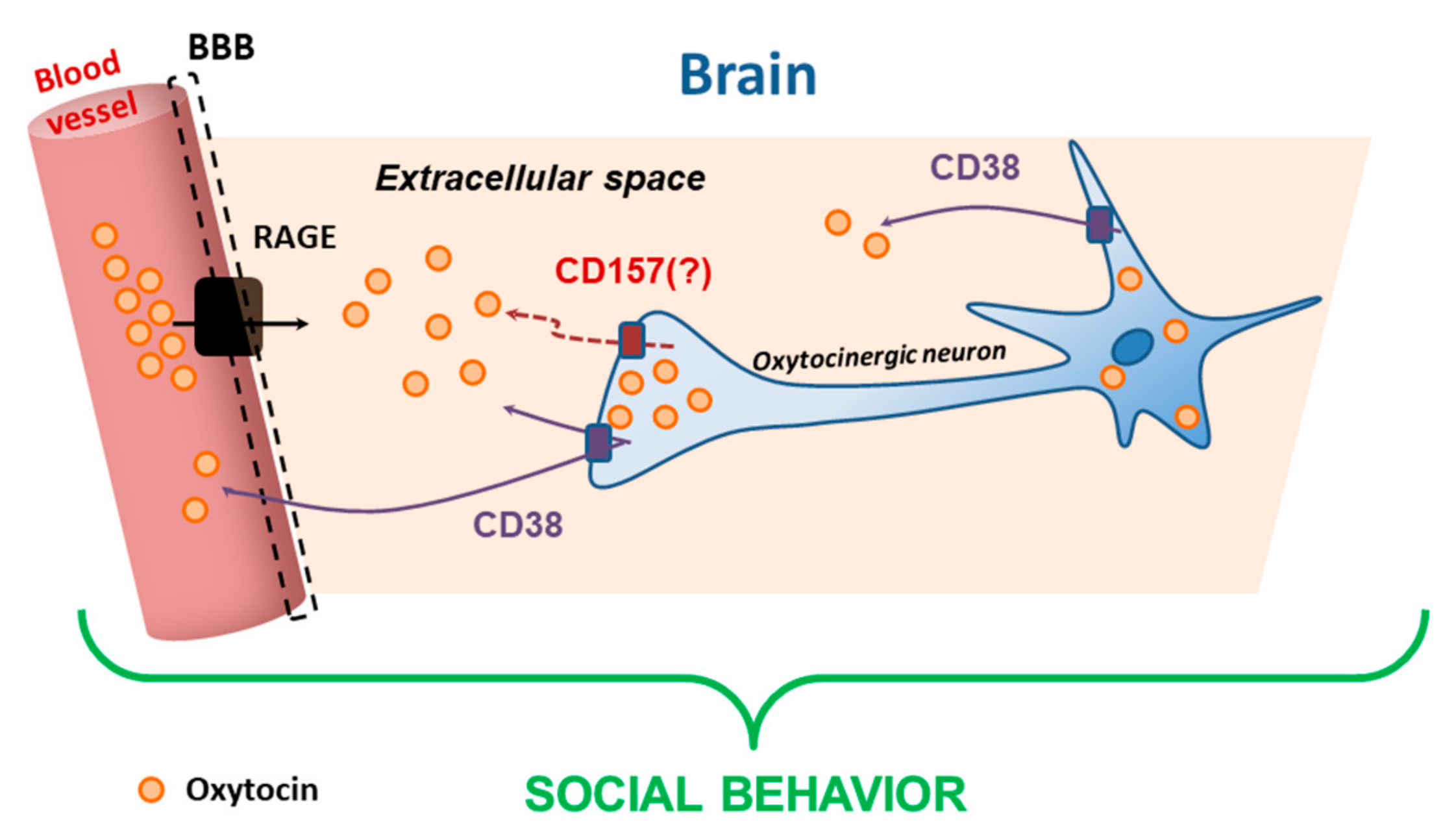
4. Proteins in the Brain
5. Immunohistochemistry of CD157 in Neural Stem Cells
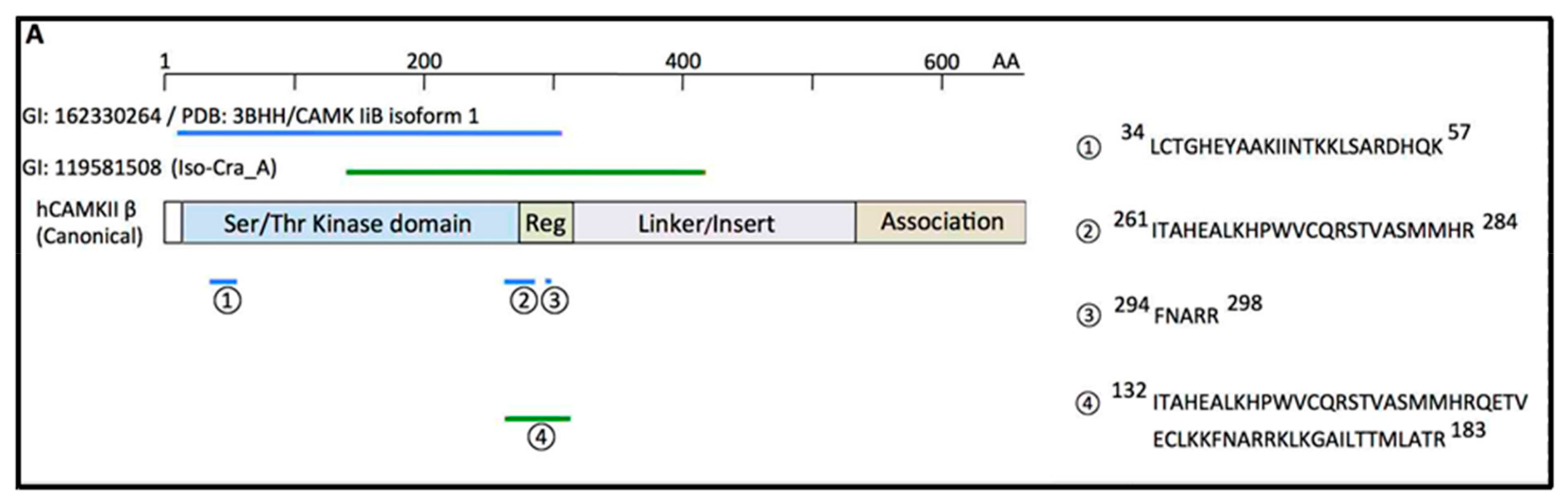
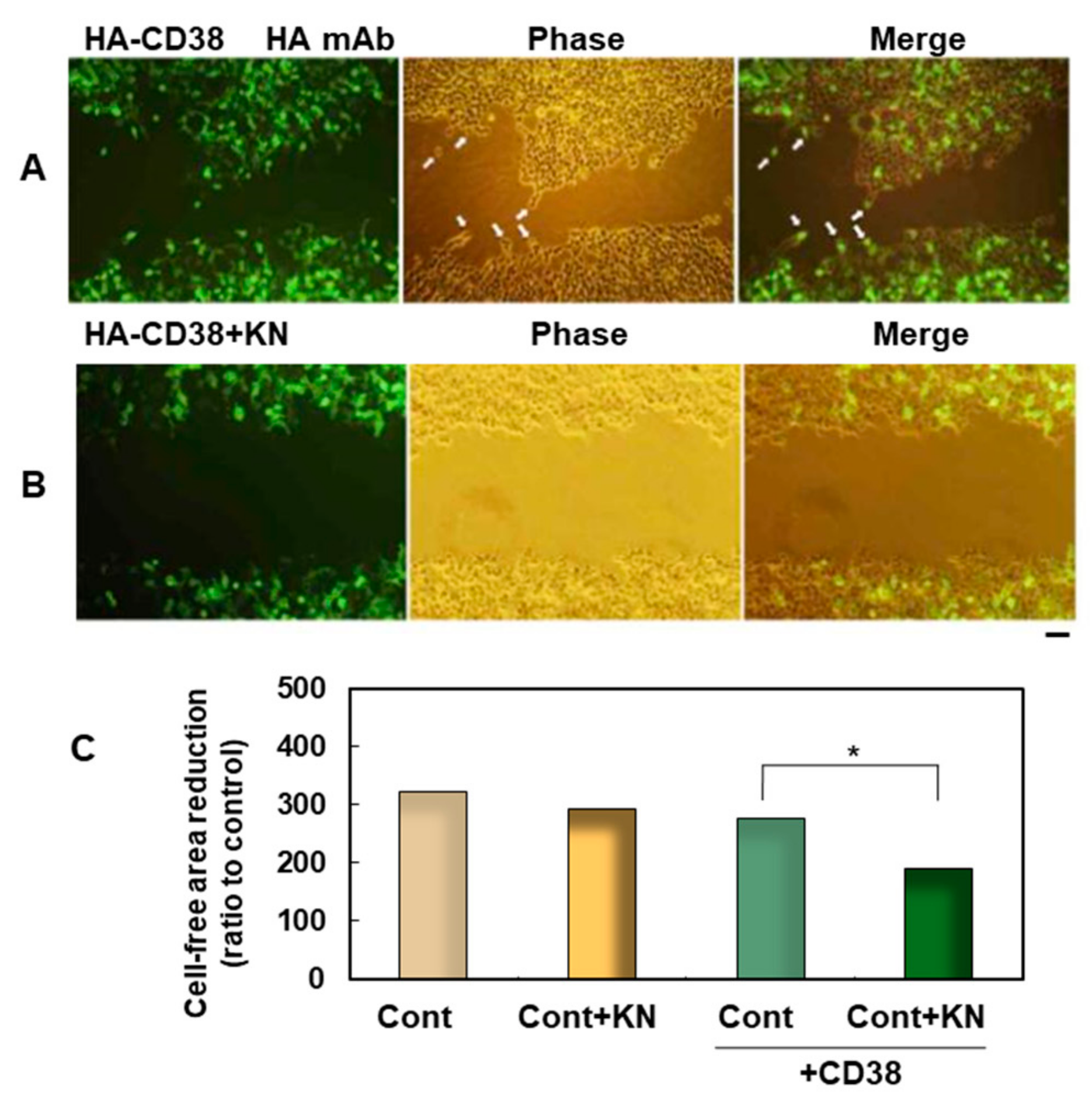
6. Binding of CD38 to Calcium Calmodulin-Dependent Kinase
7. Enzymatic Activities of CD38 and CD157
8. Contribution of TRPM2 on Ca Signaling
9. Social Behavior in Knockout Mice
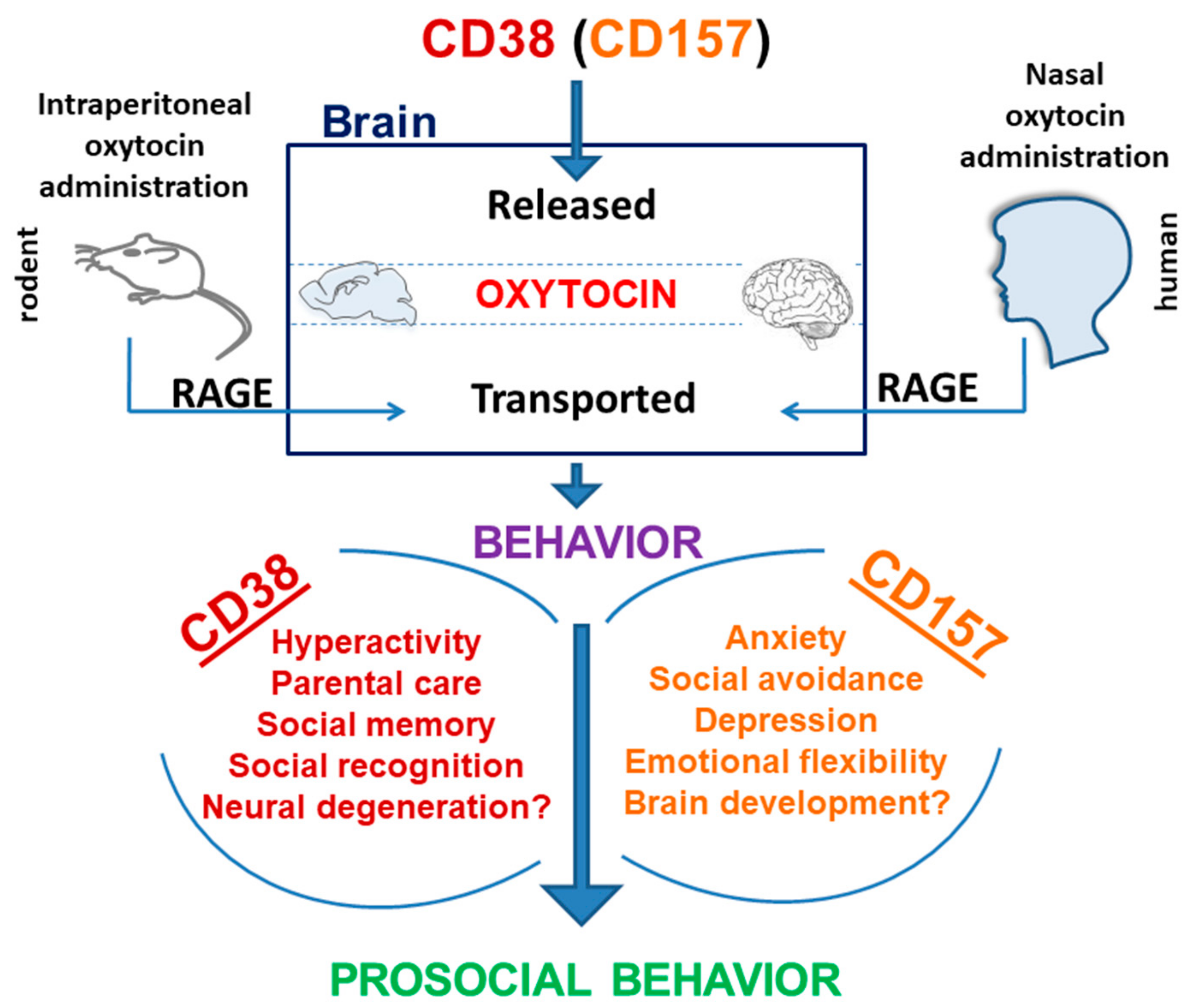
10. Differential Roles of CD38 and CD157 in Social Behavior
11. Autism Spectrum Disorder
12. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thielens, N.M.; Tedesco, F.; Bohlsn, S.S.; Gaboriaud, C.; Tenner, A.J. C1q: A fresh look upon an old molecule. Mol. Immunol. 2017, 89, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Hogan, K.A.; Chini, C.; Chini, E.N. The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front. Immunol. 2019, 10, 1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.C. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 2008, 88, 841–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, K.; Hirano, T. BST-1/CD157 regulates the humoral immune responses in vivo. Chem. Immunol. 2000, 75, 235–255. [Google Scholar]
- Ishihara, K. History and Perspectives of Research in Bone Marrow Stromal Cell Antigen-1 (BST-1)/CD157: A Relative of ADP-Ribosyl Cyclase CD38. Messenger 2014, 3, 15–20. [Google Scholar] [CrossRef]
- Quarona, V.; Zaccarello, G.; Chillemi, A.; Brunetti, E.; Singh, V.K.; Ferrero, E.; Funaro, A.; Horenstein, A.L.; Malavasi, F. CD38 and CD157: A long journey from activation markers to multifunctional molecules. Cytom. B. Clin. Cytom. 2013, 84, 207–217. [Google Scholar] [CrossRef]
- Lopatina, O.; Inzhutova, A.; Salmina, A.B.; Higashida, H. The roles of oxytocin and CD38 in social or parental behaviors. Front. Neurosci. 2013, 6, 182. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.; Liu, H.X.; Hirai, H.; Torashima, T.; Nagai, T.; Lopatina, O.; Shnayder, N.A.; Yamada, K.; Noda, M.; Seike, T.; et al. CD38 is critical for social behaviour by regulating oxytocin secretion. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 2007, 446, 41–45. [Google Scholar] [CrossRef]
- Higashida, H.; Yokoyama, S.; Huang, J.J.; Liu, L.; Ma, W.J.; Akther, S.; Higashida, C.; Kikuchi, M.; Minabe, Y.; Munesue, T. Social memory, amnesia, and autism: Brain oxytocin secretion is regulated by NAD+ metabolites and single nucleotide polymorphisms of CD38. Neurochem. Int. 2012, 61, 828–838. [Google Scholar] [CrossRef] [Green Version]
- Higashida, H. Somato-axodendritic release of oxytocin into the brain due to calcium amplification is essential for social memory. J. Physiol. Sci. 2016, 66, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashida, H.; Yokoyama, S.; Kikuchi, M.; Munesue, T. CD38 and its role in oxytocin secretion and social behavior. Horm. Behav. 2012, 61, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satake, W.; Nakabayashi, Y.; Mizuta, I.; Hirota, Y.; Ito, C.; Kubo, M.; Kawaguchi, T.; Tsunoda, T.; Watanabe, M.; Takeda, A.; et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 2009, 41, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Ioannidis, J.P.; Aasly, J.O.; Annesi, G.; Brice, A.; Van Broeckhoven, C.; Bertram, L.; Bozi, M.; Crosiers, D.; Clarke, C.; et al. Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology 2012, 79, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, S.; Yoshihara, T.; Lopatina, O.; Ishihara, K.; Higashida, H. Selegiline Ameliorates Depression-Like Behavior in Mice Lacking the CD157/BST1 Gene, a Risk Factor for Parkinson’s Disease. Front. Behav. Neurosci. 2017, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, O.; Yoshihara, T.; Nishimura, T.; Zhong, J.; Akther, S.; Fakhrul, A.A.; Liang, M.; Higashida, C.; Sumi, K.; Furuhara, K.; et al. Anxiety- and depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for Parkinson’s disease. Front. Behav. Neurosci. 2014, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ö.H.; Katajisto, P.; Lamming, D.W.; Gültekin, Y.; Bauer-Rowe, K.E.; Sengupta, S.; Birso, Y.K.; Dursun, A.; Yilmaz, V.O.; Selig, M.; et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 2012, 486, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Higashida, H.; Liang, M.; Yoshihara, T.; Akther, S.; Fakhrul, A.; Stanislav, C.; Nam, T.S.; Kim, U.H.; Kasai, S.; Nishimura, T.; et al. An immunohistochemical, enzymatic, and behavioral study of CD157/BST-1 as a neuroregulator. BMC Neurosci. 2017, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Riebold, M.; Mankuta, D.; Lerer, E.; Israel, S.; Zhong, S.; Nemanov, L.; Monakhov, M.V.; Levi, S.; Yirmiya, N.; Yaari, M.; et al. All-trans retinoic acid upregulates reduced CD38 transcription in lymphoblastoid cell lines from Autism spectrum disorder. Mol. Med. 2011, 17, 799–806. [Google Scholar] [CrossRef]
- Feldman, R.; Monakhov, M.; Pratt, M.; Ebstein, R.P. Oxytocin Pathway Genes: Evolutionary Ancient System Impacting on Human Affiliation, Sociality, and Psychopathology. Biol. Psychiatry 2016, 79, 174–184. [Google Scholar] [CrossRef]
- Munesue, T.; Yokoyama, S.; Nakamura, K.; Anitha, A.; Yamada, K.; Hayashi, K.; Asaka, T.; Liu, H.X.; Jin, D.; Koizumi, K.; et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci. Res. 2010, 67, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krol, K.M.; Monakhov, M.; Lai, P.S.; Ebstein, R.P.; Grossmann, T. Genetic variation in CD38 and breastfeeding experience interact to impact infants’ attention to social eye cues. Proc. Natl. Acad. Sci. USA 2015, 112, E5434–E5442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovey, D.; Zettergren, A.; Jonsson, L.; Melke, J.; Anckarsäter, H.; Lichtenstein, P.; Westberg, L. Associations between oxytocin-related genes and autistic-like traits. Soc. Neurosci. 2014, 9, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadgama, N.; Pittman, A.; Simpson, M.; Nirmalananthan, N.; Murray, R.; Yoshikawa, T.; De Rijk, P.; Rees, E.; Kirov, G.; Hughes, D.; et al. De novo single-nucleotide and copy number variation in discordant monozygotic twins reveals disease-related genes. Eur. J. Hum. Genet. 2019, 27, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Lerer, E.; Levi, S.; Israel, S.; Yaari, M.; Nemanov, L.; Mankuta, D.; Nurit, Y.; Ebstein, R.P. Low CD38 expression in lymphoblastoid cells and haplotypes are both associated with autism in a family-based study. Autism Res. 2010, 3, 293–302. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, R.J.; McInnis, O.A.; Matheson, K.; Anisman, H. Oxytocin and Social Sensitivity: Gene Polymorphisms in Relation to Depressive Symptoms and Suicidal Ideation. Front. Hum. Neurosci. 2016, 10, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zhang, H.F.; Han, J.S.; Han, S.P. Genes Related to Oxytocin and Arginine-Vasopressin Pathways: Associations with Autism Spectrum Disorders. Neurosci. Bull. 2017, 33, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Sauer, C.; Montag, C.; Wörner, C.; Kirsch, P.; Reuter, M. Effects of a common variant in the CD38 gene on social processing in an oxytocin challenge study: Possible links to autism. Neuropsychopharmacology 2012, 37, 1474–1482. [Google Scholar] [CrossRef] [Green Version]
- Kucharska, K.; Kot, E.; Biernacka, K.; Zimowski, J.; Rogoza, R.; Rybakowski, F.; Kostecka, B.; Bednarska-Makaruk, M. Interaction between polymorphisms of the oxytocinergic system genes and emotion perception in inpatients with anorexia nervosa. Eur. Eat. Disord. Rev. 2019, 27, 481–494. [Google Scholar] [CrossRef]
- Parris, M.S.; Grunebaum, M.F.; Galfalvy, H.C.; Andronikashvili, A.; Burke, A.K.; Yin, H.; Min, E.; Huang, Y.Y.; Mann, J.J. Attempted suicide and oxytocin-related gene polymorphisms. J. Affect. Disord. 2018, 238, 62–68. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Liu, L.; Fu, H.; Tang, L. The association between CD157/BST1 polymorphisms and the susceptibility of Parkinson’s disease: A meta-analysis. Neuropsychiatr. Dis. Treat. 2019, 15, 1089–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.L.; Lin, C.H.; Lee, M.J.; Wu, R.M. BST1 rs11724635 interacts with environmental factors to increase the risk of Parkinson’s disease in a Taiwanese population. Parkinsonism Relat. Disord. 2014, 20, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Al Mahmuda, N.; Munesue, T.; Hayashi, K.; Yagi, K.; Yamagishi, M.; Higashida, H. Association Study between the CD157/BST1 Gene and Autism Spectrum Disorders in a Japanese Population. Brain Sci. 2015, 5, 188–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashida, H.; Kamimura, S.Y.; Inoue, T.; Hori, O.; Islam, M.S.; Lopatina, O.; Tsuji, C. Cyclic ADP-ribose as an endogenous inhibitor of the mTOR pathway downstream of dopamine receptors in the mouse striatum. J. Neural. Transm. (Vienna) 2018, 125, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, M.; Otsuka, N.; Sato, M.; Ishii, Y.; Kon, S.; Yamada, M.; Nishina, H.; Katada, T.; Ikeda, K. Neuronal localization of CD38 antigen in the human brain. Brain Res. 1995, 697, 2340–2352. [Google Scholar] [CrossRef]
- Zhong, J.; Amina, S.; Liang, M.; Akther, S.; Yuhi, T.; Nishimura, T.; Tsuji, C.; Tsuji, T.; Liu, H.X.; Hashii, M.; et al. Cyclic ADP-Ribose and heat regulate oxytocin release via CD38 and TRPM2 in the hypothalamus during social or psychological stress in mice. Front. Neurosci. 2016, 10, 304. [Google Scholar] [CrossRef] [Green Version]
- Akimoto, N.; Kamiyama, Y.; Yamafuji, M.; Fujita, K.; Seike, T.; Kido, M.A.; Yokoyama, S.; Higashida, H.; Noda, M. Immunohistochemistry of CD38 in Different Cell Types in the Hypothalamus and Pituitary of Male Mice. Messenger 2013, 2, 54–61. [Google Scholar] [CrossRef]
- Hattori, T.; Kaji, M.; Ishii, H.; Jureepon, R.; Takarada-Iemata, M.; Minh Ta, H.; Manh Le, T.; Konno, A.; Hirai, H.; Shiraishi, Y.; et al. CD38 positively regulates postnatal development of astrocytes cell-autonomously and oligodendrocytes non-cell-autonomously. Glia 2017, 65, 974–989. [Google Scholar] [CrossRef]
- Roboon, J.; Hattori, T.; Ishii, H.; Takarada-Iemata, M.; Le, T.M.; Shiraishi, Y.; Ozaki, N.; Yamamoto, Y.; Sugawara, A.; Okamoto, H.; et al. Deletion of CD38 Suppresses Glial Activation and Neuroinflammation in a Mouse Model of Demyelination. Front. Cell. Neurosci. 2019, 13, 258. [Google Scholar] [CrossRef]
- Yamada, M.; Mizuguchi, M.; Otsuka, N.; Ikeda, K.; Takahashi, H. Ultrastructural localization of CD38 immunoreactivity in rat brain. Brain Res. 1997, 756, 52–60. [Google Scholar] [CrossRef]
- Lai, X.; Wu, X.; Hou, N.; Liu, S.; Li, Q.; Yang, T.; Miao, J.; Dong, Z.; Chen, J.; Li, T. Vitamin A Deficiency Induces Autistic-Like Behaviors in Rats by Regulating the RARβ-CD38-Oxytocin Axis in the Hypothalamus. Mol. Nutr. Food Res. 2018, 62, 5. [Google Scholar] [CrossRef] [PubMed]
- Martucci, L.L.; Amar, M.; Chaussenot, R.; Benet, G.; Bauer, O.; De Zélicourt, A.; Nosjean, A.; Launay, J.M.; Callebert, J.; Sebrié, C.; et al. A multiscale analysis in CD38-/- mice unveils major prefrontal cortex dysfunctions. FASEB J. 2019, 33, 5823–5835. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, T.P.; Bamford, R.A.; Tochitani, S.; Akkus, K.; Kudzinskas, A.; Yokoi, K.; Okamoto, H.; Yamamoto, Y.; Burbach, J.P.H.; Matsuzaki, H.; et al. CD38 is Required for Dendritic Organization in Visual Cortex and Hippocampus. Neuroscience 2018, 372, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Verderio, C.; Schenk, U.; Fedele, E.; Zocchi, E.; Matteoli, M.; De Flora, A. Glutamate-mediated overexpression of CD38 in astrocytes cultured with neurones. J. Neurochem. 2004, 8, 264–272. [Google Scholar] [CrossRef]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Kou, W.; Banerjee, S.; Eudy, J.; Smith, L.M.; Persidsky, R.; Borgmann, K.; Wu, L.; Sakhuja, N.; Deshpande, M.S.; Walseth, T.F.; et al. CD38 regulation in activated astrocytes: Implications for neuroinflammation and HIV-1 brain infection. J. Neurosci. Res. 2009, 87, 2326–2339. [Google Scholar] [CrossRef]
- Meditz, A.L.; Haas, M.K.; Folkvord, J.M.; Melander, K.; Young, R.; McCarter, M.; Mawhinney, S.; Campbell, T.B.; Lie, Y.; Coakley, E.; et al. HLA-DR+ CD38+ CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo. J. Virol. 2011, 85, 10189–10200. [Google Scholar] [CrossRef] [Green Version]
- Lévy, Y.; Thiébaut, R.; Gougeon, M.L.; Molina, J.M.; Weiss, L.; Girard, P.M.; Venet, A.; Morlat, P.; Poirier, B.; Lascaux, A.S.; et al. Effect of intermittent interleukin-2 therapy on CD4+ T-cell counts following antiretroviral cessation in patients with HIV. AIDS 2012, 26, 711–720. [Google Scholar] [CrossRef]
- Wu, T.J.; Tzeng, Y.K.; Chang, W.W.; Cheng, C.A.; Kuo, Y.; Chien, C.H.; Chang, H.C.; Yu, J. Tracking the engraftment and regenerative capabilities of transplanted lung stem cells using fluorescent nanodiamonds. Nat. Nanotechnol. 2013, 8, 682–689. [Google Scholar] [CrossRef]
- Pentinmikko, N.; Iqbal, S.; Mana, M.; Andersson, S.; Cognetta, A.B.; Suciu, R.M.; Roper, J.; Luopajärvi, K.; Markelin, E.; Gopalakrishnan, S.; et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 2019, 571, 398–402. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Naito, H.; Suehiro, J.I.; Lin, Y.; Kawaji, H.; Iba, T.; Kouno, T.; Ishikawa-Kato, S.; Furuno, M.; Takara, K.; et al. CD157 Marks Tissue-Resident Endothelial Stem Cells with Homeostatic and Regenerative Properties. Cell Stem Cell 2018, 22, 384–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kfir, S.H.; Barash, I. Calorie restriction and rapamycin administration induce stem cell self-renewal and consequent development and production in the mammary gland. Exp. Cell Res. 2019, 382, 111477. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, E.; Augeri, S.; Fissolo, G.; Musso, I.; Funaro, A. CD157: From immunoregulatory protein to potential therapeutic target. Immunol. Lett. 2019, 205, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Buono, N.; Parrotta, R.; Morone, S.; Bovino, P.; Nacci, G.; Ortolan, E.; Horenstein, A.L.; Inzhutova, A.; Ferrero, E.; Funaro, A. The CD157-integrin partnership controls transendothelial migration and adhesion of human monocytes. J. Biol. Chem. 2011, 286, 18681–18691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takakura, N. Discovery of a Vascular Endothelial Stem Cell (VESC) Population Required for Vascular Regeneration and Tissue Maintenance. Circ. J. 2018, 83, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Hashii, M.; Tanaka, Y.; Higuchi, Y.; Gabata, R.; Tsubomoto, M.; Iwatsuka, N.; Teramachi, M.; Kamijima, T.; Yokoyama, S.; Matsukawa, S.; et al. A Ca2+-Calmodulin-Dependent Protein Kinase II Inhibitor Suppresses Cell Migration of CD38-Expressing Human Embryonic Kidney Cells: A Preliminary Report. Messenger 2018, 6, 85–88. [Google Scholar] [CrossRef]
- Colbran, R.J.; Brown, A.M. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr. Opin. Neurobiol. 2004, 14, 318–327. [Google Scholar] [CrossRef]
- Takasawa, S.; Ishida, A.; Nata, K.; Nakagawa, K.; Noguchi, N.; Tohgo, A.; Kato, I.; Yonekura, H.; Fujisawa, H.; Okamoto, H. Requirement of calmodulin-dependent protein kinase II in cyclic ADP-ribose-mediated intracellular Ca2+ mobilization. J. Biol. Chem. 1995, 270, 30257–30259. [Google Scholar] [CrossRef] [Green Version]
- Ohta, Y.; Hartwig, J.H. Actin filament cross-linking by chicken gizzard filamin is regulated by phosphorylation in vitro. Biochemistry 1995, 34, 6745–6754. [Google Scholar] [CrossRef]
- Hoffman, L.; Li, L.; Alexov, E.; Sanabria, H.; Waxham, M.N. Cytoskeletal-like Filaments of Ca2+-Calmodulin-Dependent Protein Kinase II Are Formed in a Regulated and Zn2+-Dependent Manner. Biochemistry 2017, 56, 2149–2160. [Google Scholar] [CrossRef]
- Shi, C.; Cai, Y.; Li, Y.; Li, Y.; Hu, N.; Ma, S.; Hu, S.; Zhu, P.; Wang, W.; Zhou, H. Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 2018, 14, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Zumaquero, E.; Muñoz, P.; Cobo, M.; Lucena, G.; Pavón, E.J.; Martín, A.; Navarro, P.; García-Pérez, A.; Ariza-Veguillas, A.; Malavasi, F.; et al. Exosomes from human lymphoblastoid B cells express enzymatically active CD38 that is associated with signaling complexes containing CD81, Hsc-70 and Lyn. Exp. Cell Res. 2010, 316, 2692–2706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Zhu, W.J.; Wang, X.W.; Zhang, L.H.; Lee, H.C. Determinants of the membrane orientation of a calcium signaling enzyme CD38. Biochim. Biophys. Acta. 2015, 1853, 2095–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhao, Y.J.; Li, W.H.; Hou, Y.N.; Li, T.; Zhao, Z.Y.; Fang, C.; Li, S.L.; Lee, H.C. Cytosolic interaction of type III human CD38 with CIB1 modulates cellular cyclic ADP-ribose levels. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Q.W.; Zhang, J.; Li, T.; He, W.M.; Fang, L.; Lee, H.C.; Zhao, Y.J. The transferrin receptor CD71 regulates type II CD38, revealing tight topological compartmentalization of intracellular cyclic ADP-ribose production. J. Biol. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Li, T.; Li, Y.; Xu, G.J.; Deng, Q.W.; Chen, Y.J.; Hou, Y.N.; Lee, H.C.; Zhao, Y.J. CD38 produces nicotinic acid adenosine dinucleotide phosphate in the lysosome. J. Biol. Chem. 2018, 293, 8151–8160. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.K.; Bolton, E.L.; Cortopassi, W.A.; Wang, Y.; O’Brien, F.; Maciejewska, M.; Jacobson, M.P.; Garnham, C.; Ruas, M.; Parrington, J.; et al. Synthesis of the Ca2+-mobilizing messengers NAADP and cADPR by intracellular CD38 enzyme in the mouse heart: Role in β-adrenoceptor signaling. J. Biol. Chem. 2017, 292, 13243–13257. [Google Scholar] [CrossRef] [Green Version]
- Kashio, M.; Tominaga, M. The TRPM2 channel: A thermo-sensitive metabolic sensor. Channels (Austin) 2017, 11, 426–433. [Google Scholar] [CrossRef]
- Uchida, K.; Dezaki, K.; Yoneshiro, T.; Watanabe, T.; Yamazaki, J.; Saito, M.; Yada, T.; Tominaga, M.; Iwasaki, Y. Involvement of thermosensitive TRP channels in energy metabolism. J. Physiol. Sci. 2017, 67, 549–560. [Google Scholar]
- Fliegert, R.; Bauche, A.; Wolf Pérez, A.M.; Watt, J.M.; Rozewitz, M.D.; Winzer, R.; Janus, M.; Gu, F.; Rosche, A.; Harneit, A.; et al. 2’-Deoxyadenosine 5’-diphosphoribose is an endogenous TRPM2 superagonist. Nat. Chem. Biol. 2017, 13, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Higashida, H.; Yuhi, T.; Akther, S.; Amina, S.; Zhong, J.; Liang, M.; Nishimura, T.; Liu, H.X.; Lopatina, O. Oxytocin release via activation of TRPM2 and CD38 in the hypothalamus during hyperthermia in mice: Implication for autism spectrum disorder. Neurochem. Int. 2018, 119, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rah, S.Y.; Kwak, J.Y.; Chung, Y.J.; Kim, U.H. ADP-ribose/TRPM2-mediated Ca2+ signaling is essential for cytolytic degranulation and antitumor activity of natural killer cells. Sci. Rep. 2015, 5, 9482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, R.; Sun, H.Y.; Lau, C.P.; Tse, H.F.; Lee, H.C.; Li, G.R. Cyclic ADP ribose is a novel regulator of intracellular Ca2+ oscillations in human bone marrow mesenchymal stem cells. J. Cell. Mol. Med. 2011, 15, 2684–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, I.; Yamamoto, S.; Partida-Sanchez, S.; Mori, Y.; Fleig, A.; Penner, R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci. Signal. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiner, I.; Radukina, N.; Eisfeld, J.; Kühn, F.; Lückhoff, A. Regulation of TRPM2 channels in neutrophil granulocytes by ADP-ribose: A promising pharmacological target. Naunyn Schmiedebergs Arch. Pharmacol. 2005, 371, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Rodríguez, S.; Rosal-Vela, A.; Botta, D.; Cumba Garcia, L.M.; Zumaquero, E.; Prados-Maniviesa, V.; Cerezo-Walli, S.D.; Lo Buono, N.; Robles-Guirado, J.Á.; Guerrero, S.; et al. CD38 promotes pristane-induced chronic inflammation and increases susceptibility to experimental lupus by an apoptosis-driven and TRPM2-dependent mechanism. Sci. Rep. 2018, 8, 3357. [Google Scholar]
- Lopatina, O.L.; Furuhara, K.; Ishihara, K.; Salmina, A.B.; Higashida, H. Communication Impairment in Ultrasonic Vocal Repertoire during the Suckling Period of Cd157 Knockout Mice: Transient Improvement by Oxytocin. Front. Neurosci. 2017, 11, 266. [Google Scholar] [CrossRef]
- Mizuno, A.; Cherepanov, S.M.; Kikuchi, Y.; Fakhrul, A.A.; Akther, S.; Deguchi, K.; Yoshihara, T.; Ishihara, K.; Shuto, S.; Higashida, H. Lipo-oxytocin-1, a Novel Oxytocin Analog Conjugated with Two Palmitoyl Groups, Has Long-Lasting Effects on Anxiety-Related Behavior and Social Avoidance in CD157 Knockout Mice. Brain Sci. 2015, 5, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Ichinose, W.; Cherepanov, S.M.; Shabalova, A.A.; Yokoyama, S.; Yuhi, T.; Yamaguchi, H.; Watanabe, A.; Yamamoto, Y.; Okamoto, H.; Horike, S.; et al. Development of a Highly Potent Analogue and a Long-Acting Analogue of Oxytocin for the Treatment of Social Impairment-Like Behaviors. J. Med. Chem. 2019, 62, 3297–3310. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Liang, M.; Munesue, S.; Deguchi, K.; Harashima, A.; Furuhara, K.; Yuhi, T.; Zhong, J.; Akther, S.; Goto, H.; et al. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun. Biol. 2019, 2, 76. [Google Scholar] [CrossRef]
- Cherepanov, S.M.; Akther, S.; Nishimura, T.; Shabalova, A.A.; Mizuno, A.; Ichinose, W.; Shuto, S.; Yamamoto, Y.; Yokoyama, S.; Higashida, H. Effects of Three Lipidated Oxytocin Analogs on Behavioral Deficits in CD38 Knockout Mice. Brain Sci. 2017, 7, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriaan Bouwknecht, J.; Olivier, B.; Paylor, R.E. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: A review of pharmacological and genetic studies in the mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.; Harker, C.T.; Vander, A.J.; Kluger, M.J. Hyperthermia induced by open-field stress is blocked by salicylate. Physiol. Behav. 1986, 36, 1179–1182. [Google Scholar] [CrossRef] [Green Version]
- Vinkers, C.H.; Penning, R.; Hellhammer, J.; Verster, J.C.; Klaessens, J.H.; Olivier, B.; Kalkman, C.J. The effect of stress on core and peripheral body temperature in humans. Stress 2013, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Groenink, L.; Vinkers, C.; van Oorschot, R.; Olivier, B. Models of anxiety: Stress-induced hyperthermia (SIH) in singly housed mice. Curr. Protoc. Pharmacol. 2009, 45, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Mehler, M.F.; Purpura, D.P. Autism, fever, epigenetics and the locus coeruleus. Brain Res. Rev. 2009, 59, 388–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curran, L.K.; Newschaffer, C.J.; Lee, L.C.; Crawford, S.O.; Johnston, M.V.; Zimmerman, A.W. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics 2007, 120, e1386–e1392. [Google Scholar] [CrossRef]
- Naviaux, R.K. Antipurinergic therapy for autism-An in-depth review. Mitochondrion 2018, 43, 1–15. [Google Scholar] [CrossRef]
- Owada, K.; Okada, T.; Munesue, T.; Kuroda, M.; Fujioka, T.; Uno, Y.; Matsumoto, K.; Kuwabara, H.; Mori, D.; Okamoto, Y.; et al. Quantitative facial expression analysis revealed the efficacy and time course of oxytocin in autism. Brain 2019, 142, 2127–2136. [Google Scholar] [CrossRef]
- Yamasue, H.; Okada, T.; Munesue, T.; Kuroda, M.; Fujioka, T.; Uno, Y.; Matsumoto, K.; Kuwabara, H.; Mori, D.; Okamoto, Y.; et al. Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: A randomized clinical trial. Mol. Psychiatry 2018. [Google Scholar] [CrossRef]
- Kosaka, H.; Okamoto, Y.; Munesue, T.; Yamasue, H.; Inohara, K.; Fujioka, T.; Anme, T.; Orisaka, M.; Ishitobi, M.; Jung, M.; et al. Oxytocin efficacy is modulated by dosage and oxytocin receptor genotype in young adults with high-functioning autism: A 24-week randomized clinical trial. Transl. Psychiatry 2016, 6, e872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munesue, T.; Nakamura, H.; Kikuchi, M.; Miura, Y.; Takeuch, N.; Anme, T.; Nanba, E.; Adachi, K.; Tsubouchi, K.; Sai, Y.; et al. Oxytocin for Male Subjects with Autism Spectrum Disorder and Comorbid Intellectual Disabilities: A Randomized Pilot Study. Front. Psychiatry. 2016, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, K.J.; Oztan, O.; Libove, R.A.; Sumiyoshi, R.D.; Jackson, L.P.; Karhson, D.S.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. USA 2017, 114, 8119–8124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashida, H.; Munesue, T.; Kosaka, H.; Yamasue, H.; Yokoyama, S.; Kikuchi, M. Social Interaction Improved by Oxytocin in the Subclass of Autism with Comorbid Intellectual Disabilities. Diseases 2019, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Higashida, H.; Furuhara, K.; Yamauchi, A.M.; Deguchi, K.; Harashima, A.; Munesue, S.; Lopatina, O.; Gerasimenko, M.; Salmina, A.B.; Zhang, J.S.; et al. Intestinal transepithelial permeability of oxytocin into the blood is dependent on the receptor for advanced glycation end products in mice. Sci. Rep. 2017, 7, 7883. [Google Scholar] [CrossRef]
- Shindler, A.E.; Hill-Yardin, E.L.; Petrovski, S.; Bishop, N.; Franks, A.E. Towards Identifying Genetic Biomarkers for Gastrointestinal Dysfunction in Autism. J. Autism Dev. Disord. 2019. [Google Scholar] [CrossRef]
- Lee, H.C.; Zhao, Y.J. Resolving the topological enigma in Ca2+-signaling by cyclic ADP-ribose and NAADP. J. Biol. Chem. 2019, 294, 19447. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.Y.; Xie, X.J.; Li, W.H.; Liu, J.; Chen, Z.; Zhang, B.; Li, T.; Li, S.L.; Lu, J.G.; Zhang, L.; et al. A Cell-Permeant Mimetic of NMN Activates SARM1 to Produce Cyclic ADP-Ribose and Induce Non-apoptotic Cell Death. iScience. 2019, 15, 452–466. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.W.; Smith, C.B.; Schmidt, M.S.; Cambronne, X.A.; Cohen, M.S.; Migaud, M.E.; Brenner, C.; Goodman, R.H. Pharmacological bypass of NAD+ salvage pathway protects neurons from chemotherapy-induced degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, 10654–10659. [Google Scholar] [CrossRef] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashida, H.; Hashii, M.; Tanaka, Y.; Matsukawa, S.; Higuchi, Y.; Gabata, R.; Tsubomoto, M.; Seishima, N.; Teramachi, M.; Kamijima, T.; et al. CD38, CD157, and RAGE as Molecular Determinants for Social Behavior. Cells 2020, 9, 62. https://doi.org/10.3390/cells9010062
Higashida H, Hashii M, Tanaka Y, Matsukawa S, Higuchi Y, Gabata R, Tsubomoto M, Seishima N, Teramachi M, Kamijima T, et al. CD38, CD157, and RAGE as Molecular Determinants for Social Behavior. Cells. 2020; 9(1):62. https://doi.org/10.3390/cells9010062
Chicago/Turabian StyleHigashida, Haruhiro, Minako Hashii, Yukie Tanaka, Shigeru Matsukawa, Yoshihiro Higuchi, Ryosuke Gabata, Makoto Tsubomoto, Noriko Seishima, Mitsuyo Teramachi, Taiki Kamijima, and et al. 2020. "CD38, CD157, and RAGE as Molecular Determinants for Social Behavior" Cells 9, no. 1: 62. https://doi.org/10.3390/cells9010062
APA StyleHigashida, H., Hashii, M., Tanaka, Y., Matsukawa, S., Higuchi, Y., Gabata, R., Tsubomoto, M., Seishima, N., Teramachi, M., Kamijima, T., Hattori, T., Hori, O., Tsuji, C., Cherepanov, S. M., Shabalova, A. A., Gerasimenko, M., Minami, K., Yokoyama, S., Munesue, S.-i., ... Lopatina, O. (2020). CD38, CD157, and RAGE as Molecular Determinants for Social Behavior. Cells, 9(1), 62. https://doi.org/10.3390/cells9010062






