Extracellular Vesicles from Mesenchymal Stem Cells as Novel Treatments for Musculoskeletal Diseases
Abstract
:1. Introduction
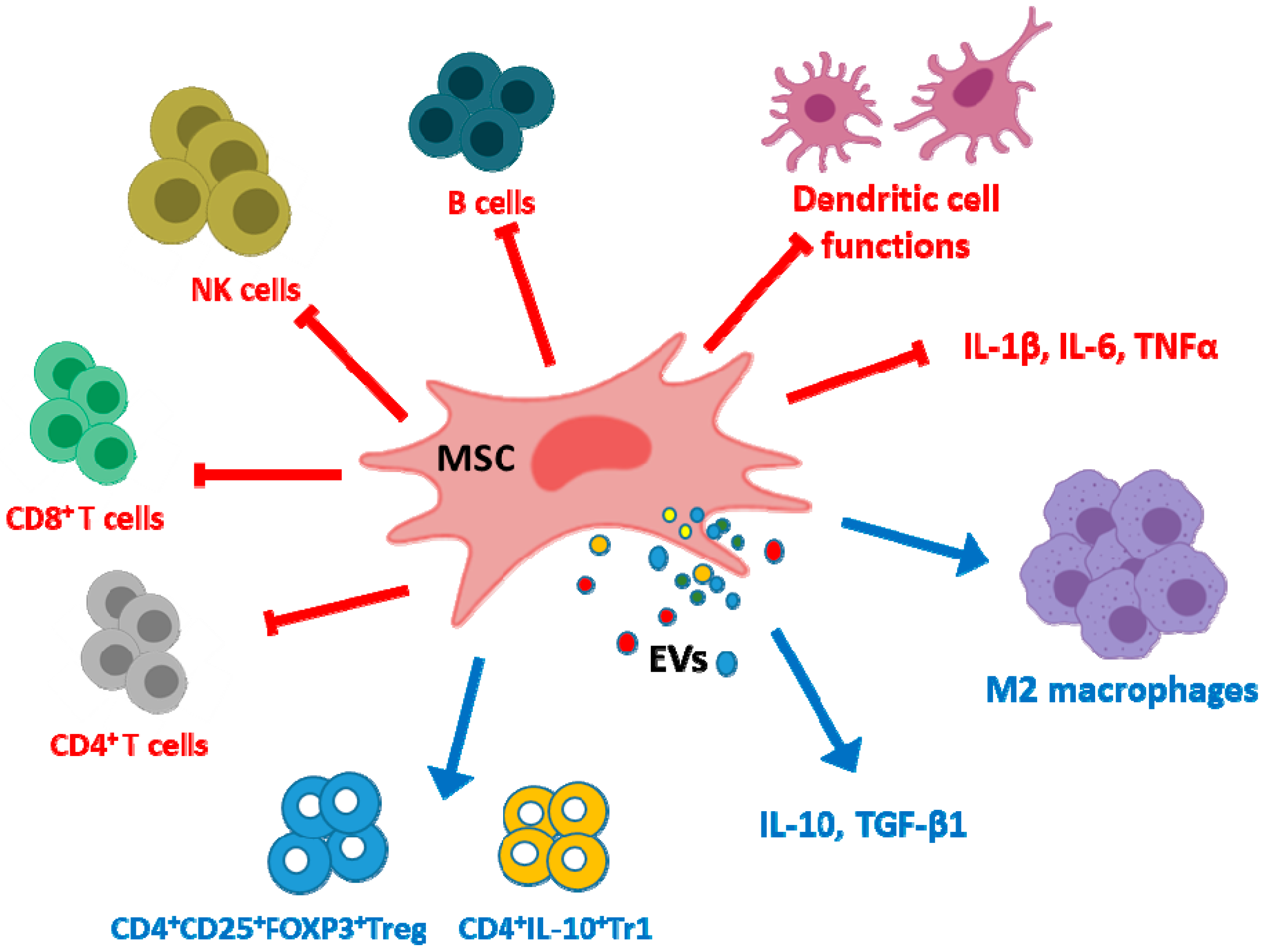
2. Immune Regulation and Rheumatoid Arthritis
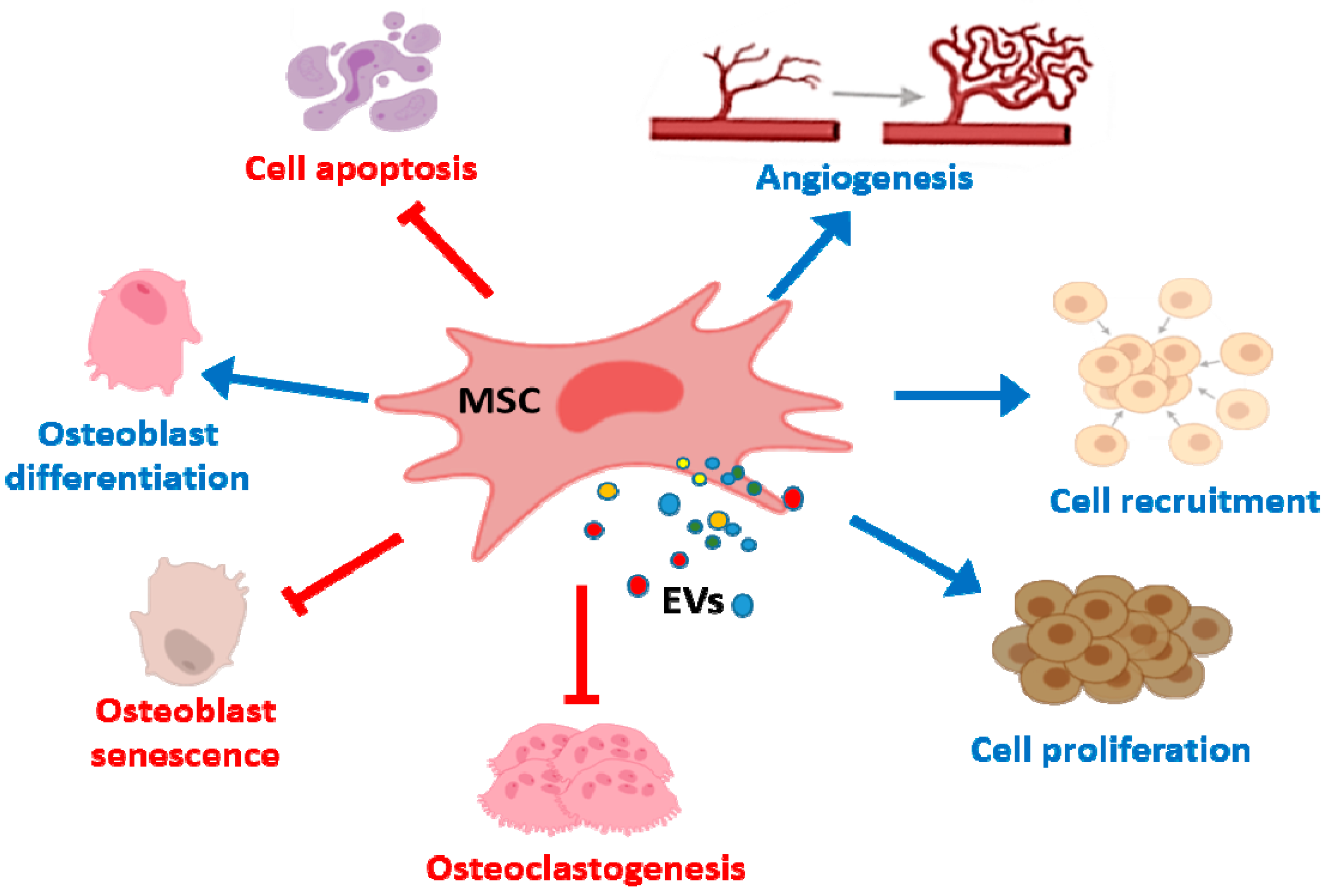
3. Bone Repair and Bone Diseases
4. Cartilage Repair and Osteoarthritis
5. Intervertebral Disk Degeneration
6. Skeletal Muscle and Tendon Repair
7. Conclusions
Funding
Conflicts of Interest
References
- World Health Organization. Musculoskeletal Conditions. Available online: https://www.who. int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 21 November 2019).
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, L.; Cong, X.; Liu, G.; Zhou, J.; Bai, B.; Li, Y.; Bai, W.; Li, M.; Ji, H.; et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: Safety and efficacy. Stem Cells Dev. 2013, 22, 3192–3202. [Google Scholar] [CrossRef] [Green Version]
- Franceschetti, T.; De, B.C. The potential role of adult stem cells in the management of the rheumatic diseases. Ther. Adv. Musculoskelet. Dis. 2017, 9, 165–179. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Boyd, R.; Shah, K.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety and efficacy—A review. BMC Musculoskelet. Disord. 2016, 17, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, A.; Martin-Ferrero, M.A.; Del, C.F.; Alberca, M.; Garcia, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Wang, Y.Y.; Li, C.J.; Shi, C.H.; Wang, W.S. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp. Ther. Med. 2016, 12, 3390–3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult stem cells for bone regeneration and repair. Front. Cell Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marolt, P.D.; Traweger, A.; Gimona, M.; Redl, H. Mesenchymal stromal cell-based bone regeneration therapies: From cell transplantation and tissue engineering to therapeutic secretomes and extracellular vesicles. Front. Bioeng. Biotechnol. 2019, 7, 352. [Google Scholar] [CrossRef]
- Jo, C.H.; Yoon, K.S.; Chai, J.W.; Jeong, E.C.; Oh, S. Intratendinous injection of mesenchymal stem cells for the treatment of rotator cuff disease: A 2-year follow-up study. Arthroscopy 2019. [Google Scholar] [CrossRef]
- Klimczak, A.; Kozlowska, U.; Kurpisz, M. Muscle stem/progenitor cells and mesenchymal stem cells of bone marrow origin for skeletal muscle regeneration in muscular dystrophies. Arch. Immunol. Ther. Exp. 2018, 66, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Goncalves, R.M. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Chabannes, D.; Hill, M.; Merieau, E.; Rossignol, J.; Brion, R.; Soulillou, J.P.; Anegon, I.; Cuturi, M.C. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 2007, 110, 3691–3694. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Gunawardena, T.N.A.; Rahman, M.T.; Abdullah, B.J.J.; Abu Kasim, N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019, 13, 569–586. [Google Scholar] [CrossRef]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell Biochem. 2010, 111, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhao, W.L.; Ye, Y.Y.; Bai, X.C.; Liu, R.Q.; Chang, L.F.; Zhou, Q.; Sui, S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.; Hsu, C.Y.; Gao, Y.; Meyers, C.A.; Chang, L.; Zhang, L.; Broderick, K.; Ding, C.; Peault, B.; et al. Human perivascular stem cell-derived extracellular vesicles mediate bone repair. eLife 2019, 8, e48191. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res. Ther. 2018, 9, 320. [Google Scholar] [CrossRef]
- Van, N.G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar]
- Lai, R.C. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 2016, 5, 29828. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Tofiño-Vian, M.; Guillen, M.I.; Alcaraz, M.J. Extracellular vesicles: A new therapeutic strategy for joint conditions. Biochem. Pharmacol. 2018, 153, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.E.; Banyard, D.A.; Santos, P.J.F.; Sayadi, L.R.; Evans, G.R.D.; Widgerow, A.D. Adipose-derived stem cell extracellular vesicles: A systematic review. J Plast. Reconstr. Aesthet. Surg. 2019, 72, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, O.G.; van Balkom, B.W.; Schiffelers, R.M.; Bouten, C.V.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem cell-derived extracellular vesicles and immune-modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef] [Green Version]
- Siegel, G.; Schafer, R.; Dazzi, F. The immunosuppressive properties of mesenchymal stem cells. Transplantation 2009, 87, S45–S49. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; James, V.; Rizvanov, A.A. Therapeutic application of mesenchymal stem cells derived extracellular vesicles for immunomodulation. Front. Immunol. 2019, 10, 2663. [Google Scholar] [CrossRef]
- Fierabracci, A.; Del, F.A.; Luciano, R.; Muraca, M.; Teti, A.; Muraca, M. Recent advances in mesenchymal stem cell immunomodulation: The role of microvesicles. Cell Transplant. 2015, 24, 133–149. [Google Scholar] [CrossRef]
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.A.; Mardani, K. Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunol. Lett. 2012, 147, 47–54. [Google Scholar] [CrossRef]
- Conforti, A.; Scarsella, M.; Starc, N.; Giorda, E.; Biagini, S.; Proia, A.; Carsetti, R.; Locatelli, F.; Bernardo, M.E. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 2014, 23, 2591–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreras-Planella, L.; Monguio-Tortajada, M.; Borras, F.E.; Franquesa, M. Immunomodulatory effect of MSC on B cells is independent of secreted extracellular vesicles. Front. Immunol. 2019, 10, 1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Harting, M.T.; Srivastava, A.K.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Toledano Furman, N.E.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S., Jr.; Olson, S.D. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells 2018, 36, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X.N. Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Front. Immunol. 2018, 9, 2538. [Google Scholar] [CrossRef]
- Ji, L.; Bao, L.; Gu, Z.; Zhou, Q.; Liang, Y.; Zheng, Y.; Xu, Y.; Zhang, X.; Feng, X. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol. Res. 2019, 67, 432–442. [Google Scholar] [CrossRef]
- Blazquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Alvarez, V.; Tarazona, R.; Casado, J.G. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front. Immunol. 2014, 5, 556. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.; Lim, S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef]
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, S.K. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy 2018, 20, 687–696. [Google Scholar] [CrossRef]
- Toh, W.S.; Zhang, B.; Lai, R.C.; Lim, S.K. Immune regulatory targets of mesenchymal stromal cell exosomes/small extracellular vesicles in tissue regeneration. Cytotherapy 2018, 20, 1419–1426. [Google Scholar] [CrossRef]
- Budoni, M.; Fierabracci, A.; Luciano, R.; Petrini, S.; Di Ciommo, V.; Muraca, M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013, 22, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 2016, 6, 24120. [Google Scholar] [CrossRef] [PubMed]
- Henao Agudelo, J.S.; Braga, T.T.; Amano, M.T.; Cenedeze, M.A.; Cavinato, R.A.; Peixoto-Santos, A.R.; Muscará, M.N.; Teixeira, S.A.; Cruz, M.C.; Castoldi, A.; et al. Mesenchymal stromal cell-derived microvesicles regulate an internal pro-inflammatory program in activated macrophages. Front. Immunol. 2017, 8, 881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, 249. [Google Scholar] [CrossRef] [Green Version]
- Casado, J.G.; Blazquez, R.; Vela, F.J.; Alvarez, V.; Tarazona, R.; Sanchez-Margallo, F.M. Mesenchymal stem cell-derived exosomes: Immunomodulatory evaluation in an antigen-induced synovitis porcine model. Front. Vet. Sci. 2017, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noel, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef]
- Yang, Y.; Hutchinson, P.; Morand, E.F. Inhibitory effect of annexin I on synovial inflammation in rat adjuvant arthritis. Arthritis Rheum. 1999, 42, 1538–1544. [Google Scholar] [CrossRef]
- Headland, S.E.; Jones, H.R.; Norling, L.V.; Kim, A.; Souza, P.R.; Corsiero, E.; Gil, C.D.; Nerviani, A.; Dell’Accio, F.; Pitzalis, C.; et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl. Med. 2015, 7, 315ra190. [Google Scholar] [CrossRef] [PubMed]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez Del Caz, M.D.; Silvestre, A.; Alcaraz, M.J. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Matsubara, K.; Ishikawa, J.; Fujio, M.; Shohara, R.; Hibi, H.; Ueda, M.; Yamamoto, A. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 2014, 61, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, Y.; Dunstan, C.; Roohani-Esfahani, S.; Zreiqat, H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng. Part A 2017, 23, 1212–1220. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekstrom, K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE 2018, 13, e0193059. [Google Scholar] [CrossRef]
- Shirley, D.; Marsh, D.; Jordan, G.; McQuaid, S.; Li, G. Systemic recruitment of osteoblastic cells in fracture healing. J. Orthop. Res. 2005, 23, 1013–1021. [Google Scholar] [CrossRef]
- Lee, D.Y.; Cho, T.J.; Kim, J.A.; Lee, H.R.; Yoo, W.J.; Chung, C.Y.; Choi, I.H. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone 2008, 42, 932–941. [Google Scholar] [CrossRef]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces. 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Li, S.; Roy, K. Induced pluripotent stem cells for regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 277–294. [Google Scholar] [CrossRef] [Green Version]
- Sabapathy, V.; Kumar, S. hiPSC-derived IMSCs: Nextgen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J. Cell Mol. Med. 2016, 20, 1571–1588. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, R.; Huang, C.C.; Ravindran, S. Hijacking the cellular mail: Exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016, 2016, 3808674. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Jia, Y.; Wang, Y.; Xu, J.; Chai, Y. Impaired bone regenerative effect of exosomes derived from bone marrow mesenchymal stem cells in type 1 diabetes. Stem Cells Transl. Med. 2019, 8, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.; Porter, R.M. Harnessing extracellular vesicles to direct endochondral repair of large bone defects. Bone Joint Res. 2018, 7, 263–273. [Google Scholar] [CrossRef]
- Moya, A.; Paquet, J.; Deschepper, M.; Larochette, N.; Oudina, K.; Denoeud, C.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Human mesenchymal stem cell failure to adapt to glucose shortage and rapidly use intracellular energy reserves through glycolysis explains poor cell survival after implantation. Stem Cells 2018, 36, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Moya, A.; Larochette, N.; Paquet, J.; Deschepper, M.; Bensidhoum, M.; Izzo, V.; Kroemer, G.; Petite, H.; Logeart-Avramoglou, D. Quiescence preconditioned human multipotent stromal cells adopt a metabolic profile favorable for enhanced survival under ischemia. Stem Cells 2017, 35, 181–196. [Google Scholar] [CrossRef]
- Potier, E.; Ferreira, E.; Andriamanalijaona, R.; Pujol, J.P.; Oudina, K.; Logeart-Avramoglou, D.; Petite, H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 2007, 40, 1078–1087. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Zhu, Y.; Qiu, S.; Xu, J.; Chai, Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res. Ther. 2019, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1alpha-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019, 52, e12570. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Rao, S.S.; Tan, Y.J.; Luo, M.J.; Hu, X.K.; Yin, H.; Huang, J.; Hu, Y.; Luo, Z.W.; Liu, Z.Z.; et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Xu, R.; Chen, C.Y.; Rao, S.S.; Xia, K.; Huang, J.; Yin, H.; Wang, Z.X.; Cao, J.; Liu, Z.Z.; et al. Extracellular vesicles from human umbilical cord blood ameliorate bone loss in senile osteoporotic mice. Metabolism 2019, 95, 93–101. [Google Scholar] [CrossRef]
- Ren, L.; Song, Z.J.; Cai, Q.W.; Chen, R.X.; Zou, Y.; Fu, Q.; Ma, Y.Y. Adipose mesenchymal stem cell-derived exosomes ameliorate hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis in vitro. Biochem. Biophys. Res. Commun. 2019, 508, 138–144. [Google Scholar] [CrossRef]
- Guo, S.C.; Tao, S.C.; Yin, W.J.; Qi, X.; Sheng, J.G.; Zhang, C.Q. Exosomes from human synovial-derived mesenchymal stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head in the rat. Int. J. Biol. Sci. 2016, 12, 1262–1272. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, Q.; Niu, X.; Hu, B.; Chen, S.; Song, W.; Ding, J.; Zhang, C.; Wang, Y. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Biol. Sci. 2017, 13, 232–244. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Bouxsein, M.L.; Faugere, M.C.; Guldberg, R.E.; Gerstenfeld, L.C.; et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Invest. 2007, 117, 1616–1626. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, S.E.; Chung, W.J.; Choi, Y.; Kwack, K.; Kim, S.W.; Kim, M.S.; Park, H.; Lee, Z.H. Stabilization of hypoxia-inducible factor-1alpha is involved in the hypoxic stimuli-induced expression of vascular endothelial growth factor in osteoblastic cells. Cytokine 2002, 17, 14–27. [Google Scholar] [CrossRef]
- Li, H.; Liu, D.; Li, C.; Zhou, S.; Tian, D.; Xiao, D.; Zhang, H.; Gao, F.; Huang, J. Exosomes secreted from mutant-HIF-1alpha-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol. Int. 2017, 41, 1379–1390. [Google Scholar] [CrossRef]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of stem cells on periodontal regeneration: Systematic review of pre-clinical studies. J. Periodontal Res. 2017, 52, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.P.; et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006, 169, 987–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Katagiri, W.; Osugi, M.; Sugimura, Y.; Hibi, H.; Ueda, M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 2015, 17, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Osugi, M.; Kawai, T.; Hibi, H. First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face. Med. 2016, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu, J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264. [Google Scholar] [CrossRef]
- Petho, A.; Chen, Y.; George, A. Exosomes in extracellular matrix bone biology. Curr. Osteoporos. Rep. 2018, 16, 58–64. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Zhang, Q. Emerging role of extracellular vesicles in bone remodeling. J. Dent. Res. 2018, 97, 859–868. [Google Scholar] [CrossRef]
- Cooke, M.E.; Allon, A.A.; Cheng, T.; Kuo, A.C.; Kim, H.T.; Vail, T.P.; Marcucio, R.S.; Schneider, R.A.; Lotz, J.C.; Alliston, T. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthr. Cartil. 2011, 19, 1210–1218. [Google Scholar] [CrossRef] [Green Version]
- De Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.; van den Broek, M.P.; Nizak, R.; van Rijen, M.H.; de Weger, R.A.; Dhert, W.J.; Saris, D.B. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells 2017, 35, 256–264. [Google Scholar] [CrossRef]
- Kim, M.; Steinberg, D.R.; Burdick, J.A.; Mauck, R.L. Extracellular vesicles mediate improved functional outcomes in engineered cartilage produced from MSC/chondrocyte cocultures. Proc. Natl. Acad. Sci. USA 2019, 116, 1569–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sward, P.; Frobell, R.; Englund, M.; Roos, H.; Struglics, A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)—A cross-sectional analysis. Osteoarthr. Cartil. 2012, 20, 1302–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sward, P.; Struglics, A.; Englund, M.; Roos, H.P.; Frobell, R.B. Soft tissue knee injury with concomitant osteochondral fracture is associated with higher degree of acute joint inflammation. Am. J. Sports Med. 2014, 42, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C.R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef] [Green Version]
- Ragni, E.; Perucca, O.C.; De, L.P.; Lugano, G.; Vigano, M.; Colombini, A.; Valli, F.; Zacchetti, D.; Bollati, V.; de, G.L. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res. Ther. 2019, 10, 109. [Google Scholar] [CrossRef]
- Vonk, L.A.; van Dooremalen, S.; Liv, N.; Klumperman, J.; Coffer, P.; Saris, D.; Lorenowicz, M. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez del Caz, M.D.; Castejón, M.A.; Alcaraz, M.J. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid. Med. Cell Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Wang, A.T.; Feng, Y.; Jia, H.H.; Zhao, M.; Yu, H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: A concise review. World J. Stem Cells 2019, 11, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torrillas, M.; Rubio, M.; Damia, E.; Cuervo, B.; Del, R.A.; Pelaez, P.; Chicharro, D.; Miguel, L.; Sopena, J.J. Adipose-derived mesenchymal stem cells: A promising tool in the treatment of musculoskeletal diseases. Int. J. Mol. Sci. 2019, 20, 3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Kim, W.S.; Park, H.-A.; Kim, S.Y.; Cho, Y.W. Exosomes secreted during chondrogenic differentiation of human adipose-derived stem cells for osteoarthritis treatment. J. Extracell. Vesicles 2019, 8 (Suppl. 1), 217–218. [Google Scholar]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. MiR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Cui, D.; Li, H.; Xu, X.; Ye, L.; Zhou, X.; Zheng, L.; Zhou, Y. Mesenchymal stem cells for cartilage regeneration of TMJ osteoarthritis. Stem Cells Int. 2017, 2017, 5979741. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.D.; Zhang, J.N.; Gan, Y.H.; Zhou, Y.H. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 2015, 94, 666–673. [Google Scholar] [CrossRef]
- Chen, K.; Man, C.; Zhang, B.; Hu, J.; Zhu, S.S. Effect of in vitro chondrogenic differentiation of autologous mesenchymal stem cells on cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int. J. Oral Maxillofac. Surg. 2013, 42, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Jiang, C.; Ji, P.; Wang, M.; Xu, J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via mir-100-5p/mtor. Stem Cell Res. Ther. 2019, 10, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.S.; Bandeira, E.; Shelke, G.V.; Lasser, C.; Lotvall, J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, Z.; Chen, W.; Huang, G.; He, A.; Hou, C.; Long, Y.; Yang, Z.; Zhang, Z.; Liao, W. MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1beta-induced chondrocyte responses. Osteoarthr. Cartil. 2016, 24, 932–941. [Google Scholar] [CrossRef]
- Sun, H.; Hu, S.; Zhang, Z.; Lun, J.; Liao, W.; Zhang, Z. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J. Cell Biochem. 2019, 120, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting Wnt5a. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Xu, B.; Xu, H. TGF-beta1 promoted chondrocyte proliferation by regulating SP1 through MSC-exosomes derived mir-135b. Cell Cycle 2018, 17, 2756–2765. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Qiao, F.; Xue, P. The protective role of microRNA-140-5p in synovial injury of rats with knee osteoarthritis via inactivating the TLR4/Myd88/NF-kappaB signaling pathway. Cell Cycle 2019, 18, 2344–2358. [Google Scholar] [CrossRef]
- Tao, S.C.; Yuan, T.; Zhang, Y.L.; Yin, W.J.; Guo, S.C.; Zhang, C.Q. Exosomes derived from mir-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, R.; Wang, Z.; Wen, C.; Zhang, F.; Lin, F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J. 2018, 475, 3629–3638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, L.; Zou, R.; Wen, C.; Wang, Z.; Lin, F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/mir-206/GIT1 axis in osteoarthritis. Cell Cycle 2018, 17, 2411–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, S.C.; Guo, S.C. Hsa_circ_0000077-overexpressing extracellular vesicle: A new tool to prevent cartilage degeneration. J. Extracell. Vesicles 2019, 8 (Suppl. 1), 101–102. [Google Scholar]
- Arai, Y.; Park, H.; Park, S.; Bello, A.; Ahn, J.; Kim, D.; Kim, B.J.; Park, H.; Lee, S.H. Bile acids hybrid extracellular vesicles derived from mesenchymal stem cells for cartilage tissue regeneration. J. Extracell. Vesicles 2019, 8 (Suppl. 1), 121–122. [Google Scholar]
- Kim, S.; Lee, J.; Park, J.; Lee, J.; Kim, S.; Moon, H.; Bae, S. Human adipose stem cells-derived vesicles improve pain and reduce cartilage destruction in an osteoarthritis rat model. J. Extracell. Vesicles 2019, 8 (Suppl. 1), 216–217. [Google Scholar]
- D’Atri, D.; Garcia, J.; Creemers, L.; Machluf, M. Nano-ghosts: Mesenchymal stem cells derived nanoparticles as a novel approach for cartilage regeneration. J. Extracell. Vesicles 2019, 8 (Suppl. 1), 263. [Google Scholar]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Heathfield, S.K.; Le Maitre, C.L.; Hoyland, J.A. Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration. Arthritis Res. Ther. 2008, 10, R87. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tian, Y.; Phillips, K.L.; Chiverton, N.; Haddock, G.; Bunning, R.A.; Cross, A.K.; Shapiro, I.M.; Le Maitre, C.L.; Risbud, M.V. Tumor necrosis factor alpha- and interleukin-1beta-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013, 65, 832–842. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Yan, M.; Yang, M.; Wang, S.; Pan, J.; Li, L.; Tan, J. Oxidative damage induces apoptosis and promotes calcification in disc cartilage endplate cell through ROS/MAPK/NF-kappaB pathway: Implications for disc degeneration. Biochem. Biophys. Res. Commun. 2019, 516, 1026–1032. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M.; Vaccaro, A.R.; Albert, T.J. Stem cell regeneration of the nucleus pulposus. Spine J. 2004, 4, 348S–353S. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, G.; Zhang, L.; Hu, Y.; Zhang, K.; Sun, X.; Zhao, C.; Li, H.; Li, Y.M.; Zhao, J. Mesenchymal stem cells deliver exogenous mir-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J. Cell Mol. Med. 2018, 22, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.J.; Liu, X.; Che, L.; Liu, Z.H.; Samartzis, D.; Wang, H.Q. Stem cell therapies for intervertebral disc degeneration: Immune privilege reinforcement by Fas/Fasl regulating machinery. Curr. Stem Cell Res. Ther. 2015, 10, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Krock, E.; Rosenzweig, D.H.; Haglund, L. The inflammatory milieu of the degenerate disc: Is mesenchymal stem cell-based therapy for intervertebral disc repair a feasible approach? Curr. Stem Cell Res. Ther. 2015, 10, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Luo, R.; Li, G.; Song, Y.; Zhan, S.; Zhao, K.; Hua, W.; Zhang, Y.; Wu, X.; Yang, C. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 2019, 9, 4084–4100. [Google Scholar] [CrossRef]
- Lan, W.R.; Pan, S.; Li, H.Y.; Sun, C.; Chang, X.; Lu, K.; Jiang, C.Q.; Zuo, R.; Zhou, Y.; Li, C.Q. Inhibition of the Notch1 pathway promotes the effects of nucleus pulposus cell-derived exosomes on the differentiation of mesenchymal stem cells into nucleus pulposus-like cells in rats. Stem Cells Int. 2019, 2019, 8404168. [Google Scholar] [CrossRef]
- Lu, K.; Li, H.Y.; Yang, K.; Wu, J.L.; Cai, X.W.; Zhou, Y.; Li, C.Q. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: In-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 108. [Google Scholar] [CrossRef]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.; Ide, C.; Nabeshima, Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005, 309, 314–317. [Google Scholar] [CrossRef]
- Mellows, B.; Mitchell, R.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Denecke, B.; Musante, L.; Ramachandra, D.L.; Debacq-Chainiaux, F.; et al. Protein and molecular characterization of a clinically compliant amniotic fluid stem cell-derived extracellular vesicle fraction capable of accelerating muscle regeneration through enhancement of angiogenesis. Stem Cells Dev. 2017, 26, 1316–1333. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Song, W.; Chen, B.; Liu, X.; He, Y. Exosomes isolated from adipose-derived stem cells: A new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am. J. Sports Med. 2019, 47, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yoneda, S.; Abu-Amer, Y.; Guilak, F.; Gelberman, R.H. Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J. Orthop. Res. 2020, 38, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, C.S.; Clements, A.E.B.; Kink, J.A.; Choi, U.; Baer, G.S.; Halanski, M.A.; Hematti, P.; Vanderby, R. Extracellular vesicle-educated macrophages promote early achilles tendon healing. Stem Cells 2019, 37, 652–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, B.K.; Davies, L.C. MSCs-cells with many sides. Cytotherapy 2018, 20, 273–278. [Google Scholar]
- Christy, B.A.; Herzig, M.C.; Montgomery, R.K.; Delavan, C.; Bynum, J.A.; Reddoch, K.M.; Cap, A.P. Procoagulant activity of human mesenchymal stem cells. J. Trauma Acute Care Surg. 2017, 83, S164–S169. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Hui, J.H.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell. Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef]
- Witwer, K.W.; van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; de, K.D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaraz, M.J.; Compañ, A.; Guillén, M.I. Extracellular Vesicles from Mesenchymal Stem Cells as Novel Treatments for Musculoskeletal Diseases. Cells 2020, 9, 98. https://doi.org/10.3390/cells9010098
Alcaraz MJ, Compañ A, Guillén MI. Extracellular Vesicles from Mesenchymal Stem Cells as Novel Treatments for Musculoskeletal Diseases. Cells. 2020; 9(1):98. https://doi.org/10.3390/cells9010098
Chicago/Turabian StyleAlcaraz, María José, Alvaro Compañ, and María Isabel Guillén. 2020. "Extracellular Vesicles from Mesenchymal Stem Cells as Novel Treatments for Musculoskeletal Diseases" Cells 9, no. 1: 98. https://doi.org/10.3390/cells9010098
APA StyleAlcaraz, M. J., Compañ, A., & Guillén, M. I. (2020). Extracellular Vesicles from Mesenchymal Stem Cells as Novel Treatments for Musculoskeletal Diseases. Cells, 9(1), 98. https://doi.org/10.3390/cells9010098




