Cells 2020, 9(1), 109; https://doi.org/10.3390/cells9010109 - 2 Jan 2020
Cited by 136 | Viewed by 18006
Abstract
DAF-16, the only forkhead box transcription factors class O (FoxO) homolog in Caenorhabditis elegans, integrates signals from upstream pathways to elicit transcriptional changes in many genes involved in aging, development, stress, metabolism, and immunity. The major regulator of DAF-16 activity is the
[...] Read more.
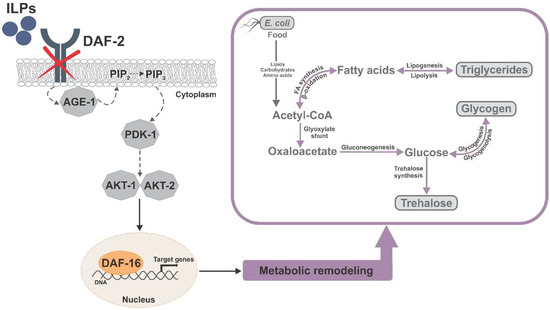
DAF-16, the only forkhead box transcription factors class O (FoxO) homolog in Caenorhabditis elegans, integrates signals from upstream pathways to elicit transcriptional changes in many genes involved in aging, development, stress, metabolism, and immunity. The major regulator of DAF-16 activity is the insulin/insulin-like growth factor 1 (IGF-1) signaling (IIS) pathway, reduction of which leads to lifespan extension in worms, flies, mice, and humans. In C. elegans daf-2 mutants, reduced IIS leads to a heterochronic activation of a dauer survival program during adulthood. This program includes elevated antioxidant defense and a metabolic shift toward accumulation of carbohydrates (i.e., trehalose and glycogen) and triglycerides, and activation of the glyoxylate shunt, which could allow fat-to-carbohydrate conversion. The longevity of daf-2 mutants seems to be partially supported by endogenous trehalose, a nonreducing disaccharide that mammals cannot synthesize, which points toward considerable differences in downstream mechanisms by which IIS regulates aging in distinct groups.
Full article
(This article belongs to the Special Issue The FoxO Transcription Factors and Metabolic Regulation)
►
Show Figures