Presenilin-2 and Calcium Handling: Molecules, Organelles, Cells and Brain Networks
Abstract
:1. Presenilin-2 in Physiology and Pathology
2. PS2 and Ca2+ Homeostasis
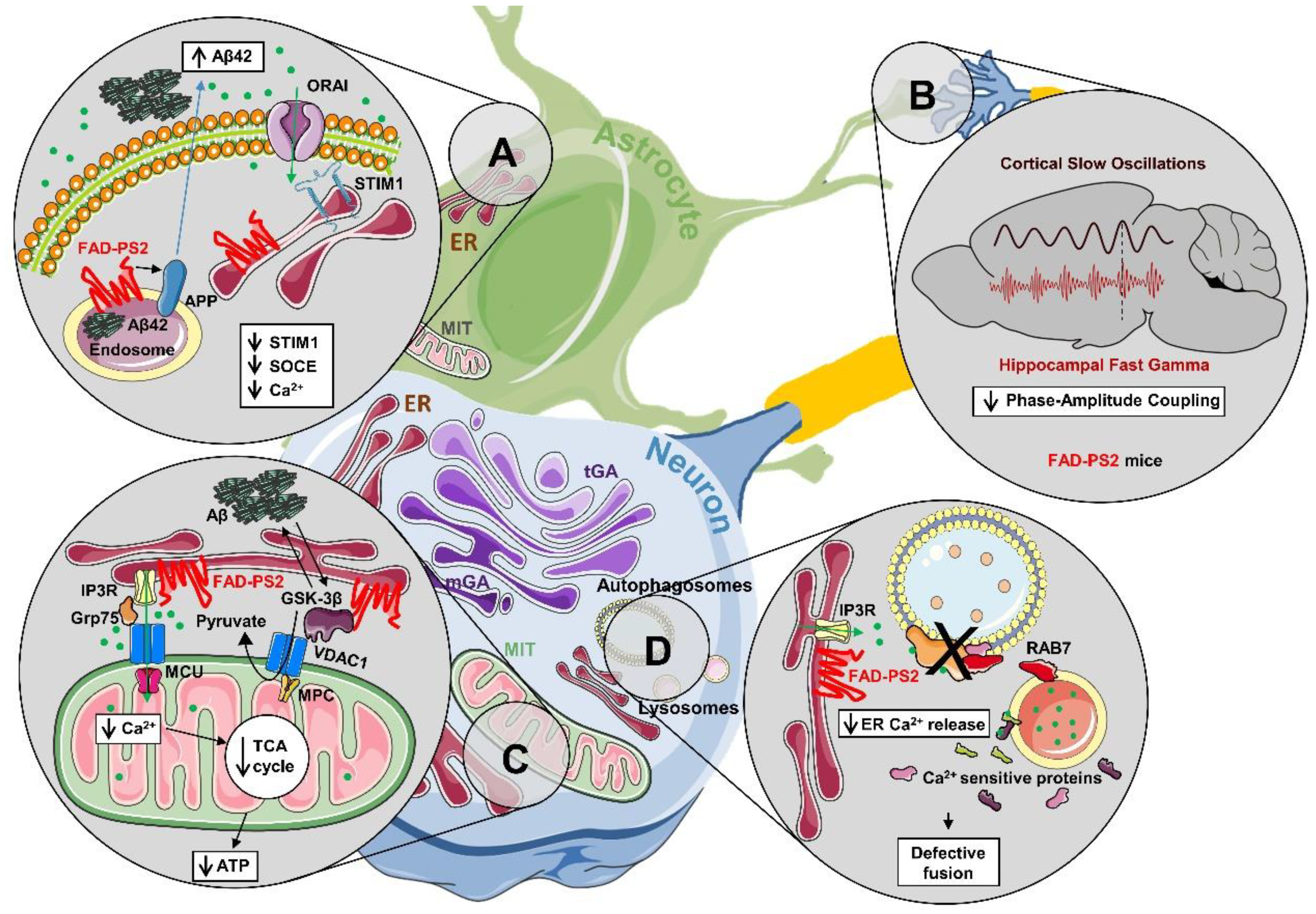
2.1. Alterations of Ca2+ Homeostasis in FAD-PS2 Cell Models
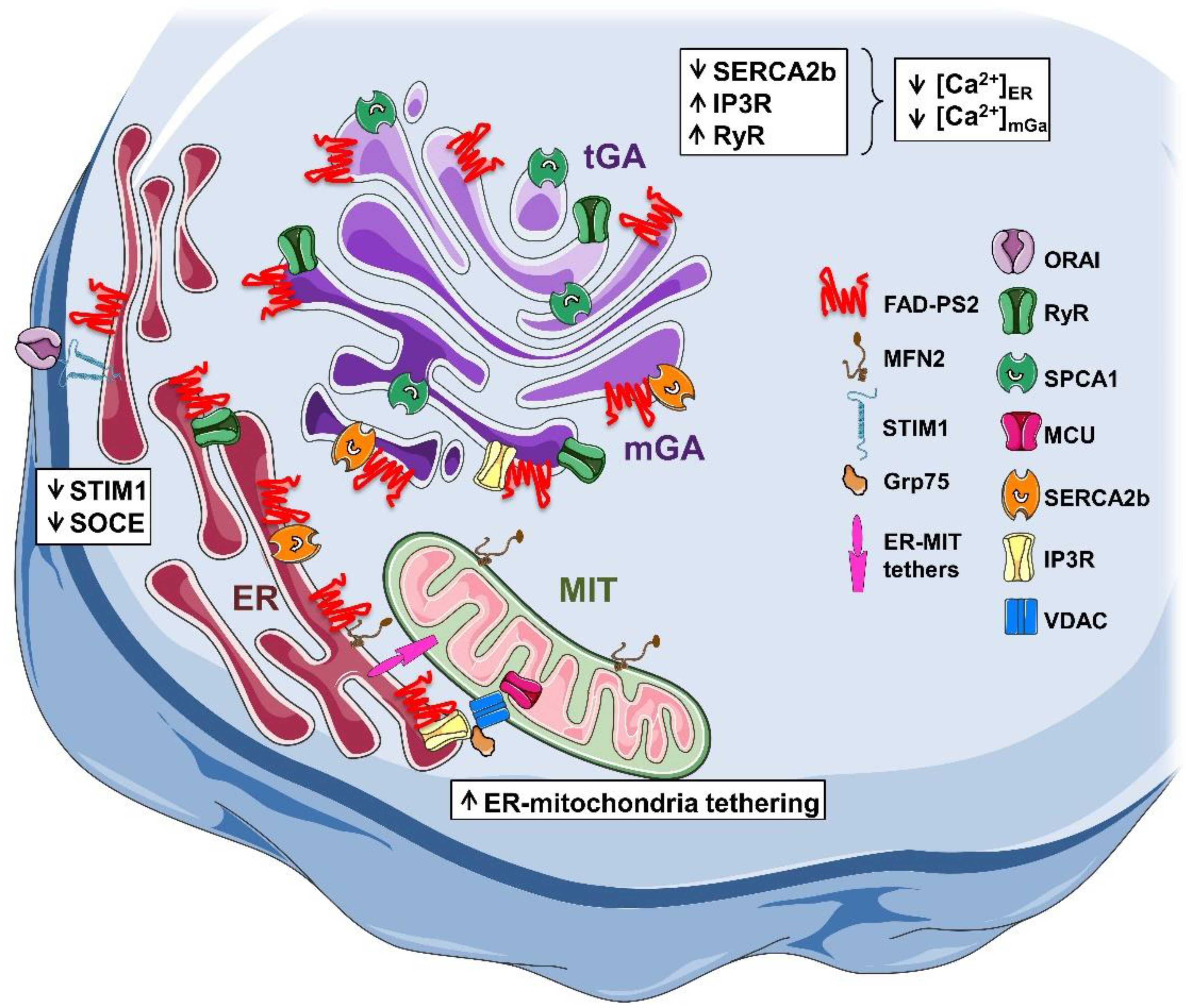
2.2. Calcium Handling in AD Mouse Models Expressing PS2-N141I
3. Functional Effects of Ca2+ Dysregulation by FAD-PS2
3.1. Autophagy
3.2. Cell Metabolism and Bioenergetics
3.3. Brain Network Activity
4. Concluding Remarks and Possible Therapeutic Targets
5. Box 1: AD Mouse Models Based on PS2
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jayadev, S.; Leverenz, J.B.; Steinbart, E.; Stahl, J.; Klunk, W.; Yu, C.E.; Bird, T.D. Alzheimer’s disease phenotypes and genotypes associated with mutations in presenilin 2. Brain 2010, 133, 1143–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunkan, A.L.; Goate, A.M. Presenilin function and gamma-secretase activity. J. Neurochem. 2005, 93, 769–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Slunt, H.H.; Martin, L.J.; Thinakaran, G.; Kim, G.; Gandy, S.E.; Seeger, M.; Koo, E.; Price, D.L.; Sisodia, S.S. Expression of presenilin 1 and 2 (PS1 and PS2) in human and murine tissues. J. Neurosci. 1996, 16, 7513–7525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chévez-Gutiérrez, L.; Bammens, L.; Benilova, I.; Vandersteen, A.; Benurwar, M.; Borgers, M.; Lismont, S.; Zhou, L.; Van Cleynenbreugel, S.; Esselmann, H.; et al. The Mechanism of γ-Secretase Dysfunction in Familial Alzheimer Disease. EMBO J. 2012, 31, 2261–2274. [Google Scholar] [CrossRef]
- Chávez-Gutiérrez, L.; Szaruga, M. Mechanisms of neurodegeneration-insights from familial alzheimer’s disease. In Seminars in Cell and Developmental Biology; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 75–85. [Google Scholar]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of alzheimer’s disease at 25years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer disease: An update on pathobiology and treatment strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Walker, E.S.; Martinez, M.; Brunkan, A.L.; Goate, A. Presenilin 2 familial alzheimer’s disease mutations result in partial loss of function and dramatic changes in abeta 42/40 ratios. J. Neurochem. 2005, 92, 294–301. [Google Scholar] [CrossRef]
- Güner, G.; Lichtenthaler, S.F. The substrate repertoire of γ-secretase/presenilin. In Seminars in Cell and Developmental Biology; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 27–42. [Google Scholar]
- Bolduc, D.M.; Montagna, D.R.; Gu, Y.; Selkoe, D.J.; Wolfe, M.S. Nicastrin functions to sterically hinder γ-secretase-substrate interactions driven by substrate transmembrane domain. Proc. Natl. Acad. Sci. USA 2016, 113, E509–E518. [Google Scholar] [CrossRef] [Green Version]
- Holmes, O.; Paturi, S.; Selkoe, D.J.; Wolfe, M.S. Pen-2 is essential for γ-secretase complex stability and trafficking but partially dispensable for endoproteolysis. Biochemistry 2014, 53, 4393–4406. [Google Scholar] [CrossRef]
- Kim, S.H.; Sisodia, S.S. Evidence that the “NF” motif in transmembrane domain 4 of presenilin 1 is critical for binding with PEN-2. J. Biol. Chem. 2005, 280, 41953–41996. [Google Scholar] [CrossRef] [Green Version]
- Prokop, S.; Shirotani, K.; Edbauer, D.; Haass, C.; Steiner, H. Requirement of PEN-2 for stabilization of the presenilin n-/c-terminal fragment heterodimer within the γ-secretase complex. J. Biol. Chem. 2004, 279, 23255–23261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardossi-Piquard, R.; Yang, S.P.; Kanemoto, S.; Gu, Y.; Chen, F.; Böhm, C.; Sevalle, J.; Li, T.; Wong, P.C.; Checler, F.; et al. APH1 polar transmembrane residues regulate the assembly and activity of presenilin complexes. J. Biol. Chem. 2009, 284, 16298–16307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Chen, F.; Sanjo, N.; Kawarai, T.; Hasegawa, H.; Duthie, M.; Li, W.; Ruan, X.; Luthra, A.; Mount, H.T.; et al. APH-1 interacts with mature and immature forms of presenilins and nicastrin and may play a role in maturation of presenilin.nicastrin complexes. J. Biol. Chem. 2003, 278, 7374–7380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serneels, L.; Van Biervliet, J.; Craessaerts, K.; Dejaegere, T.; Horré, K.; Van Houtvin, T.; Esselmann, H.; Paul, S.; Schäfer, M.K.; Berezovska, O.; et al. γ-Secretase heterogeneity in the aph1 subunit: Relevance for alzheimer’s disease. Science 2009, 324, 629–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, M.S.; Xia, W.; Ostaszewski, B.L.; Diehl, T.S.; Kimberly, W.T.; Selkoe, D.J. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and g-secretase activity. Nature 1999, 398, 513–517. [Google Scholar] [CrossRef]
- Wolfe, M.S. Substrate recognition and processing by γ-secretase. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183016. [Google Scholar] [CrossRef]
- Sannerud, R.; Esselens, C.; Ejsmont, P.; Mattera, R.; Rochin, L.; Tharkeshwar, A.K.; De Baets, G.; De Wever, V.; Habets, R.; Baert, V.; et al. Restricted location of PSEN2/gamma-secretase determines substrate specificity and generates an intracellular abeta pool. Cell 2016, 166, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Meckler, X.; Checler, F. Presenilin 1 and presenilin 2 target gamma-secretase complexes to distinct cellular compartments. J. Biol. Chem. 2016, 291, 12821–12837. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Newman, M.; Lardelli, M. The zebrafish orthologue of familial alzheimer’s disease gene PRESENILIN 2 is required for normal adult melanotic skin pigmentation. PLoS ONE 2018, 13, e0206155. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Gong, J.; Sviderskaya, E.V.; Wei, A.; Li, W. Mitochondrial NCKX5 regulates melanosomal biogenesis and pigment production. J. Cell Sci. 2019, 132, jcs232009. [Google Scholar] [CrossRef] [Green Version]
- Murayama, M.; Tanaka, S.; Palacino, J.; Murayama, O.; Honda, T.; Sun, X.; Yasutake, K.; Nihonmatsu, N.; Wolozin, B.; Takashima, A. Direct association of presenilin-1 with β-catenin. FEBS Lett. 1998, 433, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Hartmann, H.; Do, V.M.; Abramowski, D.; Sturchler-Pierrat, C.; Staufenbiel, M.; Sommer, B.; Van De Wetering, M.; Clevers, H.; Saftig, P.; et al. Destabilization of β-Catenin by mutations in Presenilin-1 potentiates neuronal apoptosis. Nature 1998, 395, 698–702. [Google Scholar] [CrossRef]
- Soriano, S.; Kang, D.E.; Fu, M.; Pestell, R.; Chevallier, N.; Zheng, H.; Koo, E.H. Presenilin 1 negatively regulates β-Catenin/T cell factor/Lymphoid enhancer factor-1 signaling independently of β-Amyloid precursor protein and notch processing. J. Cell Biol. 2001, 52, 785–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.E.; Soriano, S.; Frosch, M.P.; Collins, T.; Naruse, S.; Sisodia, S.S.; Leibowitz, G.; Levine, F.; Koo, E.H. Presenilin 1 facilitates the constitutive turnover of β-Catenin: Differential activity of alzheimer’s disease-linked PS1 mutants in the β-catenin-signaling pathway. J. Neurosci. 1999, 19, 4229–4237. [Google Scholar] [CrossRef] [PubMed]
- Dolma, K.; Iacobucci, G.J.; Hong Zheng, K.; Shandilya, J.; Toska, E.; White, J.A.; Spina, E.; Gunawardena, S. Presenilin influences glycogen synthase kinase-3 beta (GSK-3beta) for Kinesin-1 and dynein function during axonal transport. Hum. Mol. Genet. 2014, 23, 1121–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthet, G.; Dunys, J.; Shao, Z.; Xuan, Z.; Ren, Y.; Xu, J.; Arbez, N.; Mauger, G.; Bruban, J.; Georgakopoulos, A.; et al. Presenilin mediates neuroprotective functions of ephrinb and brain-derived neurotrophic factor and regulates ligand-induced internalization and metabolism of EphB2 and TrkB receptors. Neurobiol. Aging 2013, 34, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFerla, F.M. Calcium dyshomeostasis and intracellular signalling in alzheimer’s disease. Nat. Rev. Neurosci. 2002, 3, 862–872. [Google Scholar] [CrossRef]
- Leissring, M.A.; Paul, B.A.; Parker, I.; Cotman, C.W.; LaFerla, F.M. Alzheimer’s presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in xenopus oocytes. J. Neurochem. 1999, 72, 1061–1068. [Google Scholar] [CrossRef]
- Chan, S.L.; Mayne, M.; Holden, C.P.; Geiger, J.D.; Mattson, M.P. Presenilin-1 Mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000, 275, 18195–18200. [Google Scholar] [CrossRef] [Green Version]
- Leissring, M.A.; Akbari, Y.; Fanger, C.M.; Cahalan, M.D.; Mattson, M.P.; LaFerla, F.M. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J. Cell Biol. 2000, 149, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Smith, I.F.; Boyle, J.P.; Vaughan, P.F.; Pearson, H.A.; Cowburn, R.F.; Peers, C.S. Ca2+ stores and capacitative Ca2+ entry in human neuroblastoma (SH- SY5Y) cells expressing a familial alzheimer’s disease presenilin-1 mutation. Brain Res. 2002, 949, 105–111. [Google Scholar] [CrossRef]
- Green, K.N.; Demuro, A.; Akbari, Y.; Hitt, B.D.; Smith, I.F.; Parker, I.; LaFerla, F.M. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid β production. J. Cell Biol. 2008, 181, 1107–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, D.; Netzer, W.J.; Zhong, M.; Lin, Y.; Du, G.; Frohman, M.; Foster, D.A.; Sisodia, S.S.; Xu, H.; Gorelick, F.S.; et al. Presenilin-1 uses phospholipase d1 as a negative regulator of β-amyloid formation. Proc. Natl. Acad. Sci. USA 2006, 103, 1941–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayrapetyan, V.; Rybalchenko, V.; Rybalchenko, N.; Koulen, P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium 2008, 44, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Reverse, D.; Dewachter, I.; Ris, L.; Caluwaerts, N.; Kuiperi, C.; Gilis, M.; Geerts, H.; Kretzschmar, H.; Godaux, E.; et al. Mutant presenilins disturb neuronal calcium homeostasis in the brain of transgenic mice, decreasing the threshold for excitotoxicity and facilitating long-term potentiation. J. Biol. Chem. 2001, 276, 11539–11544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, H.; Nelson, O.; Bezprozvanny, A.; Wang, Z.; Lee, S.F.; Hao, Y.H.; Serneels, L.; De Strooper, B.; Yu, G.; Bezprozvanny, I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial alzheimer’s disease-linked mutations. Cell 2006, 126, 981–993. [Google Scholar] [CrossRef] [Green Version]
- Zatti, G.; Ghidoni, R.; Barbiero, L.; Binetti, G.; Pozzan, T.; Fasolato, C.; Pizzo, P. The presenilin 2 M239I mutation associated with familial alzheimer’s disease reduces Ca2+ release from intracellular stores. Neurobiol. Dis. 2004, 15, 269–278. [Google Scholar] [CrossRef]
- Giacomello, M.; Barbiero, L.; Zatti, G.; Squitti, R.; Binetti, G.; Pozzan, T.; Fasolato, C.; Ghidoni, R.; Pizzo, P. Reduction of Ca2+ stores and capacitative Ca2+ entry is associated with the familial alzheimer’s disease presenilin-2 T122R mutation and anticipates the onset of dementia. Neurobiol. Dis. 2005, 18, 638–648. [Google Scholar] [CrossRef]
- Zatti, G.; Burgo, A.; Giacomello, M.; Barbiero, L.; Ghidoni, R.; Sinigaglia, G.; Florean, C.; Bagnoli, S.; Binetti, G.; Sorbi, S.; et al. Presenilin mutations linked to familial alzheimer’s disease reduce endoplasmic reticulum and golgi apparatus calcium levels. Cell Calcium 2006, 39, 539–550. [Google Scholar] [CrossRef]
- Zampese, E.; Fasolato, C.; Kipanyula, M.J.; Bortolozzi, M.; Pozzan, T.; Pizzo, P. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc. Natl. Acad. Sci. USA 2011, 108, 2777–2782. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.H.; Shineman, D.; Muller, M.; Cardenas, C.; Mei, L.; Yang, J.; Tomita, T.; Iwatsubo, T.; Lee, V.M.; Foskett, J.K. Mechanism of Ca2+ disruption in alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron 2008, 58, 871–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, K.H.; Mei, L.; Mak, D.O.; Hayashi, I.; Iwatsubo, T.; Kang, D.E.; Foskett, J.K. Gain-of-function enhancement of IP3 receptor modal gating by familial alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci. Signal. 2010, 3, ra22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilling, D.; Mak, D.O.; Kang, D.E.; Foskett, J.K. Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. J. Biol. Chem. 2012, 14, 10933–10944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greotti, E.; Wong, A.; Pozzan, T.; Pendin, D.; Pizzo, P. Characterization of the ER-targeted low affinity Ca2+ probe D4ER. Sensors 2016, 16, 1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lissandron, V.; Podini, P.; Pizzo, P.; Pozzan, T. Unique characteristics of Ca2+ homeostasis of the trans-golgi compartment. Proc. Natl. Acad. Sci. USA 2010, 107, 9198–9203. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.K.; Capitanio, P.; Lissandron, V.; Bortolozzi, M.; Pozzan, T.; Pizzo, P. Heterogeneity of Ca2+ handling among and within golgi compartments. J. Mol. Cell Biol. 2013, 5, 266–276. [Google Scholar] [CrossRef]
- Greotti, E.; Capitanio, P.; Wong, A.; Pozzan, T.; Pizzo, P.; Pendin, D. Familial alzheimer’s disease-linked presenilin mutants and intracellular Ca2+ handling: A single-organelle, FRET-based analysis. Cell Calcium 2019, 79, 44–56. [Google Scholar] [CrossRef]
- Nelson, O.; Tu, H.; Lei, T.; Bentahir, M.; de Strooper, B.; Bezprozvanny, I. Familial alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J. Clin. Investig. 2007, 117, 1230–1239. [Google Scholar] [CrossRef] [Green Version]
- Brunello, L.; Zampese, E.; Florean, C.; Pozzan, T.; Pizzo, P.; Fasolato, C. Presenilin-2 dampens intracellular Ca2+ stores by increasing Ca2+ leakage and reducing Ca2+ uptake. J. Cell. Mol. Med. 2009, 13, 3358–3369. [Google Scholar] [CrossRef] [Green Version]
- Pack-Chung, E.; Meyers, M.B.; Pettingell, W.P.; Moir, R.D.; Brownawell, A.M.; Cheng, I.; Tanzi, R.E.; Kim, T.W. Presenilin 2 interacts with sorcin, a modulator of the ryanodine receptor. J. Biol. Chem. 2000, 275, 14440–14445. [Google Scholar] [CrossRef] [Green Version]
- Chakroborty, S.; Goussakov, I.; Miller, M.B.; Stutzmann, G.E. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3×Tg-AD mice. J. Neurosci. 2009, 29, 9458–9470. [Google Scholar] [CrossRef] [PubMed]
- Kipanyula, M.J.; Contreras, L.; Zampese, E.; Lazzari, C.; Wong, A.K.C.; Pizzo, P.; Fasolato, C.; Pozzan, T. Ca2+ dysregulation in neurons from transgenic mice expressing mutant presenilin 2. Aging Cell 2012, 11, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, I.F.; Hitt, B.; Green, K.N.; Oddo, S.; LaFerla, F.M. Enhanced caffeine-induced Ca2+ release in the 3×Tg-AD mouse model of alzheimer’s disease. J. Neurochem. 2005, 94, 1711–1718. [Google Scholar] [CrossRef]
- Del Prete, D.; Checler, F.; Chami, M. Ryanodine receptors: Physiological function and deregulation in alzheimer disease. Mol. Neurodegener. 2014, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilling, D.; Muller, M.; Takano, H.; Mak, D.O.; Abel, T.; Coulter, D.A.; Foskett, J.K. Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial alzheimer’s disease pathogenesis. J. Neurosci. 2014, 34, 6910–6923. [Google Scholar] [CrossRef] [Green Version]
- Vance, J.E. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990, 265, 7248–7256. [Google Scholar]
- Filadi, R.; Theurey, P.; Pizzo, P. The endoplasmic reticulum-mitochondria coupling in health and disease: Molecules, functions and significance. Cell Calcium 2017, 62, 1–15. [Google Scholar] [CrossRef]
- Hedskog, L.; Pinho, C.M.; Filadi, R.; Ronnback, A.; Hertwig, L.; Wiehager, B.; Larssen, P.; Gellhaar, S.; Sandebring, A.; Westerlund, M.; et al. Modulation of the endoplasmic reticulum-mitochondria interface in alzheimer’s disease and related models. Proc. Natl. Acad. Sci. USA 2013, 110, 7916–7921. [Google Scholar] [CrossRef] [Green Version]
- Area-Gomez, E.; Del Carmen Lara Castillo, M.; Tambini, M.D.; Guardia-Laguarta, C.; de Groof, A.J.; Madra, M.; Ikenouchi, J.; Umeda, M.; Bird, T.D.; Sturley, S.L.; et al. Upregulated function of mitochondria-associated ER membranes in alzheimer disease. EMBO J. 2012, 31, 4106–4123. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda-Falla, D.; Barrera-Ocampo, A.; Hagel, C.; Korwitz, A.; Vinueza-Veloz, M.F.; Zhou, K.; Schonewille, M.; Zhou, H.; Velazquez-Perez, L.; Rodriguez-Labrada, R.; et al. Familial alzheimer’s disease-associated presenilin-1 alters cerebellar activity and calcium homeostasis. J. Clin. Investig. 2014, 124, 1552–1567. [Google Scholar] [CrossRef] [Green Version]
- Filadi, R.; Greotti, E.; Turacchio, G.; Luini, A.; Pozzan, T.; Pizzo, P. Presenilin 2 modulates endoplasmic reticulum-mitochondria coupling by tuning the antagonistic effect of mitofusin 2. Cell Rep. 2016, 15, 2226–2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhu, X. Endoplasmic reticulum-mitochondria tethering in neurodegenerative diseases. Transl. Neurodegener. 2017, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Area-Gomez, E.; de Groof, A.J.; Boldogh, I.; Bird, T.D.; Gibson, G.E.; Koehler, C.M.; Yu, W.H.; Duff, K.E.; Yaffe, M.P.; Pon, L.A.; et al. Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am. J. Pathol. 2009, 175, 1810–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiner, B.; Hedskog, L.; Wiehager, B.; Ankarcrona, M. Amyloid-beta peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J. Alzheimer’s Dis. 2015, 43, 369–374. [Google Scholar] [CrossRef]
- Filadi, R.; Greotti, E.; Turacchio, G.; Luini, A.; Pozzan, T.; Pizzo, P. Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc. Natl. Acad. Sci. USA 2015, 112, E2174–E2181. [Google Scholar] [CrossRef] [Green Version]
- Hogan, P.G.; Rao, A. Store-operated calcium entry: Mechanisms and modulation. Biochem. Biophys. Res. Commun. 2015, 460, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Putney, J.W. Forms and functions of store-operated calcium entry mediators, stim and orai. Adv. Biol. Regul. 2018, 68, 88–96. [Google Scholar] [CrossRef]
- Yoo, A.S.; Cheng, I.; Chung, S.; Grenfell, T.Z.; Lee, H.; Pack-Chung, E.; Handler, M.; Shen, J.; Xia, W.; Tesco, G.; et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron 2000, 27, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Tong, B.C.; Lee, C.S.; Cheng, W.H.; Lai, K.O.; Foskett, J.K.; Cheung, K.H. Familial alzheimer’s disease-associated presenilin 1 mutants promote gamma-secretase cleavage of STIM1 to impair store-operated Ca2+ entry. Sci. Signal. 2016, 9, ra89. [Google Scholar] [CrossRef] [Green Version]
- Herms, J.; Schneider, I.; Dewachter, I.; Caluwaerts, N.; Kretzschmar, H.; Van Leuven, F. Capacitive calcium entry is directly attenuated by mutant presenilin-1, independent of the expression of the amyloid precursor protein. J. Biol. Chem. 2003, 278, 2484–2489. [Google Scholar] [CrossRef] [Green Version]
- Bojarski, L.; Pomorski, P.; Szybinska, A.; Drab, M.; Skibinska-Kijek, A.; Gruszczynska-Biegala, J.; Kuznicki, J. Presenilin-dependent expression of STIM proteins and dysregulation of capacitative Ca2+ entry in familial Alzheimer’s disease. Biochim. Biophys. Acta 2009, 1793, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascual-Caro, C.; Berrocal, M.; Lopez-Guerrero, A.M.; Alvarez-Barrientos, A.; Pozo-Guisado, E.; Gutierrez-Merino, C.; Mata, A.M.; Martin-Romero, F.J. STIM1 deficiency is linked to Alzheimer’s disease and triggers cell death in SH-SY5Y cells by upregulation of L-Type voltage-operated Ca2+ entry. J. Mol. Med. 2018, 96, 1061–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Aron, L.; Zullo, J.; Pan, Y.; Kim, H.; Chen, Y.; Yang, T.H.; Kim, H.M.; Drake, D.; Liu, X.S.; et al. Rest and stress resistance in ageing and Alzheimer’s disease. Nature 2014, 507, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozmen, L.; Albientz, A.; Czech, C.; Jacobsen, H. Expression of transgenic APP MRNA is the key determinant for beta-amyloid deposition in PS2APP transgenic mice. Neurodegener. Dis. 2009, 6, 29–36. [Google Scholar] [CrossRef]
- Zott, B.; Busche, M.A.; Sperling, R.A.; Konnerth, A. What happens with the circuit in Alzheimer’s disease in mice and humans? Annu. Rev. Neurosci. 2018, 41, 277–297. [Google Scholar] [CrossRef]
- Stargardt, A.; Swaab, D.F.; Bossers, K. Storm before the quiet: Neuronal hyperactivity and ab in the presymptomatic stages of alzheimer’s Disease. Neurobiol. Aging 2015, 36, 1–11. [Google Scholar] [CrossRef]
- Wilson, C.A.; Murphy, D.D.; Giasson, B.I.; Zhang, B.; Trojanowski, J.Q.; Lee, V.M. Degradative organelles containing mislocalized alpha-and beta-synuclein proliferate in presenilin-1 null neurons. J. Cell Biol. 2004, 165, 335–346. [Google Scholar] [CrossRef]
- Esselens, C.; Oorschot, V.; Baert, V.; Raemaekers, T.; Spittaels, K.; Serneels, L.; Zheng, H.; Saftig, P.; De Strooper, B.; Klumperman, J.; et al. Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway. J. Cell Biol. 2004, 166, 1041–1054. [Google Scholar] [CrossRef]
- Lee, J.H.; Yu, W.H.; Kumar, A.; Lee, S.; Mohan, P.S.; Peterhoff, C.M.; Wolfe, D.M.; Martinez-Vicente, M.; Massey, A.C.; Sovak, G.; et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by alzheimer-related PS1 mutations. Cell 2010, 141, 1146–1158. [Google Scholar] [CrossRef] [Green Version]
- Coen, K.; Flannagan, R.S.; Baron, S.; Carraro-Lacroix, L.R.; Wang, D.; Vermeire, W.; Michiels, C.; Munck, S.; Baert, V.; Sugita, S.; et al. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J. Cell Biol. 2012, 198, 23–35. [Google Scholar] [CrossRef]
- Zhang, X.; Garbett, K.; Veeraraghavalu, K.; Wilburn, B.; Gilmore, R.; Mirnics, K.; Sisodia, S.S. A role for presenilins in autophagy revisited: Normal acidification of lysosomes in cells lacking PSEN1 and PSEN2. J. Neurosci. 2012, 32, 8633–8648. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Cusack, C.L.; Nnah, I.C.; Khayati, K.; Saqcena, C.; Huynh, T.B.; Noggle, S.A.; Ballabio, A.; Dobrowolski, R. Dysregulation of nutrient sensing and CLEARance in presenilin deficiency. Cell Rep. 2016, 14, 2166–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filadi, R.; Pizzo, P. Defective autophagy and alzheimer’s disease: Is calcium the key? In Neural Regeneration Research; Wolters Kluwer Medknow Publications: Mumbai, India, 2019; pp. 2081–2082. [Google Scholar] [CrossRef]
- Fedeli, C.; Filadi, R.; Rossi, A.; Mammucari, C.; Pizzo, P. PSEN2 (Presenilin 2) mutants linked to familial alzheimer disease impair autophagy by altering Ca2+ homeostasis. Autophagy 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.G.; Munafo, D.B.; Beron, W.; Colombo, M.I. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J. Cell Sci. 2004, 117, 2687–2697. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; McBrayer, M.K.; Wolfe, D.M.; Haslett, L.J.; Kumar, A.; Sato, Y.; Lie, P.P.; Mohan, P.; Coffey, E.E.; Kompella, U.; et al. Presenilin 1 maintains lysosomal Ca2+ homeostasis via TRPML1 by regulating VATPase-mediated lysosome acidification. Cell Rep. 2015, 12, 1430–1444. [Google Scholar] [CrossRef] [Green Version]
- Behbahani, H.; Shabalina, I.G.; Wiehager, B.; Concha, H.; Hultenby, K.; Petrovic, N.; Nedergaard, J.; Winblad, B.; Cowburn, R.F.; Ankarcrona, M. Differential role of presenilin-1 and -2 on mitochondrial membrane potential and oxygen consumption in mouse embryonic fibroblasts. J. Neurosci. Res. 2006, 84, 891–902. [Google Scholar] [CrossRef]
- Contino, S.; Porporato, P.E.; Bird, M.; Marinangeli, C.; Opsomer, R.; Sonveaux, P.; Bontemps, F.; Dewachter, I.; Octave, J.N.; Bertrand, L.; et al. Presenilin 2-dependent maintenance of mitochondrial oxidative capacity and morphology. Front. Physiol. 2017, 8, 796. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef]
- Rangaraju, V.; Calloway, N.; Ryan, T.A. Activity-driven local ATP synthesis is required for synaptic function. Cell 2014, 156, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Weidling, I.W.; Swerdlow, R.H. Mitochondria in alzheimer’s disease and their potential role in alzheimer’s proteostasis. In Experimental Neurology; Academic Press Inc.: Cambridge, MA, USA, 2020. [Google Scholar]
- Jadiya, P.; Kolmetzky, D.W.; Tomar, D.; Di Meco, A.; Lombardi, A.A.; Lambert, J.P.; Luongo, T.S.; Ludtmann, M.H.; Praticò, D.; Elrod, J.W. Impaired mitochondrial calcium efflux contributes to disease progression in models of alzheimer’s disease. Nat. Commun. 2019, 10, 3885. [Google Scholar] [CrossRef]
- Toglia, P.; Cheung, K.H.; Mak, D.O.D.; Ullah, G. Impaired mitochondrial function due to familial alzheimer’s disease-causing presenilins mutants via Ca2+ disruptions. Cell Calcium 2016, 59, 240–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flannery, P.J.; Trushina, E. Mitochondrial dynamics and transport in alzheimer’s disease. In Molecular and Cellular Neuroscience; Academic Press Inc.: Cambridge, MA, USA, 2019; pp. 109–120. [Google Scholar]
- Theurey, P.; Connolly, N.M.C.; Fortunati, I.; Basso, E.; Lauwen, S.; Ferrante, C.; Moreira Pinho, C.; Joselin, A.; Gioran, A.; Bano, D.; et al. Systems biology identifies preserved integrity but impaired metabolism of mitochondria due to a glycolytic defect in alzheimer’s disease neurons. Aging Cell 2019, 18, e12924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.; Rigotto, G.; Valente, G.; Giorgio, V.; Basso, E.; Filadi, R.; Pizzo, P. Defective mitochondrial pyruvate flux affects cell bioenergetics in alzheimer’s disease-related models. Cell Rep. 2020, 30, 2332–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorens-Martin, M.; Jurado, J.; Hernandez, F.; Avila, J. GSK-3beta, a pivotal kinase in alzheimer disease. Front. Mol. Neurosci. 2014, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, R.; Agostini, M.; Murana, E.; Mahmud, M.; Scremin, E.; Rubega, M.; Sparacino, G.; Vassanelli, S.; Fasolato, C. Early hippocampal hyperexcitability in PS2APP mice: Role of mutant PS2 and APP. Neurobiol. Aging 2017, 50, 64–76. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Salat, D.H.; Greve, D.N.; Chua, E.F.; Rand-Giovannetti, E.; Rentz, D.M.; Bertram, L.; Mullin, K.; Tanzi, R.E.; Blacker, D.; et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 2005, 65, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Bakker, A.; Albert, M.S.; Krauss, G.; Speck, C.L.; Gallagher, M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by FMRI and memory task performance. Neuroimage Clin. 2015, 7, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Palop, J.J.; Mucke, L. Network abnormalities and interneuron dysfunction in alzheimer disease. Nat. Rev. Neurosci. 2016, 17, 777–792. [Google Scholar] [CrossRef]
- Lam, A.D.; Deck, G.; Goldman, A.; Eskandar, E.N.; Noebels, J.; Cole, A.J. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in alzheimer’s disease. Nat. Med. 2017, 23, 678–680. [Google Scholar] [CrossRef]
- Mitra, A.; Snyder, A.Z.; Hacker, C.D.; Pahwa, M.; Tagliazucchi, E.; Laufs, H.; Leuthardt, E.C.; Raichle, M.E. Human cortical–hippocampal dialogue in wake and slow-wave sleep. Proc. Natl. Acad. Sci. USA 2016, 113, E6868–E6876. [Google Scholar] [CrossRef] [Green Version]
- Busche, M.A.; Grienberger, C.; Keskin, A.D.; Song, B.; Neumann, U.; Staufenbiel, M.; Forstl, H.; Konnerth, A. Decreased amyloid-beta and increased neuronal hyperactivity by immunotherapy in alzheimer’s models. Nat. Neurosci. 2015, 18, 1725–1727. [Google Scholar] [CrossRef] [PubMed]
- Kastanenka, K.V.; Hou, S.S.; Shakerdge, N.; Logan, R.; Feng, D.; Wegmann, S.; Chopra, V.; Hawkes, J.M.; Chen, X.; Bacskai, B.J. Optogenetic restoration of disrupted slow oscillations halts amyloid deposition and restores calcium homeostasis in an animal model of alzheimer’s disease. PLoS ONE 2017, 12, e0170275. [Google Scholar] [CrossRef] [PubMed]
- Leparulo, A.; Mahmud, M.; Scremin, E.; Pozzan, T.; Vassanelli, S.; Fasolato, C. Dampened slow oscillation connectivity anticipates amyloid deposition in the PS2APP mouse model of alzheimer’s disease. Cells 2019, 9, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Born, J. Slow-wave sleep and the consolidation of long-term memory. World J. Biol. Psychiatry 2010, 11, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Fasolato, C. When, Where and How? Focus on neuronal calcium dysfunctions in alzheimer’s disease. Cell Calcium 2016, 60, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Lazzari, C.; Kipanyula, M.J.; Agostini, M.; Pozzan, T.; Fasolato, C. Ab42 oligomers selectively disrupt neuronal calcium release. Neurobiol. Aging 2015, 36, 877–885. [Google Scholar] [CrossRef]
- Tong, B.C.; Wu, A.J.; Li, M.; Cheung, K.H. Calcium signaling in alzheimer’s disease & therapies. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1745–1760. [Google Scholar] [CrossRef] [PubMed]
- Brawek, B.; Garaschuk, O. Network-wide dysregulation of calcium homeostasis in alzheimer’s disease. In Cell and Tissue Research; Springer: Berlin/Heidelberg, Germany, 2014; pp. 427–438. [Google Scholar]
- Lee, Y.J.; Choi, I.S.; Park, M.H.; Lee, Y.M.; Song, J.K.; Kim, Y.H.; Kim, K.H.; Hwang, D.Y.; Jeong, J.H.; Yun, Y.P.; et al. 4-O-methylhonokiol attenuates memory impairment in presenilin 2 mutant mice through reduction of oxidative damage and inactivation of astrocytes and the ERK pathway. Free Radic. Biol. Med. 2011, 50, 66–77. [Google Scholar] [CrossRef]
- Yuk, D.Y.; Lee, Y.K.; Nam, S.Y.; Yun, Y.W.; Hwang, D.Y.; Choi, D.Y.; Oh, K.W.; Hong, J.T. Reduced anxiety in the mice expressing mutant (N141I) presenilin 2. J. Neurosci. Res. 2009, 87, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Verret, L.; Mann, E.O.; Hang, G.B.; Barth, A.M.; Cobos, I.; Ho, K.; Devidze, N.; Masliah, E.; Kreitzer, A.C.; Mody, I.; et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in alzheimer model. Cell 2012, 149, 708–721. [Google Scholar] [CrossRef] [Green Version]
- Hahn, T.T.G.; Sakmann, B.; Mehta, M.R. Phase-locking of hippocampal interneurons’ membrane potential to neocortical up-down states. Nat. Neurosci. 2006, 9, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, S.; Heistek, T.S.; van der Loo, R.; Mansvelder, H.D.; Smit, A.B.; van Kesteren, R.E. Hyperexcitable parvalbumin interneurons render hippocampal circuitry vulnerable to amyloid beta. Iscience 2020, 23, 101271. [Google Scholar] [CrossRef] [PubMed]
- Fellin, T.; Halassa, M.M.; Terunuma, M.; Succol, F.; Takano, H.; Frank, M.; Moss, S.J.; Haydon, P.G. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 15037–15042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poskanzer, K.E.; Yuste, R. Astrocytic regulation of cortical Up states. Proc. Natl. Acad. Sci. USA 2011, 108, 18453–18458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef] [Green Version]
- Durkee, C.A.; Araque, A. Diversity and specificity of astrocyte-neuron communication. Neuroscience 2019, 396, 73–78. [Google Scholar] [CrossRef]
- Busche, M.A.; Chen, X.; Henning, H.A.; Reichwald, J.; Staufenbiel, M.; Sakmann, B.; Konnerth, A. Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2012, 109, 8740–8745. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Fivaz, M. Neuronal SOCE: Myth or reality? In Trends in Cell Biology; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 890–893. [Google Scholar]
- Majewski, L.; Kuznicki, J. SOCE in Neurons: Signaling or just refilling? In Biochimica et Biophysica Acta-Molecular Cell Research; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1940–1952. [Google Scholar]
- Sun, S.; Zhang, H.; Liu, J.; Popugaeva, E.; Xu, N.J.; Feske, S.; White, C.L.; Bezprozvanny, I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron 2014, 82, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Sun, S.; Wu, L.; Pchitskaya, E.; Zakharova, O.; Tacer, K.F.; Bezprozvanny, I. Store-operated calcium channel complex in postsynaptic spines: A new therapeutic target for alzheimer’s disease treatment. J. Neurosci. 2016, 36, 11837–11850. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, L.; Pchitskaya, E.; Zakharova, O.; Saito, T.; Saido, T.; Bezprozvanny, I. Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of alzheimer’s disease. J. Neurosci. 2015, 35, 13275–13286. [Google Scholar] [CrossRef] [Green Version]
- Park, C.Y.; Shcheglovitov, A.; Dolmetsch, R. The CRAC channel activator STIM1 binds and inhibits L-Type voltage-gated calcium channels. Science 2010, 330, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Deng, X.X.; Mancarella, S.; Hendron, E.; Eguchi, S.; Soboloff, J.; Tang, X.A.D.; Gill, D.L. The calcium store sensor, STIM1, reciprocally controls orai and Ca(V)1.2 channels. Science 2010, 330, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moccia, F.; Zuccolo, E.; Soda, T.; Tanzi, F.; Guerra, G.; Mapelli, L.; Lodola, F.; D’Angelo, E. Stim and orai proteins in neuronal Ca2+ signaling and excitability. Front. Cell. Neurosci. 2015, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Ia, J.; Gao, R.; Gao, I.; Munoz, F.M.; Wei, D.; Tian, Y.; Barrett, J.E.; Ajit, S.; Meucci, O.; et al. Orai1 plays a crucial role in central sensitization by modulating neuronal excitability. J. Neurosci. 2018, 38, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Pizzo, P.; Burgo, A.; Pozzan, T.; Fasolato, C. Role of capacitative calcium entry on glutamate-induced calcium influx in Type-I rat cortical astrocytes. J. Neurochem. 2001, 79, 98–109. [Google Scholar] [CrossRef]
- Okubo, Y.; Iino, M.; Hirose, K. Store-operated Ca2+ entry-dependent Ca2+ refilling in the endoplasmic reticulum in astrocytes. Biochem. Biophys. Res. Commun. 2020, 522, 1003–1008. [Google Scholar] [CrossRef]
- Toth, A.B.; Hori, K.; Novakovic, M.M.; Bernstein, N.G.; Lambot, L.; Prakriya, M. CRAC channels regulate astrocyte Ca2+ signaling and gliotransmitter release to modulate hippocampal GABAergic transmission. Sci. Signal. 2019, 12, 5450. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Rodríguez-Arellano, J.J.; Parpura, V.; Zorec, R. Astroglial calcium signalling in alzheimer’s disease. In Biochemical and Biophysical Research Communications; Elsevier, B.V.: Amsterdam, The Netherlands, 2017; pp. 1005–1012. [Google Scholar]
- Jayadev, S.; Case, A.; Eastman, A.J.; Nguyen, H.; Pollak, J.; Wiley, J.C.; Möller, T.; Morrison, R.S.; Garden, G.A. Presenilin 2 is the predominant γ-secretase in microglia and modulates cytokine release. PLoS ONE 2010, 5, e15743. [Google Scholar] [CrossRef] [Green Version]
- Scremin, E.; Agostini, M.; Leparulo, A.; Pozzan, T.; Greotti, E.; Fasolato, C. ORAI2 down-regulation potentiates SOCE and decreases Aβ42 accumulation in human neuroglioma cells. Int. J. Mol. Sci. 2020, 21, 5288. [Google Scholar] [CrossRef]
- Zeiger, W.; Vetrivel, K.S.; Buggia-Prevot, V.; Nguyen, P.D.; Wagner, S.L.; Villereal, M.L.; Thinakaran, G. Ca2+ influx through store-operated Ca2+ channels reduces alzheimer disease b-amyloid peptide secretion. J. Biol. Chem. 2013, 288, 26955–26966. [Google Scholar] [CrossRef] [Green Version]
- Vaeth, M.; Yang, J.; Yamashita, M.; Zee, I.; Eckstein, M.; Knosp, C.; Kaufmann, U.; Karoly Jani, P.; Lacruz, R.S.; Flockerzi, V.; et al. ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat. Commun. 2017, 8, 14714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, G.R.; Li, Y.M. The role of astrocytes in amyloid production and alzheimer’s disease. In Open Biology; Royal Society Publishing: London, UK, 2017. [Google Scholar]
- Filadi, R.; Pizzo, P. Mitochondrial calcium handling and neurodegeneration: When a good signal goes wrong. Curr. Opin. Physiol. 2020. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: Pathogenesis and treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Biophys. Acta 2014, 1842, 1219–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazzari, F.H.; Abdallah, D.M.; El-Abhar, H.S. Pharmacological interventions to attenuate alzheimer’s disease progression: The story so far. Curr. Alzheimer Res. 2019, 16, 261–277. [Google Scholar] [CrossRef]
- Oules, B.; Del Prete, D.; Greco, B.; Zhang, X.; Lauritzen, I.; Sevalle, J.; Moreno, S.; Paterlini-Brechot, P.; Trebak, M.; Checler, F.; et al. Ryanodine receptor blockade reduces amyloid-b load and memory impairments in Tg2576 mouse model of alzheimer disease. J. Neurosci. 2012, 32, 11820–11834. [Google Scholar] [CrossRef]
- Chakroborty, S.; Stutzmann, G.E. Calcium channelopathies and alzheimer’s disease: Insight into therapeutic success and failures. Eur. J. Pharmacol. 2014, 739, 83–95. [Google Scholar] [CrossRef]
- Facchinetti, F.; Fasolato, C.; Del Giudice, E.; Burgo, A.; Furegato, S.; Fusco, M.; Basso, E.; Seraglia, R.; D’Arrigo, A.; Leon, A. Nimodipine selectively stimulates b-amyloid 1-42 secretion by a mechanism independent of calcium influx blockage. Neurobiol. Aging 2006, 27, 218–227. [Google Scholar] [CrossRef]
- Bantug, G.R.; Fischer, M.; Grahlert, J.; Balmer, M.L.; Unterstab, G.; Develioglu, L.; Steiner, R.; Zhang, L.; Costa, A.S.H.; Gubser, P.M.; et al. Mitochondria-endoplasmic reticulum contact sites function as immunometabolic hubs that orchestrate the rapid recall response of memory CD8(+) T cells. Immunity 2018, 48, 542–555. [Google Scholar] [CrossRef] [Green Version]
- Trushina, E.; Dutta, T.; Persson, X.M.; Mielke, M.M.; Petersen, R.C. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and alzheimer’s disease using metabolomics. PLoS ONE 2013, 8, e63644. [Google Scholar] [CrossRef] [Green Version]
- Parnetti, L.; Gaiti, A.; Polidori, M.C.; Brunetti, M.; Palumbo, B.; Chionne, F.; Cadini, D.; Cecchetti, R.; Senin, U. Increased cerebrospinal fluid pyruvate levels in alzheimer’s disease. Neurosci. Lett. 1995, 199, 231–233. [Google Scholar] [CrossRef]
- Sasaguri, H.; Nilsson, P.; Hashimoto, S.; Nagata, K.; Saito, T.; De Strooper, B.; Hardy, J.; Vassar, R.; Winblad, B.; Saido, T.C. APP mouse models for alzheimer’s disease preclinical studies. EMBO J. 2017, 36, 2473–2487. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Zheng, H. Practical considerations for choosing a mouse model of alzheimer’s disease. Mol. Neurodegener. 2017, 12, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of alzheimer’s disease with plaques and tangles: Intracellular abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.G.; Higgins, G.A.; Ouagazzal, A.M.; Ozmen, L.; Kew, J.N.; Bohrmann, B.; Malherbe, P.; Brockhaus, M.; Loetscher, H.; Czech, C.; et al. PS2APP transgenic mice, coexpressing HPS2mut and HAPPswe, show age-related cognitive deficits associated with discrete brain amyloid deposition and inflammation. J. Neurosci. 2003, 23, 8989–9003. [Google Scholar] [CrossRef] [Green Version]
- Poirier, R.; Veltman, I.; Pflimlin, M.C.; Knoflach, F.; Metzger, F. Enhanced dentate gyrus synaptic plasticity but reduced neurogenesis in a mouse model of amyloidosis. Neurobiol. Dis. 2010, 40, 386–393. [Google Scholar] [CrossRef]
- Weidensteiner, C.; Metzger, F.; Bruns, A.; Bohrmann, B.; Kuennecke, B.; Von Kienlin, M. Cortical hypoperfusion in the B6.PS2APP mouse model for alzheimer’s disease: Comprehensive phenotyping of vascular and tissular parameters by MRI. Magn. Reson. Med. 2009, 62, 35–45. [Google Scholar] [CrossRef]
- Brendel, M.; Jaworska, A.; Grießinger, E.; Rötzer, C.; Burgold, S.; Gildehaus, F.J.; Carlsen, J.; Cumming, P.; Baumann, K.; Haass, C.; et al. Cross-sectional comparison of small animal [18f]-florbetaben amyloid-PET between transgenic AD mouse models. PLoS ONE 2015, 10, e0116678. [Google Scholar] [CrossRef] [Green Version]
- Grueninger, F.; Bohrmann, B.; Czech, C.; Ballard, T.M.; Frey, J.R.; Weidensteiner, C.; von Kienlin, M.; Ozmen, L. Phosphorylation of Tau at S422 is enhanced by Aβ in TauPS2APP triple transgenic mice. Neurobiol. Dis. 2010, 37, 294–306. [Google Scholar] [CrossRef]
- Loreth, D.; Ozmen, L.; Revel, F.G.; Knoflach, F.; Wetzel, P.; Frotscher, M.; Metzger, F.; Kretz, O. Selective degeneration of septal and hippocampal GABAergic neurons in a mouse model of amyloidosis and tauopathy. Neurobiol. Dis. 2012, 47, 1–12. [Google Scholar] [CrossRef]
- Kins, S.; Crameri, A.; Evans, D.R.H.; Hemmings, B.A.; Nitsch, R.M.; Götz, J. Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of Tau in transgenic mice. J. Biol. Chem. 2001, 276, 38193–38200. [Google Scholar] [CrossRef] [PubMed]
- Nyabi, O.; Pype, S.; Mercken, M.; Herreman, A.; Saftig, P.; Craessaerts, K.; Serneels, L.; Annaert, W.; De Strooper, B. No endogenous ab production in presenilin-deficient fibroblasts. Nat. Cell Biol. 2002, 4, E164. [Google Scholar] [CrossRef] [PubMed]
- Herreman, A.; Hartmann, D.; Annaert, W.; Saftig, P.; Craessaerts, K.; Serneels, L.; Umans, L.; Schrijvers, V.; Checler, F.; Vanderstichele, H.; et al. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc. Natl. Acad. Sci. USA 1999, 96, 11872–11877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Lutz, D.; Mossalam, M.; Bolshakov, V.Y.; Frotscher, M.; Shen, J. Presenilins regulate synaptic plasticity and mitochondrial calcium homeostasis in the hippocampal mossy fiber pathway. Mol. Neurodegener. 2017, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Gratuze, M.; Leyns, C.E.G.; Sauerbeck, A.D.; St-Pierre, M.-K.; Xiong, M.; Kim, N.; Remolina Serrano, J.; Tremblay, M.-È.; Kummer, T.T.; Colonna, M.; et al. Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J. Clin. Investig. 2020. [Google Scholar] [CrossRef]
- Balu, D.; Karstens, A.J.; Loukenas, E.; Maldonado Weng, J.; York, J.M.; Valencia-Olvera, A.C.; LaDu, M.J. The role of APOE in transgenic mouse models of AD. Neurosci. Lett. 2019, 707, 134285. [Google Scholar] [CrossRef]
- Hargis, K.E.; Blalock, E.M. Transcriptional signatures of brain aging and alzheimer’s disease: What are our rodent models telling us? Behav. Brain Res. 2017, 322, 311–328. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzo, P.; Basso, E.; Filadi, R.; Greotti, E.; Leparulo, A.; Pendin, D.; Redolfi, N.; Rossini, M.; Vajente, N.; Pozzan, T.; et al. Presenilin-2 and Calcium Handling: Molecules, Organelles, Cells and Brain Networks. Cells 2020, 9, 2166. https://doi.org/10.3390/cells9102166
Pizzo P, Basso E, Filadi R, Greotti E, Leparulo A, Pendin D, Redolfi N, Rossini M, Vajente N, Pozzan T, et al. Presenilin-2 and Calcium Handling: Molecules, Organelles, Cells and Brain Networks. Cells. 2020; 9(10):2166. https://doi.org/10.3390/cells9102166
Chicago/Turabian StylePizzo, Paola, Emy Basso, Riccardo Filadi, Elisa Greotti, Alessandro Leparulo, Diana Pendin, Nelly Redolfi, Michela Rossini, Nicola Vajente, Tullio Pozzan, and et al. 2020. "Presenilin-2 and Calcium Handling: Molecules, Organelles, Cells and Brain Networks" Cells 9, no. 10: 2166. https://doi.org/10.3390/cells9102166
APA StylePizzo, P., Basso, E., Filadi, R., Greotti, E., Leparulo, A., Pendin, D., Redolfi, N., Rossini, M., Vajente, N., Pozzan, T., & Fasolato, C. (2020). Presenilin-2 and Calcium Handling: Molecules, Organelles, Cells and Brain Networks. Cells, 9(10), 2166. https://doi.org/10.3390/cells9102166







