Epac1 Is Crucial for Maintenance of Endothelial Barrier Function through A Mechanism Partly Independent of Rac1
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Isolation of Endothelial Cells
2.2. Ethical Approval
2.3. Antibodies and Test Reagents
2.4. Western Blots
2.5. Transendothelial Electrical Resistance Measurements
2.6. Immunofluorescence and Densitometric Measurements
2.7. Rac1 and RhoA GLISA Measurements
2.8. Non-Radioactive Rap1 Pull-Down Activity Assay
2.9. Quantitative Determination of Cyclic AMP
2.10. PDE Activity Assay
2.11. PCR Analysis
2.12. Data Analysis and Statistic
3. Results
3.1. Epac1 Contributes to Baseline Endothelial Barrier Function and Is Crucial for the cAMP-Mediated Barrier Stabilization
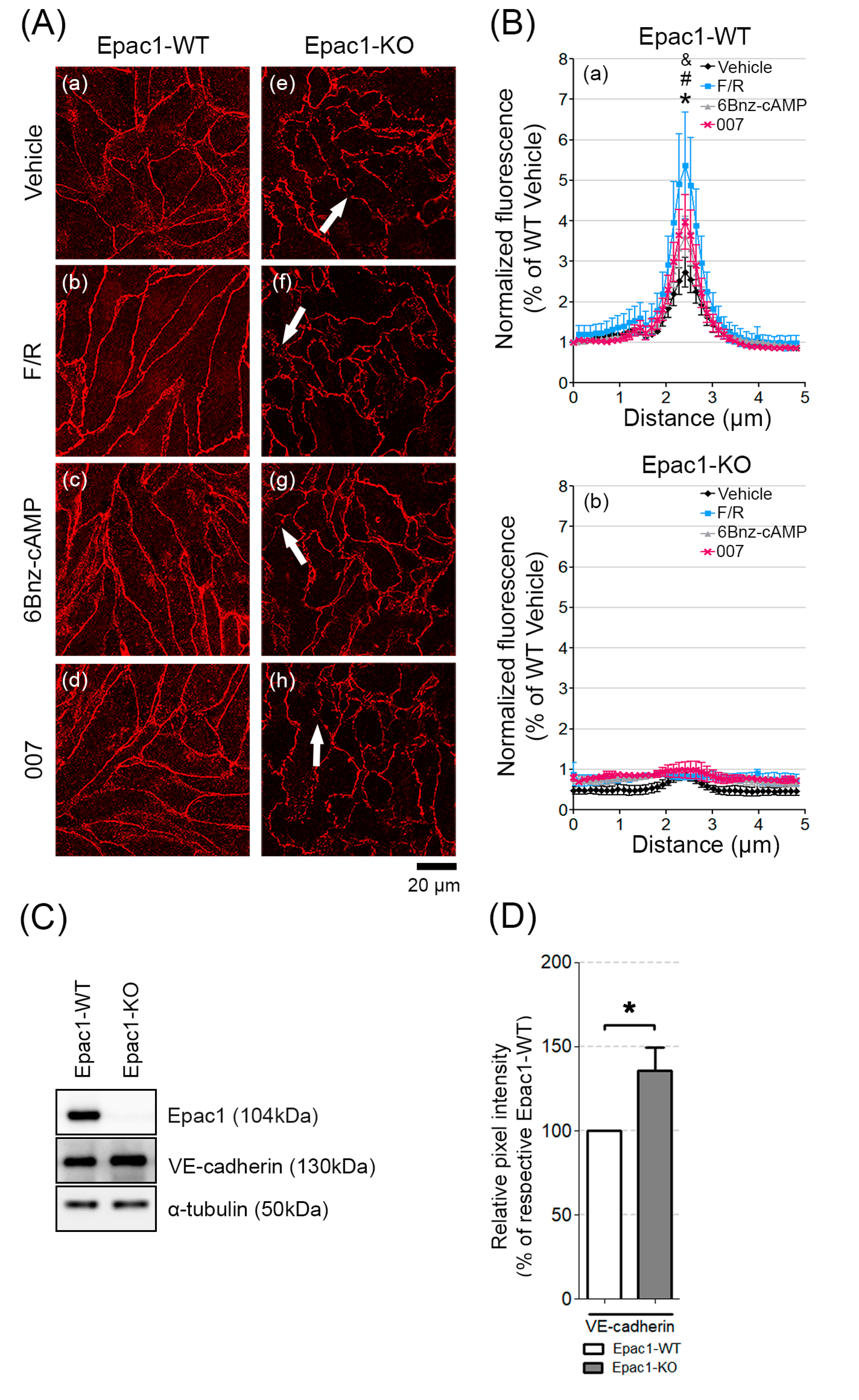
3.2. Epac1 Mediates the Recruitment of VE-Cadherin to AJs upon cAMP Elevation
3.3. Loss of Epac1 Affects the Expression of the Junctional Adhesion Protein VE-Cadherin
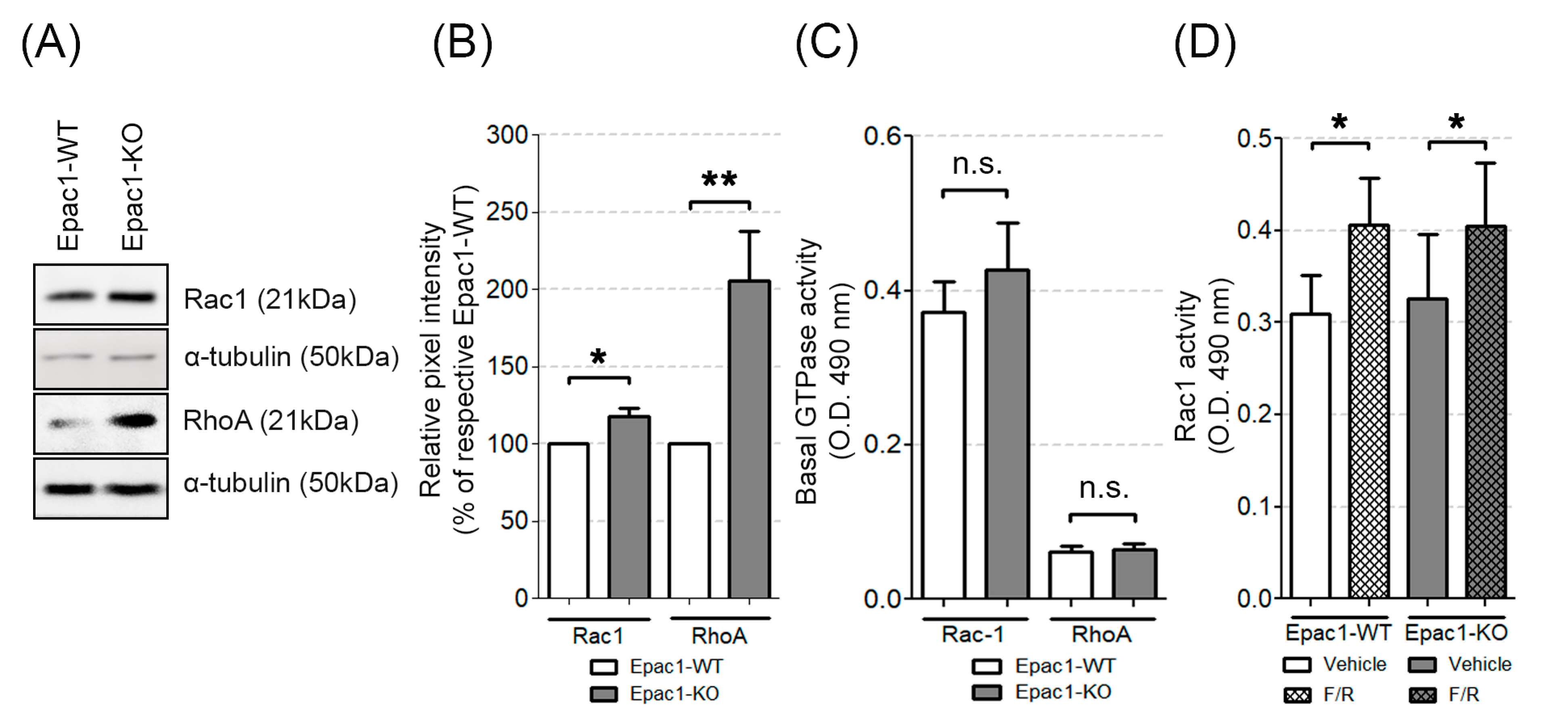
3.4. The Expression and Activity of Rap1, Rac1, and RhoA in the WT and Epac1 Null Cells
3.5. Epac1 Appears Not to Be Required for cAMP-Mediated Rac1 Activation
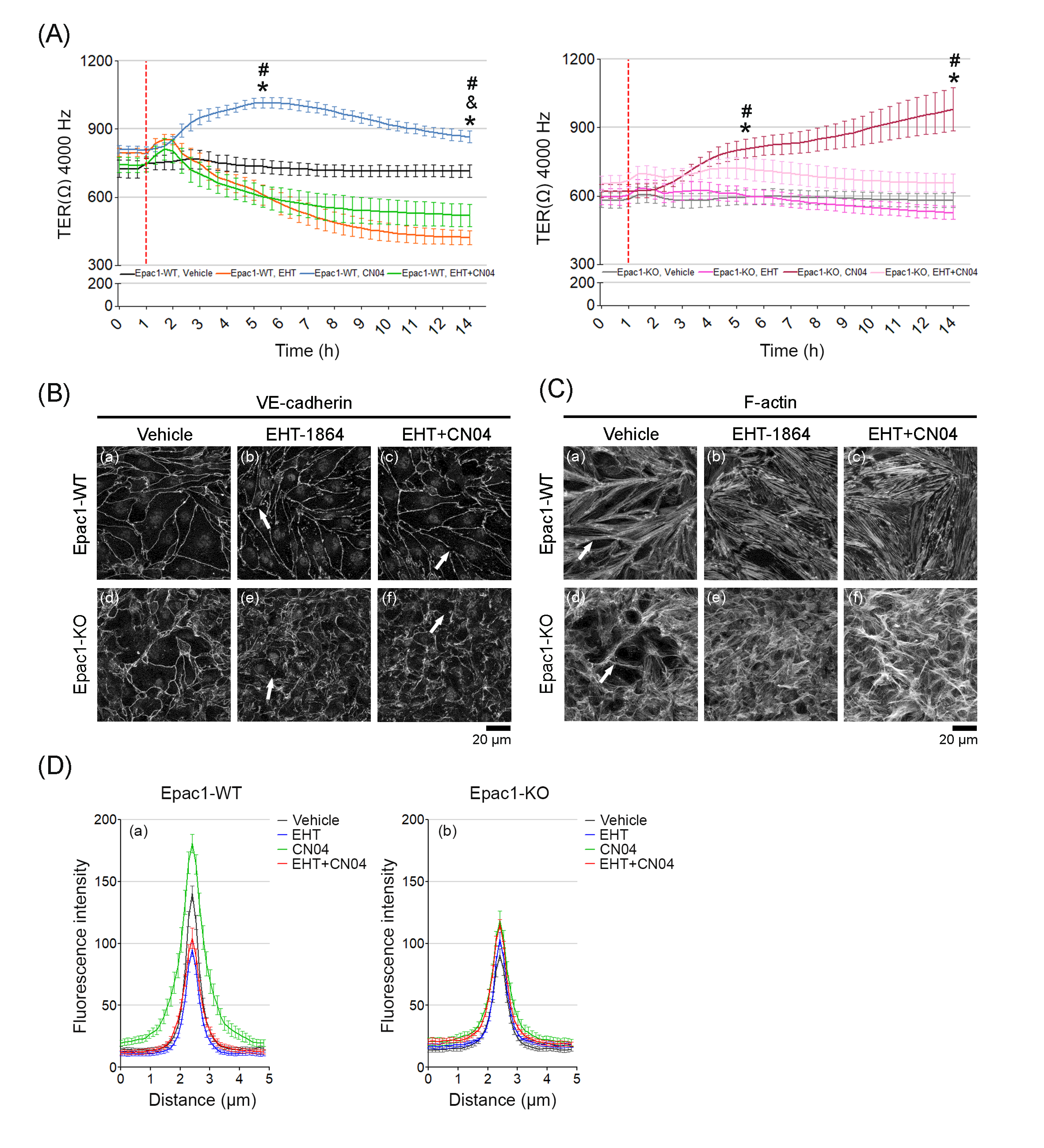
3.6. Simultaneous Direct Activation of Rac1 and RhoA Enhanced Barrier Function in the Absence of Epac1
4. Discussion
4.1. Epac1 Is Required for Baseline Integrity and cAMP-Mediated Regulation of Endothelial Adherens Junctions
4.2. Epac1 Is Required for cAMP-Mediated Rap1, but Not for Rac1 and RhoA Activity
4.3. Epac1 Deletion Might Regulate cAMP Levels in a PDE4-Dependent Manner
4.4. Roles of Rac1 and RhoA for Epac1-Mediated Barrier Regulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Nomenclature |
| AJ | Adherens junction |
| AC | Adenylyl Cyclase |
| CNF-1 | Cytotoxic necrotizing factor 1 |
| cAMP | Cyclic adenosine monophosphate |
| Epac1 | Exchange protein activated by cAMP 1 |
| GEFs | Guanosine nucleotide exchange factors |
| GTPases | Guanosine triphosphatases |
| G-LISA | G-protein ELISA |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| HDMEC | Human Dermal Microvascular Endothelial Cells |
| MyEnds | Myocardial endothelial cells |
| PKA | Protein Kinase A |
| PDE | Phosphodiesterase |
| TER | Transendothelial electrical resistance |
| VASP | Vasodilator-stimulated phosphoprotein |
| 007 | 8-pCPT-2′-O-Me-cAMP |
References
- Garcia-Ponce, A.; Citalan-Madrid, A.F.; Velazquez-Avila, M.; Vargas-Robles, H.; Schnoor, M. The role of actin-binding proteins in the control of endothelial barrier integrity. Thromb. Haemost. 2015, 113, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, M.; Garcia Ponce, A.; Vadillo, E.; Pelayo, R.; Rossaint, J.; Zarbock, A. Actin dynamics in the regulation of endothelial barrier functions and neutrophil recruitment during endotoxemia and sepsis. Cell. Mol. Life Sci. 2017, 74, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Kopperud, R.K.; Rygh, C.B.; Karlsen, T.V.; Krakstad, C.; Kleppe, R.; Hoivik, E.A.; Bakke, M.; Tenstad, O.; Selheim, F.; Liden, A.; et al. Increased microvascular permeability in mice lacking Epac1 (RapGef3). Acta Physiol. (Oxf.) 2017, 219, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Curry, F.E.; Taxt, T.; Rygh, C.B.; Pavlin, T.; Bjonrstad, R.; Doskeland, S.O.; Reed, R.K. Epac1(-/-) mice have elevated baseline permeability and do not respond to histamine as measured with dynamic contrast-enhanced magnetic resonance imaging with contrast agents of different molecular weights. Acta Physiol. (Oxf.) 2019, 225, e13199. [Google Scholar] [CrossRef]
- Nygaard, G.; Herfindal, L.; Asrud, K.S.; Bjornstad, R.; Kopperud, R.K.; Oveland, E.; Berven, F.S.; Myhren, L.; Hoivik, E.A.; Lunde, T.H.F.; et al. Epac1-deficient mice have bleeding phenotype and thrombocytes with decreased GPIbbeta expression. Sci. Rep. 2017, 7, 8725. [Google Scholar] [CrossRef]
- Okumura, S.; Fujita, T.; Cai, W.; Jin, M.; Namekata, I.; Mototani, Y.; Jin, H.; Ohnuki, Y.; Tsuneoka, Y.; Kurotani, R.; et al. Epac1-dependent phospholamban phosphorylation mediates the cardiac response to stresses. J. Clin. Investig. 2014, 124, 2785–2801. [Google Scholar] [CrossRef]
- Spindler, V.; Schlegel, N.; Waschke, J. Role of GTPases in control of microvascular permeability. Cardiovasc. Res. 2010, 87, 243–253. [Google Scholar] [CrossRef]
- Harris, E.S.; Nelson, W.J. VE-cadherin: At the front, center, and sides of endothelial cell organization and function. Curr. Opin. Cell Biol. 2010, 22, 651–658. [Google Scholar] [CrossRef]
- Hatanaka, K.; Simons, M.; Murakami, M. Phosphorylation of VE-cadherin controls endothelial phenotypes via p120-catenin coupling and Rac1 activation. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H162–H172. [Google Scholar] [CrossRef]
- Lampugnani, M.G.; Zanetti, A.; Breviario, F.; Balconi, G.; Orsenigo, F.; Corada, M.; Spagnuolo, R.; Betson, M.; Braga, V.; Dejana, E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol. Biol. Cell 2002, 13, 1175–1189. [Google Scholar] [CrossRef]
- Curry, F.R.; Adamson, R.H. Tonic regulation of vascular permeability. Acta Physiol. 2013, 207, 628–649. [Google Scholar] [CrossRef] [PubMed]
- Radeva, M.Y.; Waschke, J. Mind the gap: Mechanisms regulating the endothelial barrier. Acta Physiol. (Oxf.) 2017, 222, e12860. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Ghosh, C.C.; Mukherjee, A.; Parikh, S.M. Angiopoietin-1 requires IQ domain GTPase-activating protein 1 to activate Rac1 and promote endothelial barrier defense. Arter. Thromb. Vasc. Biol. 2011, 31, 2643–2652. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, J.; Zwartkruis, F.J.; Verheijen, M.H.; Cool, R.H.; Nijman, S.M.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, W.G., 3rd; Cheng, X. Intracellular cAMP Sensor EPAC: Physiology, Pathophysiology, and Therapeutics Development. Physiol. Rev. 2018, 98, 919–1053. [Google Scholar] [CrossRef]
- Schnoor, M.; Lai, F.P.; Zarbock, A.; Klaver, R.; Polaschegg, C.; Schulte, D.; Weich, H.A.; Oelkers, J.M.; Rottner, K.; Vestweber, D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J. Exp. Med. 2011, 208, 1721–1735. [Google Scholar] [CrossRef]
- Chrzanowska-Wodnicka, M. Rap1 in endothelial biology. Curr. Opin. Hematol. 2017, 24, 248–255. [Google Scholar] [CrossRef]
- Wittchen, E.S.; Worthylake, R.A.; Kelly, P.; Casey, P.J.; Quilliam, L.A.; Burridge, K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J. Biol. Chem. 2005, 280, 11675–11682. [Google Scholar] [CrossRef]
- Kooistra, M.R.; Corada, M.; Dejana, E.; Bos, J.L. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005, 579, 4966–4972. [Google Scholar] [CrossRef]
- Fukuhara, S.; Sakurai, A.; Sano, H.; Yamagishi, A.; Somekawa, S.; Takakura, N.; Saito, Y.; Kangawa, K.; Mochizuki, N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell. Biol. 2005, 25, 136–146. [Google Scholar] [CrossRef]
- Spindler, V.; Peter, D.; Harms, G.S.; Asan, E.; Waschke, J. Ultrastructural Analysis Reveals cAMP-Dependent Enhancement of Microvascular Endothelial Barrier Functions via Rac1-Mediated Reorganization of Intercellular Junctions. Am. J. Pathol. 2011, 178, 2424–2436. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Fukuhara, S.; Moriya, T.; Obara, Y.; Nakahata, N.; Mochizuki, N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J. Cell Biol. 2013, 202, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Stockton, R.A.; Shenkar, R.; Awad, I.A.; Ginsberg, M.H. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J. Exp. Med. 2010, 207, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Zieba, B.J.; Artamonov, M.V.; Jin, L.; Momotani, K.; Ho, R.; Franke, A.S.; Neppl, R.L.; Stevenson, A.S.; Khromov, A.S.; Chrzanowska-Wodnicka, M.; et al. The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activity. J. Biol. Chem. 2011, 286, 16681–16692. [Google Scholar] [CrossRef] [PubMed]
- Cullere, X.; Shaw, S.K.; Andersson, L.; Hirahashi, J.; Luscinskas, F.W.; Mayadas, T.N. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 2005, 105, 1950–1955. [Google Scholar] [CrossRef]
- Post, A.; Pannekoek, W.J.; Ponsioen, B.; Vliem, M.J.; Bos, J.L. Rap1 Spatially Controls ArhGAP29 To Inhibit Rho Signaling during Endothelial Barrier Regulation. Mol. Cell. Biol. 2015, 35, 2495–2502. [Google Scholar] [CrossRef]
- Birukova, A.A.; Tian, X.; Tian, Y.; Higginbotham, K.; Birukov, K.G. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol. Biol. Cell 2013, 24, 2678–2688. [Google Scholar] [CrossRef]
- Jeyaraj, S.C.; Unger, N.T.; Eid, A.H.; Mitra, S.; Paul El-Dahdah, N.; Quilliam, L.A.; Flavahan, N.A.; Chotani, M.A. Cyclic AMP-Rap1A signaling activates RhoA to induce alpha(2c)-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2012, 303, C499–C511. [Google Scholar] [CrossRef]
- Schlegel, N.; Burger, S.; Golenhofen, N.; Walter, U.; Drenckhahn, D.; Waschke, J. The role of VASP in regulation of cAMP- and Rac 1-mediated endothelial barrier stabilization. Am. J. Physiol. Cell Physiol. 2008, 294, C178–C188. [Google Scholar] [CrossRef]
- Golenhofen, N.; Ness, W.; Wawrousek, E.F.; Drenckhahn, D. Expression and induction of the stress protein alpha-B-crystallin in vascular endothelial cells. Histochem. Cell Biol. 2002, 117, 203–209. [Google Scholar] [CrossRef]
- Baumgartner, W.; Schutz, G.J.; Wiegand, J.; Golenhofen, N.; Drenckhahn, D. Cadherin function probed by laser tweezer and single molecule fluorescence in vascular endothelial cells. J. Cell Sci. 2003, 116, 1001–1011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muller-Marschhausen, K.; Waschke, J.; Drenckhahn, D. Physiological hydrostatic pressure protects endothelial monolayer integrity. Am. J. Physiol. Cell Physiol. 2008, 294, C324–C332. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, M.; Grenacher, B.; Rohde, B.; Vogel, J. Quantifying Western blots: Pitfalls of densitometry. Electrophoresis 2009, 30, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Baumer, Y.; Drenckhahn, D.; Waschke, J. cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem. Cell Biol. 2008, 129, 765–778. [Google Scholar] [CrossRef]
- Kugelmann, D.; Waschke, J.; Radeva, M.Y. Adducin is involved in endothelial barrier stabilization. PLoS ONE 2015, 10, e0126213. [Google Scholar] [CrossRef]
- Radeva, M.Y.; Kugelmann, D.; Spindler, V.; Waschke, J. PKA compartmentalization via AKAP220 and AKAP12 contributes to endothelial barrier regulation. PLoS ONE 2014, 9, e106733. [Google Scholar] [CrossRef]
- Schlegel, N.; Waschke, J. VASP is involved in cAMP-mediated Rac 1 activation in microvascular endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C453–C462. [Google Scholar] [CrossRef]
- Heo, S.H.; Cho, J.Y. ELK3 suppresses angiogenesis by inhibiting the transcriptional activity of ETS-1 on MT1-MMP. Int. J. Biol. Sci. 2014, 10, 438–447. [Google Scholar] [CrossRef][Green Version]
- Hennenberg, M.; Strittmatter, F.; Schmetkamp, H.; Rutz, B.; Walther, S.; Stief, C.G.; Gratzke, C. The cAMP effector EPAC activates Elk1 transcription factor in prostate smooth muscle, and is a minor regulator of alpha1-adrenergic contraction. J. Biomed. Sci. 2013, 20, 46. [Google Scholar] [CrossRef]
- Waschke, J. Epac1—A tonic stabilizer of the endothelial barrier. Acta Physiol. (Oxf.) 2017, 219, 339–340. [Google Scholar] [CrossRef]
- Rampersad, S.N.; Ovens, J.D.; Huston, E.; Umana, M.B.; Wilson, L.S.; Netherton, S.J.; Lynch, M.J.; Baillie, G.S.; Houslay, M.D.; Maurice, D.H. Cyclic AMP phosphodiesterase 4D (PDE4D) Tethers EPAC1 in a vascular endothelial cadherin (VE-Cad)-based signaling complex and controls cAMP-mediated vascular permeability. J. Biol. Chem. 2010, 285, 33614–33622. [Google Scholar] [CrossRef] [PubMed]
- Baumer, Y.; Burger, S.; Curry, F.E.; Golenhofen, N.; Drenckhahn, D.; Waschke, J. Differential role of Rho GTPases in endothelial barrier regulation dependent on endothelial cell origin. Histochem. Cell Biol. 2008, 129, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.E.; Adderley, S.P.; Breslin, J.W. Activation of RhoA, but Not Rac1, Mediates Early Stages of S1P-Induced Endothelial Barrier Enhancement. PLoS ONE 2016, 11, e0155490. [Google Scholar] [CrossRef]
- Wojciak-Stothard, B.; Ridley, A.J. Rho GTPases and the regulation of endothelial permeability. Vasc. Pharmacol. 2002, 39, 187–199. [Google Scholar]
- Birukova, A.A.; Zagranichnaya, T.; Fu, P.; Alekseeva, E.; Chen, W.; Jacobson, J.R.; Birukov, K.G. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp. Cell Res. 2007, 313, 2504–2520. [Google Scholar] [CrossRef]
- Birukova, A.A.; Zagranichnaya, T.; Alekseeva, E.; Bokoch, G.M.; Birukov, K.G. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J. Cell. Physiol. 2008, 215, 715–724. [Google Scholar] [CrossRef]
- Parnell, E.; Smith, B.O.; Yarwood, S.J. The cAMP sensors, EPAC1 and EPAC2, display distinct subcellular distributions despite sharing a common nuclear pore localisation signal. Cell. Signal. 2015, 27, 989–996. [Google Scholar] [CrossRef]
- Pereira, L.; Cheng, H.; Lao, D.H.; Na, L.; van Oort, R.J.; Brown, J.H.; Wehrens, X.H.; Chen, J.; Bers, D.M. Epac2 mediates cardiac beta1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia. Circulation 2013, 127, 913–922. [Google Scholar] [CrossRef]
- Lehrke, M.; Kahles, F.; Makowska, A.; Tilstam, P.V.; Diebold, S.; Marx, J.; Stohr, R.; Hess, K.; Endorf, E.B.; Bruemmer, D.; et al. PDE4 inhibition reduces neointima formation and inhibits VCAM-1 expression and histone methylation in an Epac-dependent manner. J. Mol. Cell. Cardiol. 2015, 81, 23–33. [Google Scholar] [CrossRef]
- Sivertsen Asrud, K.; Pedersen, L.; Aesoy, R.; Muwonge, H.; Aasebo, E.; Nitschke Pettersen, I.K.; Herfindal, L.; Dobie, R.; Jenkins, S.; Berge, R.K.; et al. Mice depleted for Exchange Proteins Directly Activated by cAMP (Epac) exhibit irregular liver regeneration in response to partial hepatectomy. Sci. Rep. 2019, 9, 13789. [Google Scholar] [CrossRef]
- DiStefano, P.V.; Smrcka, A.V.; Glading, A.J. Phospholipase Cepsilon Modulates Rap1 Activity and the Endothelial Barrier. PLoS ONE 2016, 11, e0162338. [Google Scholar] [CrossRef]
- Arthur, W.T.; Quilliam, L.A.; Cooper, J.A. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J. Cell Biol. 2004, 167, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Pannekoek, W.J.; Ross, S.H.; Verlaan, I.; Brouwer, P.M.; Bos, J.L. Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proc. Natl. Acad. Sci. USA 2013, 110, 11427–11432. [Google Scholar] [CrossRef] [PubMed]
- Pannekoek, W.J.; Vliem, M.J.; Bos, J.L. Multiple Rap1 effectors control Epac1-mediated tightening of endothelial junctions. Small GTPases 2020, 11, 346–353. [Google Scholar] [CrossRef]
- Schlegel, N.; Waschke, J. cAMP with other signaling cues converges on Rac1 to stabilize the endothelial barrier- a signaling pathway compromised in inflammation. Cell Tissue Res. 2014, 355, 587–596. [Google Scholar] [CrossRef]
- Patterson, C.E.; Lum, H.; Schaphorst, K.L.; Verin, A.D.; Garcia, J.G. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium 2000, 7, 287–308. [Google Scholar] [CrossRef]
- Garcia Ponce, A.; Citalan Madrid, A.F.; Vargas Robles, H.; Chanez Paredes, S.; Nava, P.; Betanzos, A.; Zarbock, A.; Rottner, K.; Vestweber, D.; Schnoor, M. Loss of cortactin causes endothelial barrier dysfunction via disturbed adrenomedullin secretion and actomyosin contractility. Sci. Rep. 2016, 6, 29003. [Google Scholar] [CrossRef]
- Heemskerk, N.; Schimmel, L.; Oort, C.; van Rijssel, J.; Yin, T.; Ma, B.; van Unen, J.; Pitter, B.; Huveneers, S.; Goedhart, J.; et al. F-actin-rich contractile endothelial pores prevent vascular leakage during leukocyte diapedesis through local RhoA signalling. Nat. Commun. 2016, 7, 10493. [Google Scholar] [CrossRef]
- van Hooren, K.W.; van Agtmaal, E.L.; Fernandez-Borja, M.; van Mourik, J.A.; Voorberg, J.; Bierings, R. The Epac-Rap1 signaling pathway controls cAMP-mediated exocytosis of Weibel-Palade bodies in endothelial cells. J. Biol. Chem. 2012, 287, 24713–24720. [Google Scholar] [CrossRef]
- Liu, C.; Takahashi, M.; Li, Y.; Dillon, T.J.; Kaech, S.; Stork, P.J. The interaction of Epac1 and Ran promotes Rap1 activation at the nuclear envelope. Mol. Cell. Biol. 2010, 30, 3956–3969. [Google Scholar] [CrossRef]




| mEpac1-378bp-FW | CAGGTCAGCGTACGGATGAAGAAC |
| mEpac1-378bp-REV | GCTTCCACATCCTTGATGATGCG |
| mVE-cadherin-139bp-FW | CTGTCTTCCAGCGACACTTCTAC |
| mVE-cadherin-139bp-REV | GCCTCTGTCACTGGTCTTGC |
| mB2M-292+788bp-FW | CAAGTATACTCACGCCACCCAC |
| mB2M-292+788bp-REV | CATCATGATGCTTGATCACATGTCTC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Ponce, A.; Schuster, K.; Døskeland, S.-O.; Reed, R.K.; Curry, F.-R.E.; Waschke, J.; Radeva, M.Y. Epac1 Is Crucial for Maintenance of Endothelial Barrier Function through A Mechanism Partly Independent of Rac1. Cells 2020, 9, 2170. https://doi.org/10.3390/cells9102170
García-Ponce A, Schuster K, Døskeland S-O, Reed RK, Curry F-RE, Waschke J, Radeva MY. Epac1 Is Crucial for Maintenance of Endothelial Barrier Function through A Mechanism Partly Independent of Rac1. Cells. 2020; 9(10):2170. https://doi.org/10.3390/cells9102170
Chicago/Turabian StyleGarcía-Ponce, Alexander, Katharina Schuster, Stein-Ove Døskeland, Rolf K. Reed, Fitz-Roy E. Curry, Jens Waschke, and Mariya Y. Radeva. 2020. "Epac1 Is Crucial for Maintenance of Endothelial Barrier Function through A Mechanism Partly Independent of Rac1" Cells 9, no. 10: 2170. https://doi.org/10.3390/cells9102170
APA StyleGarcía-Ponce, A., Schuster, K., Døskeland, S.-O., Reed, R. K., Curry, F.-R. E., Waschke, J., & Radeva, M. Y. (2020). Epac1 Is Crucial for Maintenance of Endothelial Barrier Function through A Mechanism Partly Independent of Rac1. Cells, 9(10), 2170. https://doi.org/10.3390/cells9102170





