Apoptosis, Induced by Human α-Synuclein in Yeast, Can Occur Independent of Functional Mitochondria
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Yeast Transformation
2.3. SYTO18 Staining of mtDNA
2.4. Detection of Dead Cells with Phloxine B Dye
2.5. Detection of ROS
2.6. Assessing the Presence/Absence of Mitochondrial Membrane Potential (MMP) by Staining Live Cells with the Dye DiOC6(3)
2.7. Quantifying Mitochondrial Membrane Potential (MMP) with the JC-10 Dye
2.8. Staining with Hoechst Dye for Monitoring Live Cells
2.9. Assessing Nuclear DNA Fragmentation via the TUNEL Assay. AA
2.10. Western Blotting
2.11. Quantification of Petite formation
3. Results and Discussion
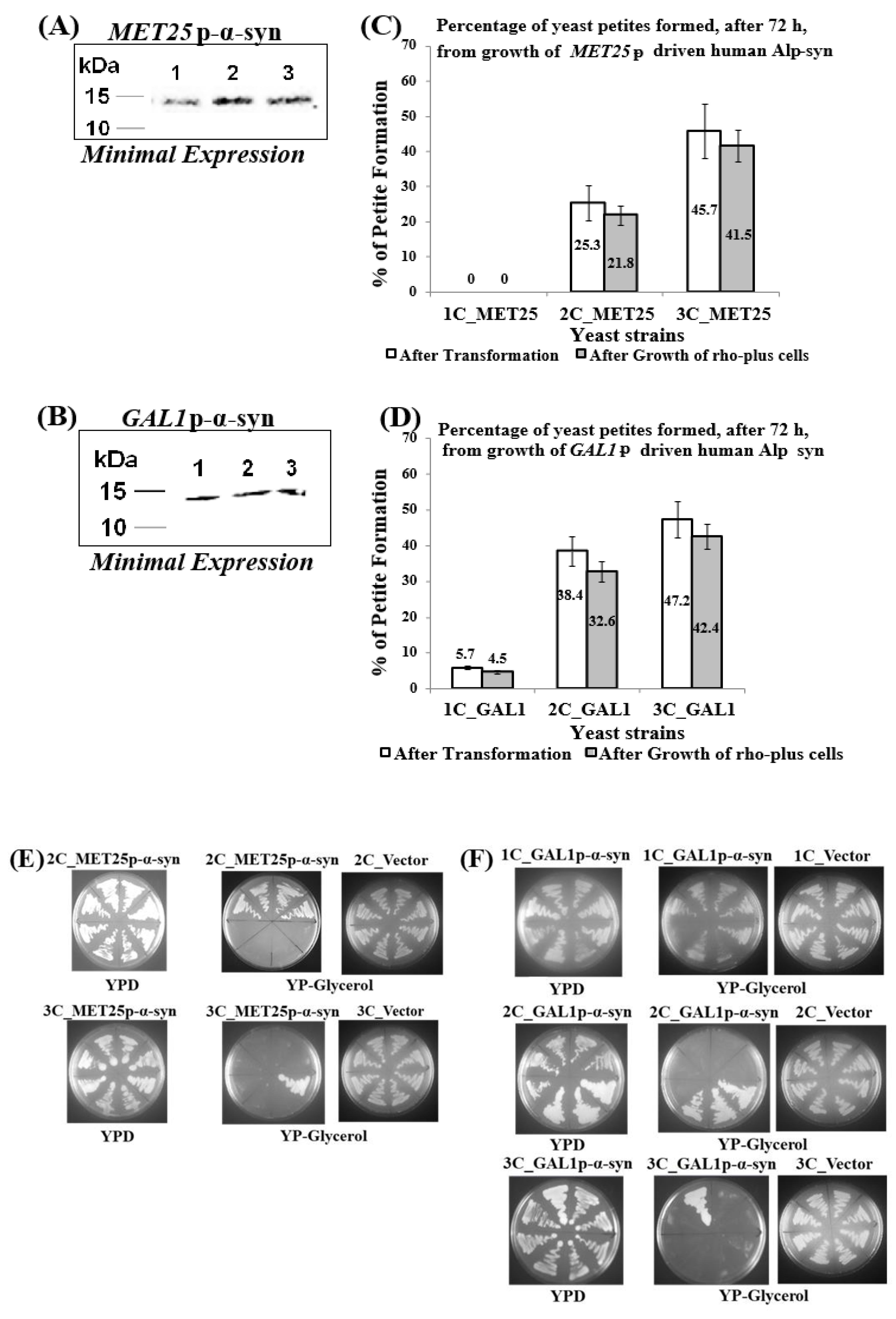
3.1. Minimal Expression of Human α-Syn in Yeast from the MET25 or GAL1 Promoters (MET25p or GAL1p), under De-Repressing Conditions, Produces Petites
3.2. Expression, in ρ− Yeast Petites, of Human α-Syn Gene from Fully-Induced MET25p/GAL1p Retards Cell Growth and Causes Cell Death
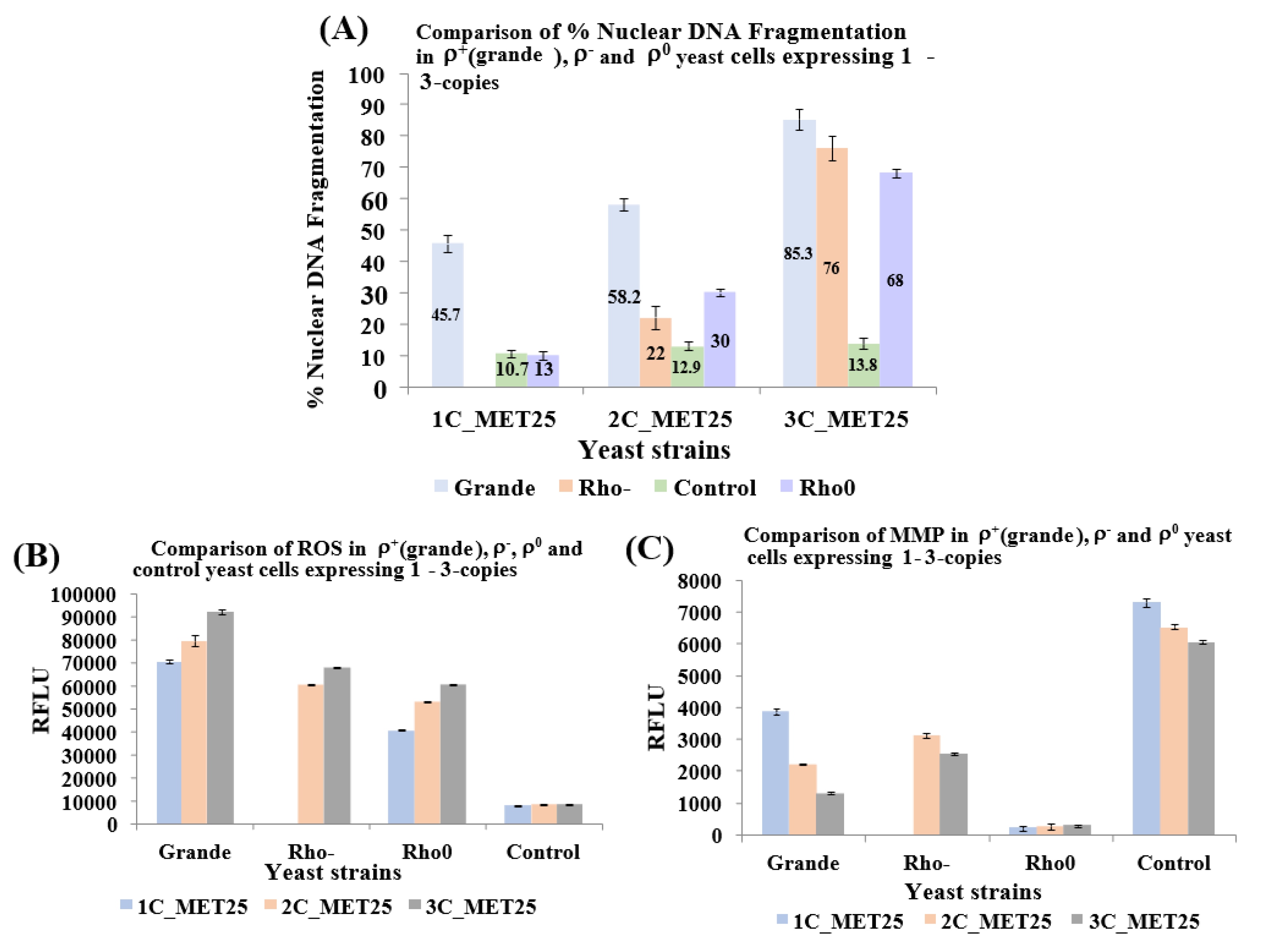
3.3. After Full Induction of MET25/GAL1 Promoter, Human α-Syn Expressing ρ− Yeast Petites Undergo Loss of MMP and Increase in Nuclear DNA Fragmentation upon Gradual Increase of α-Syn Copy Number from 2–3 or 1–3 Copies
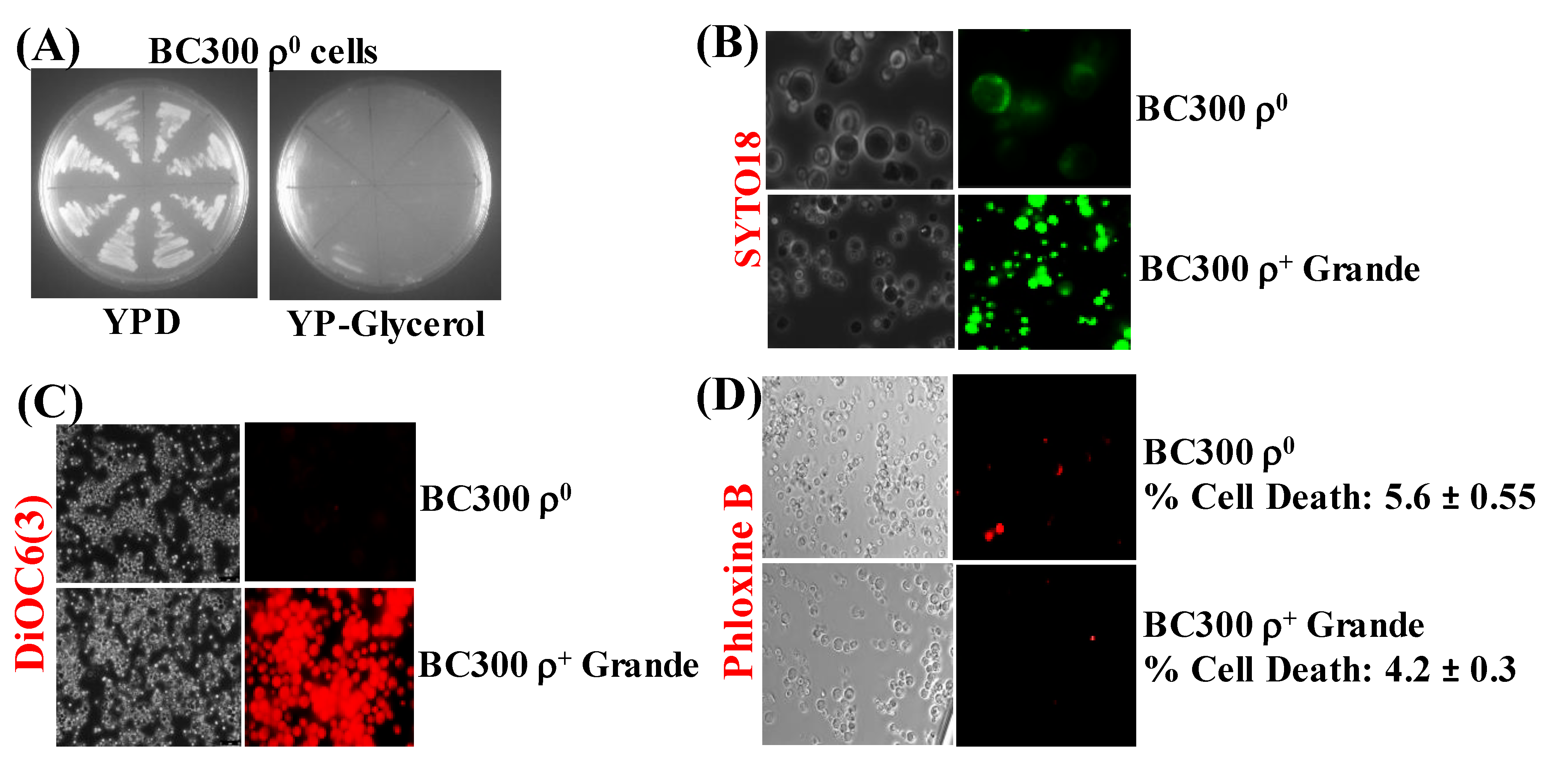
3.4. Yeast Petite Cells Generated from ρ+ Basic Yeast Strain Do Not Contain mtDNA or Functional Mitochondrial Membranes
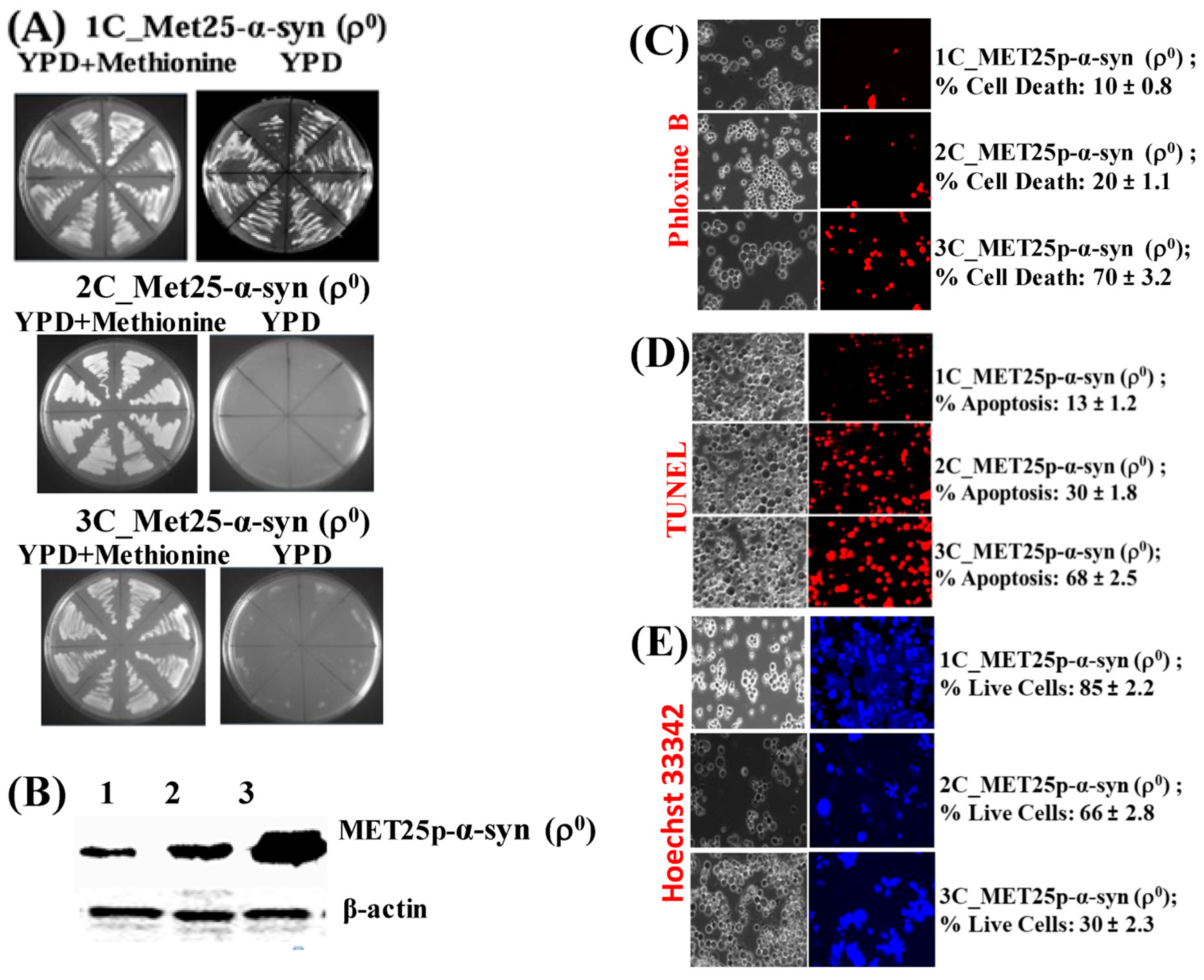
3.5. ρ0 Yeast Petite Cells, that Bear MET25p-α-Syn Integrative Plasmids, on Expression of α-Syn Undergo Cell Death and Nuclear DNA Fragmentation (Apoptosis)
3.6. Like ρ+ Cells, ρ0 and ρ− Cells Undergo Apoptosis When α-Syn is Expressed from the MET25 Promoter in Complete YPD Medium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eid, R.; Zhou, D.R.; Arab, N.T.T.; Boucher, E.; Young, P.G.; Mandato, C.A.; Greenwood, M.T. Heterologous expression of anti-apoptotic human 14-3-3β/α enhances iron-mediated programmed cell death in yeast. PLoS ONE 2017, 12, e0184151. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Acharya, L.; Zhu, N.; Cheng, J. An overview of bioinformatics methods for modeling biological pathways in yeast. Brief. Funct. Genom. 2015, 15, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Saberidokht, B.; Subramaniam, S.; Grama, A. Scope and limitations of yeast as a model organism for studying human tissue-specific pathways. BMC Syst. Boil. 2015, 9, 1–22. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, Y.; Wan, F.; Ma, K.; Guo, X.; Kou, L.; Yin, S.; Han, C.; Liu, L.; Huang, J.; et al. New Perspectives on Roles of Alpha-Synuclein in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, I.; Nakahara, A.; Ito, K. Morphology of mitochondrial nucleoids, mitochondria, and nuclei during meiosis and sporulation of the yeast Saccharomycodes ludwigii. J. Gen. Appl. Microbiol. 2012, 58, 43–51. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Gurnev, P.A.; Protchenko, O.; Hoogerheide, D.P.; Yap, T.L.; Philpott, C.C.; Lee, J.C.; Bezrukov, S.M. α-Synuclein Shows High Affinity Interaction with Voltage-dependent Anion Channel, Suggesting Mechanisms of Mitochondrial Regulation and Toxicity in Parkinson Disease. J. Boil. Chem. 2015, 290, 18467–18477. [Google Scholar] [CrossRef]
- Akintade, D.D.; Chaudhuri, B. Sensing the Generation of Intracellular Free Electrons Using the Inactive Catalytic Subunit of Cytochrome P450s as a Sink. Sensors 2020, 20, 4050. [Google Scholar] [CrossRef]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Teng, X.; Park, H.K.; Tucker, C.M.; Dunham, M.J.; Hardwick, J.M. Fis1 deficiency selects for compensatory mutations responsible for cell death and growth control defects. Cell Death Differ. 2008, 15, 1838–1846. [Google Scholar] [CrossRef]
- Roberts, G.G.; Hudson, A.P. Transcriptome profiling of Saccharomyces cerevisiae during a transition from fermentative to glycerol-based respiratory growth reveals extensive metabolic and structural remodeling. Mol. Genet. Genom. 2006, 276, 170–186. [Google Scholar] [CrossRef]
- Ernst, D.C.; Downs, D.M. Mmf1p Couples Amino Acid Metabolism to Mitochondrial DNA Maintenance in Saccharomyces cerevisiae. Mbio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Xie, X.; Liu, C.; Lim, K.-L.; Zhang, C.; Bai, L. The Sources of Reactive Oxygen Species and Its Possible Role in the Pathogenesis of Parkinson’s Disease. Park. Dis. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008, 7, 97–109. [Google Scholar] [CrossRef]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139, 216–231. [Google Scholar] [CrossRef]
- Luo, Y.; Hoffer, A.; Hoffer, B.; Qi, X. Mitochondria: A Therapeutic Target for Parkinson’s Disease? Int. J. Mol. Sci. 2015, 16, 20704–20730. [Google Scholar] [CrossRef] [PubMed]
- Benskey, M.J.; Perez, R.G.; Manfredsson, F.P. The contribution of alpha synuclein to neuronal survival and function—Implications for Parkinson’s disease. J. Neurochem. 2016, 137, 331–359. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Südhof, T.C. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J. Neurosci. 2015, 35, 5221–5232. [Google Scholar] [CrossRef]
- Games, D.; Valera, E.; Spencer, B.; Rockenstein, E.; Mante, M.; Adame, A.; Patrick, C.; Ubhi, K.; Nuber, S.; Sacayon, P.; et al. Reducing C-Terminal-Truncated Alpha-Synuclein by Immunotherapy Attenuates Neurodegeneration and Propagation in Parkinson’s Disease-Like Models. J. Neurosci. 2014, 34, 9441–9454. [Google Scholar] [CrossRef]
- Chinta, S.J.; Mallajosyula, J.K.; Rane, A.; Andersen, J.K. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci. Lett. 2010, 486, 235–239. [Google Scholar] [CrossRef]
- Ghiglieri, V.; Calabrese, V.; Calabresi, P. Alpha-Synuclein: From Early Synaptic Dysfunction to Neurodegeneration. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef]
- Flagmeier, P.; Meisl, G.; Vendruscolo, M.; Knowles, T.P.J.; Dobson, C.M.; Buell, A.K.; Galvagnion, C. Mutations associated with familial Parkinson’s disease alter the initiation and amplification steps of α-synuclein aggregation. Proc. Natl. Acad. Sci. USA 2016, 113, 10328–10333. [Google Scholar] [CrossRef] [PubMed]
- Heude, M.; Fukuhara, H.; Moustacchi, E. Spontaneous and induced rho mutants of Saccharomyces cerevisiae: Patterns of loss of mitochondrial genetic markers. J. Bacteriol. 1979, 139, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Carl, S.M.; Swerdlow, R.H. Cytoplasmic hybrid (cybrid) cell lines as a practical model for mitochondriopathies. Redox Boil. 2014, 2, 619–631. [Google Scholar] [CrossRef]
- Kukat, A.; Kukat, C.; Brocher, J.; Schäfer, I.; Krohne, G.; Trounce, I.A.; Villani, G.; Seibel, P. Generation of ρ 0 cells utilizing a mitochondrially targeted restriction endonuclease and comparative analyses. Nucleic Acids Res. 2008, 36, e44. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moreno, M.; Hermida-Gómez, T.; Gallardo, M.E.; Dalmao-Fernández, A.; Rego-Perez, I.; Garesse, R.; Blanco, F.J. Generating Rho-0 Cells Using Mesenchymal Stem Cell Lines. PLoS ONE 2016, 11, e0164199. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Silva, J.P.; Gustafsson, C.M.; Rustin, P.; Larsson, N.-G. Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc. Natl. Acad. Sci. USA 2001, 98, 4038–4043. [Google Scholar] [CrossRef]
- Marchetti, P.A.; Susin, S.; Decaudin, D.; Gamen, S.; Castedo, M.; Hirsch, T.; Zamzami, N.; Naval, J.; Senik, A.; Kroemer, G. Apoptosis-associated derangement of mitochondrial function in cells lacking mitochondrial DNA. Cancer Res. 1996, 56, 2033–2038. [Google Scholar]
- Aouida, M.; Mekid, H.; Belhadj, O.; Mir, L.M.; Tounekti, O. Mitochondria-independent morphological and biochemical apoptotic alterations promoted by the anti-tumor agent bleomycin in Saccharomyces cerevisiae. Biochem. Cell Boil. 2007, 85, 49–55. [Google Scholar] [CrossRef]
- Franssens, V.; Boelen, E.; Anandhakumar, J.; Vanhelmont, T.; Büttner, S.; Winderickx, J. Yeast unfolds the road map toward α-synuclein-induced cell death. Cell Death Differ. 2009, 17, 746–753. [Google Scholar] [CrossRef]
- Greenhalf, W.; Stephan, C.; Chaudhuri, B. Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality in Saccharomyces cerevisiae. FEBS Lett. 1996, 380, 169–175. [Google Scholar] [CrossRef]
- Côrte-Real, M.; Madeo, F. Yeast Programed Cell Death and Aging. Front. Oncol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, C.W.; Ayscough, K.R. Actin-Induced Hyperactivation of the Ras Signaling Pathway Leads to Apoptosis in Saccharomyces cerevisiae. Mol. Cell. Boil. 2006, 26, 6487–6501. [Google Scholar] [CrossRef] [PubMed]
- Ludovico, P.; Sousa, M.J.; Silva, M.T.; Leäo, C.; Côrte-Real, M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology 2001, 147, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Severin, F.; Hyman, A.A. Pheromone Induces Programmed Cell Death in S. cerevisiae. Curr. Boil. 2002, 12, R233–R235. [Google Scholar] [CrossRef]
- Narasimhan, M.L.; Damsz, B.; Coca, M.A.; Ibeas, J.I.; Yun, D.-J.; Pardo, J.M.; Hasegawa, P.M.; Bressan, R.A. A Plant Defense Response Effector Induces Microbial Apoptosis. Mol. Cell. 2001, 8, 921–930. [Google Scholar] [CrossRef]
- Silva, R.D.; Sotoca, R.; Johansson, B.; Ludovico, P.; Sansonetty, F.; Silva, M.T.; Peinado, J.M.; Côrte-Real, M. Hyperosmotic stress induces metacaspase- and mitochondria-dependent apoptosis in Saccharomyces cerevisiae. Mol. Microbiol. 2005, 58, 824–834. [Google Scholar] [CrossRef]
- Pereira, C.; Chaves, S.R.; Alves, S.; Salin, B.; Camougrand, N.; Manon, S.; Sousa, M.J.; Côrte-Real, M. Mitochondrial degradation in acetic acid-induced yeast apoptosis: The role of Pep4 and the ADP/ATP carrier. Mol. Microbiol. 2010, 76, 1398–1410. [Google Scholar] [CrossRef]
- Alic, N.; Higgins, V.J.; Pichova, A.; Breitenbach, M.; Dawes, I.W.; Yao, Y.-L.; Yang, W.-M. Lipid Hydroperoxides Activate the Mitogen-activated Protein Kinase Mpk1p in Saccharomyces cerevisiae. J. Boil. Chem. 2003, 278, 41849–41855. [Google Scholar] [CrossRef]
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef]
- Panchal, K.; Tiwari, A.K. Mitochondrial dynamics, a key executioner in neurodegenerative diseases. Mitochondrion 2019, 47, 151–173. [Google Scholar] [CrossRef]
- Akintade, D.D.; Chaudhuri, B. The effect of copy number on α-synuclein’s toxicity and its protective role in Bax-induced apoptosis, in yeast. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Büttner, S.; Faes, L.; Reichelt, W.; Broeskamp, F.; Habernig, L.; Benke, S.; Kourtis, N.; Ruli, D.; Carmona-Gutierrez, D.; Eisenberg, T.; et al. The Ca2+/Mn2+ ion-pump PMR1 links elevation of cytosolic Ca2+ levels to α-synuclein toxicity in Parkinson’s disease models. Cell Death Differ. 2013, 20, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Goldring, E.S.I.; Grossman, L.; Krupnick, D.; Cryer, D.R.; Marmur, J. The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J. Mol. Boil. 1970, 52, 323–335. [Google Scholar] [CrossRef]
- Kawai, S.; Hashimoto, W.; Murata, K. Transformation of Saccharomyces cerevisiae and other fungi: Methods and possible underlying mechanism. Bioeng. Bugs. 2010, 1, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Cottet-Rousselle, C.; Ronot, X.; Leverve, X.; Mayol, J.-F. Cytometric assessment of mitochondria using fluorescent probes. Cytom. Part A 2011, 79, 405–425. [Google Scholar] [CrossRef]
- Swayne, T.C.; Lipkin, T.G.; Pon, L.A. Live-Cell Imaging of the Cytoskeleton and Mitochondrial–Cytoskeletal Interactions in Budding Yeast. Adv. Struct. Saf. Stud. 2009, 586, 41–68. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Zadrag-Tecza, R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014, 14, 1068–1079. [Google Scholar] [CrossRef]
- Derf, A.; Mudududdla, R.; Akintade, D.; Williams, I.S.; Abdullaha, M.; Chaudhuri, B.; Bharate, S.B. Nonantioxidant Tetramethoxystilbene Abrogates α-Synuclein-Induced Yeast Cell Death but Not That Triggered by the Bax or βA4 Peptide. ACS Omega. 2018, 3, 9513–9532. [Google Scholar] [CrossRef]
- Von Der Haar, T. Optimized Protein Extraction for Quantitative Proteomics of Yeasts. PLoS ONE 2007, 2, e1078. [Google Scholar] [CrossRef]
- Maya, D.; Quintero, M.J.; Muñoz-Centeno, M.D.L.C.; Chàvez, S.; Muñoz-Centeno, M.C. Systems for applied gene control in Saccharomyces cerevisiae. Biotechnol. Lett. 2008, 30, 979–987. [Google Scholar] [CrossRef]
- Peng, B.; Williams, T.; Henry, M.; Nielsen, L.K.; Vickers, C.E. Controlling heterologous gene expression in yeast cell factories on different carbon substrates and across the diauxic shift: A comparison of yeast promoter activities. Microb. Cell Factories 2015, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, E.; Amaral, M.; Marshall, L.; Huang, S.-G. A High-Throughput Assay for Mitochondrial Membrane Potential in Permeabilized Yeast Cells. Anal. Biochem. 2001, 293, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Falcone, C.; Mazzoni, C. External and internal triggers of cell death in yeast. Cell. Mol. Life Sci. 2016, 73, 2237–2250. [Google Scholar] [CrossRef] [PubMed]
- Renvoisé, M.; Bonhomme, L.; Davanture, M.; Valot, B.; Zivy, M.; Lemaire, C. Quantitative variations of the mitochondrial proteome and phosphoproteome during fermentative and respiratory growth in Saccharomyces cerevisiae. J. Proteom. 2014, 106, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.K.; Goodell, M.A. Detection of Hematopoietic Stem Cells by Flow Cytometry. Elsevier BV 2011, 103, 21–30. [Google Scholar]
- Akintade, D.D.; Chaudhuri, B. Identification of proteins involved in transcription/translation (eEF 1A1) as an inhibitor of Bax induced apoptosis. Mol. Boil. Rep. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Morais, V.A.; De Strooper, B. Mitochondria Dysfunction and Neurodegenerative Disorders: Cause or Consequence. J. Alzheimer’s Dis. 2010, 20, S255–S263. [Google Scholar] [CrossRef]
- Ganguly, G.; Chakrabarti, S.; Chatterjee, U.; Saso, L. Proteinopathy, oxidative stress and mitochondrial dysfunction: Cross talk in Alzheimer’s disease and Parkinson’s disease. Drug Des. Dev. Ther. 2017, 11, 797–810. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.-W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain. 2017, 10, 53. [Google Scholar] [CrossRef]
- Szklarz, L.K.S.; Kozjak-Pavlovic, V.; Vögtle, F.-N.; Chacinska, A.; Milenkovic, D.; Vogel, S.; Dürr, M.; Westermann, B.; Guiard, B.; Martinou, J.-C.; et al. Preprotein Transport Machineries of Yeast Mitochondrial Outer Membrane Are not Required for Bax-induced Release of Intermembrane Space Proteins. J. Mol. Boil. 2007, 368, 44–54. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akintade, D.D.; Chaudhuri, B. Apoptosis, Induced by Human α-Synuclein in Yeast, Can Occur Independent of Functional Mitochondria. Cells 2020, 9, 2203. https://doi.org/10.3390/cells9102203
Akintade DD, Chaudhuri B. Apoptosis, Induced by Human α-Synuclein in Yeast, Can Occur Independent of Functional Mitochondria. Cells. 2020; 9(10):2203. https://doi.org/10.3390/cells9102203
Chicago/Turabian StyleAkintade, Damilare D., and Bhabatosh Chaudhuri. 2020. "Apoptosis, Induced by Human α-Synuclein in Yeast, Can Occur Independent of Functional Mitochondria" Cells 9, no. 10: 2203. https://doi.org/10.3390/cells9102203
APA StyleAkintade, D. D., & Chaudhuri, B. (2020). Apoptosis, Induced by Human α-Synuclein in Yeast, Can Occur Independent of Functional Mitochondria. Cells, 9(10), 2203. https://doi.org/10.3390/cells9102203




