The Functioning of Na+-ATPases from Protozoan Parasites: Are These Pumps Targets for Antiparasitic Drugs?
Abstract
:1. Introduction
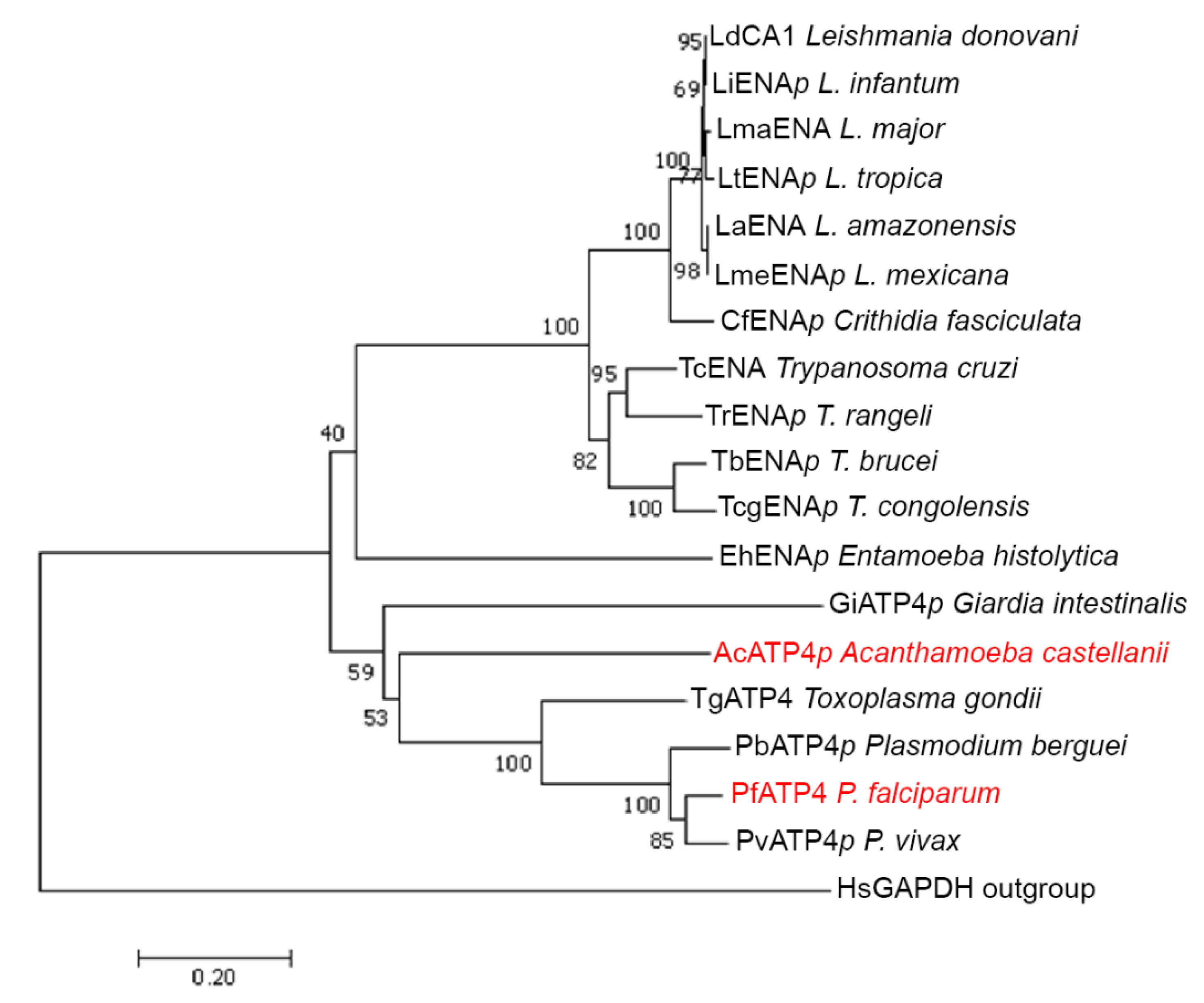
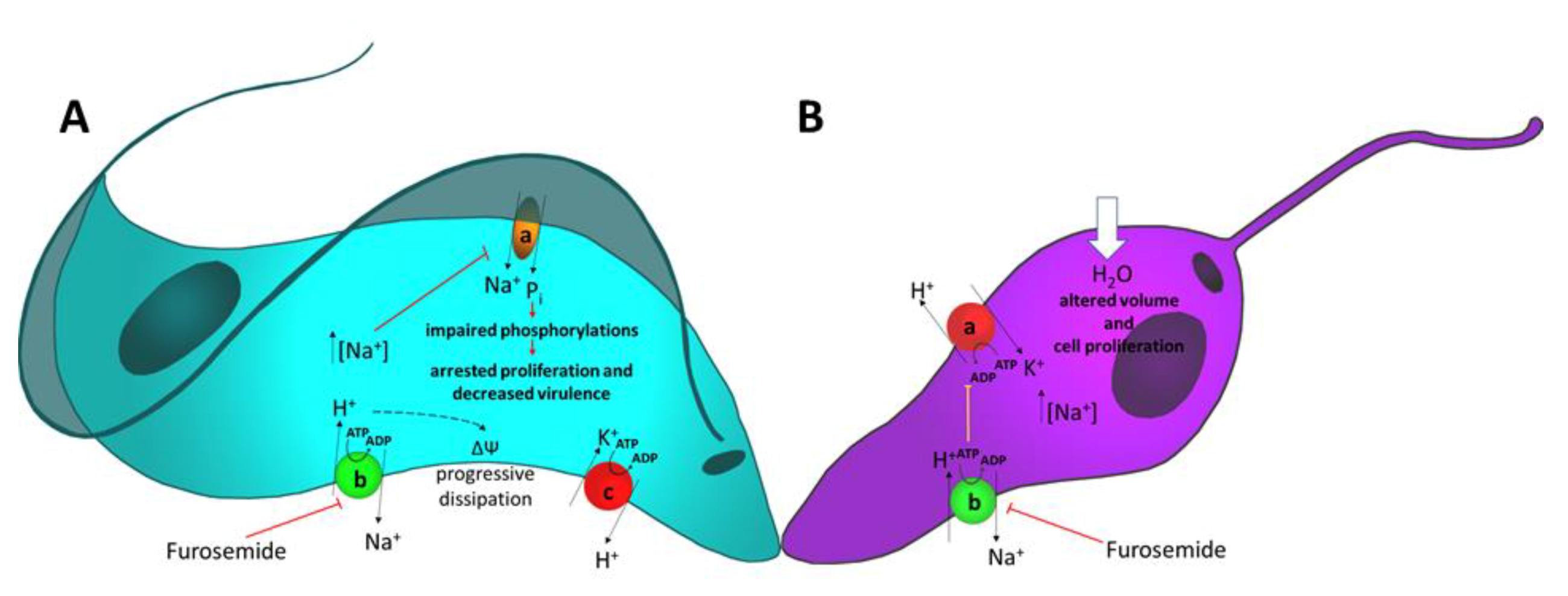
2. Trypanosomatid Parasites
3. Apicomplexan Parasites
4. Other Protozoan Parasites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beyenbach, K.W. Kidneys sans glomeruli. Am. J. Physiol. Ren. Physiol. 2004, 286, F811–F827. [Google Scholar] [CrossRef]
- Clausen, M.J.V.; Poulsen, H. Sodium/Potassium homeostasis in the cell. In Metallomics and the Cell, 1st ed.; Banci, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 41–67. [Google Scholar]
- Baldwin, E. An Introduction to Comparative Biochemistry, 4th ed.; Cambridge at the University Press: Cambridge, UK, 1964. [Google Scholar]
- Skou, J.C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta 1957, 23, 394–401. [Google Scholar] [CrossRef]
- Post, R.L.; Jolly, P.C. The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim. Biophys. Acta 1957, 25, 118–128. [Google Scholar] [CrossRef]
- Proverbio, F.; Condrescu-Guidi, M.; Whittembury, G. Ouabain-insensitive Na+ stimulation of an Mg2+-dependent ATPase in kidney tissue. Biochim. Biophys. Acta 1975, 394, 281–292. [Google Scholar] [CrossRef]
- Whittembury, G.; Proverbio, F. Two modes of Na+ extrusion in cells from guinea pig kidney cortex slices. Pflugers Arch. 1970, 316, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.E.; Burguillos, L.; del Castillo, J.R. Backdoor phosphorylation of small intestinal epithelial cells: Characterization of a furosemide-induced phosphoprotein related to the second sodium pump. Arch. Biochem. Biophys. 2003, 419, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Rocafull, M.A.; Thomas, L.E.; Del Castillo, J.R. The second sodium pump: From the function to the gene. Pflugers Arch. 2012, 463, 755–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieyra, A.; Silva, P.A.; Muzi-Filho, H.; Dick, C.F.; Araujo-dos-Santos, A.L.; Dias, J.; Vieira-Filho, L.D.; Paixão, A.D. The role of the second Na+ pump in mammals and parasites. In Regulation of Membrane Na+-K+ ATPase; Chakraborti, S., Dhalla, N.S., Eds.; Springer: Cham, Switzerland, 2016; pp. 93–112. [Google Scholar]
- Del Castillo, J.R.; Marín, R.; Proverbio, T.; Proverbio, F. Partial characterization of the ouabain- insensitive, Na+-stimulated ATPase activity of kidney basal-lateral plasma membranes. Biochim. Biophys. Acta 1982, 692, 61–68. [Google Scholar] [CrossRef]
- Rocafull, M.A.; Thomas, L.E.; Barrera, G.J.; Castillo, J.R. Differential expression of P-type ATPases in intestinal epithelial cells: Identification of putative new atp1a1 splice-variant. Biochem. Biophys. Res. Commun. 2010, 391, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Rocafull, M.A.; Romero, F.J.; Thomas, L.E.; del Castillo, J.R. Isolation and cloning of the K+-independent, ouabain-insensitive Na+-ATPase. Biochim. Biophys. Acta 2011, 1808, 1684–1700. [Google Scholar] [CrossRef] [Green Version]
- Caruso-Neves, C.; Einicker-Lamas, M.; Chagas, C.; Oliveira, M.M.; Vieyra, A.; Lopes, A.G. Ouabain-insensitive Na+-ATPase activity in Trypanosoma cruzi epimastigotes. Zeitschrift Naturforschung C 1999, 54, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Iizumi, K.; Mikami, Y.; Hashimoto, M.; Nara, T.; Hara, Y.; Aoki, T. Molecular cloning and characterization of ouabain-insensitive Na+-ATPase in the parasitic protist, Trypanosoma cruzi. Biochim. Biophys. Acta 2006, 1758, 738–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiles, J.K.; Kucerova, Z.; Sarfo, B.; Meade, C.A.; Thompson, W.; Shah, P.; Xue, L.; Meade, J.C. Identification of surface-membrane P-type ATPases resembling fungal K+- and Na+-ATPases, in Trypanosoma brucei, Trypanosoma cruzi and Leishmania donovani. Ann. Trop. Med. Parasitol. 2003, 97, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Dick, C.F.; Dos-Santos, A.L.A.; Majerowicz, D.; Gondim, K.C.; Caruso-Neves, C.; Silva, I.V.; Vieyra, A.; Meyer-Fernandes, J.R. Na+-dependent and Na+-independent mechanisms for inorganic phosphate uptake in Trypanosoma rangeli. Biochim. Biophys. Acta 2012, 1820, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Dick, C.F.; Dos-Santos, A.L.A.; Majerowicz, D.; Paes, L.S.; Giarola, N.L.; Gondim, K.C.; Vieyra, A.; Meyer-Fernandes, J.R. Inorganic phosphate uptake in Trypanosoma cruzi is coupled to K+ cycling and to active Na+ extrusion. Biochim. Biophys. Acta 2013, 1830, 4265–4273. [Google Scholar] [CrossRef]
- Spillman, N.J.; Allen, R.J.; Kirk, K. Na+ extrusion imposes an acid load on the intraerythrocytic malaria parasite. Mol. Biochem. Parasitol. 2013, 189, 1–4. [Google Scholar] [CrossRef]
- Lehane, A.M.; Dennis, A.S.M.; Bray, K.O.; Li, D.; Rajendran, E.; McCoy, J.M.; McArthur, H.M.; Winterberg, M.; Rahimi, F.; Tonkin, C.J.; et al. Characterization of the ATP4 ion pump in Toxoplasma gondii. J. Biol. Chem. 2019, 294, 5720–5734. [Google Scholar] [CrossRef] [Green Version]
- Lanza, M.; Haro, R.; Conchillo, L.B. The endophyte Serendipita indica reduces the sodium content of Arabidopsis plants exposed to salt stress: Fungal ENA ATPases are expressed and regulated at high pH and during plant co-cultivation in salinity. Environ. Microbiol. 2019, 21, 3364–3378. [Google Scholar] [CrossRef]
- Markina-Iñarrairaegui, A.; Spielvogel, A.; Etxebeste, O.; Ugalde, U.; Espeso, E.A. Tolerance to alkaline ambient pH in Aspergillus nidulans depends on the activity of ENA proteins. Sci. Rep. 2020, 10, 14325. [Google Scholar] [CrossRef]
- Axelsen, K.B.; Palmgren, M.G. Evolution of substrates specificities in the P-type ATPase superfamily. J. Mol. Evol. 1998, 46, 84–101. [Google Scholar] [CrossRef]
- Inesi, G.; Nakamoto, R.K. Special issue on transport ATPases. Arch. Biochem. Biophys. 2008, 476, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Navarro, A.; Benito, B. Sodium or potassium efflux ATPase a fungal, bryophyte, and protozoal ATPase. Biochim. Biophys. Acta 2010, 1798, 1841–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, L.A.; Sternberg, M.J.E. Protein structure prediction on the Web: A case study using the Phyre server. Nat. Protoc. 2009, 4, 363–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer-Fernandes, J.R.; Saad-Nehme, J.; Peres-Sampaio, C.E.; Belmont-Firpo, R.; Bisaggio, F.R.; do Couto, L.C.; Fonseca de Souza, A.L.; Lopes, A.H.S.C.; Souto-Padrón, T. A Mg-dependent ecto-ATPase is increased in the infective stages of Trypanosoma cruzi. Parasitol. Res. 2004, 93, 567–576. [Google Scholar] [CrossRef]
- Van Der Heyden, N.; Docampo, R. Proton and sodium pumps regulate the plasma membrane potential of different stages of Trypanosoma cruzi. Mol. Biochem. Parasitol. 2002, 120, 127–139. [Google Scholar] [CrossRef]
- Van der Heyden, N.; Docampo, R. Significant differences between procyclic and bloodstream forms of Trypanosoma brucei in the maintenance of their plasma membrane potential. J. Eukaryot. Microbiol. 2002, 49, 407–413. [Google Scholar] [CrossRef]
- Benito, B.; Garciadeblás, B.; Rodríguez-Navarro, A. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology 2002, 148, 933–941. [Google Scholar] [CrossRef] [Green Version]
- Steverding, D.; Sexton, D.W. Trypanocidal activity of salinomycin is due to sodium influx followed by cell swelling. Parasit. Vectors 2013, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- De Almeida-Amaral, E.E.; Caruso-Neves, C.; Pires, V.M.; Meyer-Fernandes, J.R. Leishmania amazonensis: Characterization of an ouabain-insensitive Na+-ATPase activity. Exp. Parasitol. 2008, 118, 165–171. [Google Scholar] [CrossRef]
- Arruda-Costa, N.; Escrivani, D.; Almeida-Amaral, E.E.; Meyer-Fernandes, J.R.; Rossi-Bergmann, B. Anti-parasitic effect of the diuretic and Na+-ATPase inhibitor furosemide in cutaneous leishmaniasis. Parasitology 2017, 144, 1375–1383. [Google Scholar] [CrossRef]
- Kirk, K.; Horner, H.A. Novel anion dependence of induced cation transport in malaria-infected erythrocytes. J. Biol. Chem. 1995, 270, 24270–24275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirk, K. Membrane transport in the malaria-infected erythrocyte. Physiol. Rev. 2001, 81, 495–537. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.; Lehane, A.M. Membrane transport in the malaria parasite and its host erythrocyte. Biochem. J. 2014, 457, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K. Ion Regulation in the Malaria Parasite. Annu. Rev. Microbiol. 2015, 69, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.D.; Addo, R.; Sharma, P.; Nguitragool, W.; Srinivasan, P.; Desai, S.A. Malaria parasites tolerate a broad range of ionic environments and do not require host cation remodelling. Mol. Microbiol. 2013, 88, 20–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staines, H.M.; Ellory, J.C.; Kirk, K. Perturbation of the pump-leak balance for Na+ and K+ in malaria-infected erythrocytes. Am. J. Physiol. Cell Physiol. 2001, 280, C1576–C1587. [Google Scholar] [CrossRef] [Green Version]
- Saliba, K.J.; Martin, R.E.; Bröer, A.; Henry, R.I.; McCarthy, C.S.; Downie, M.J.; Allen, R.J.; Mullin, K.A.; McFadden, G.I.; Bröer, S.; et al. Sodium-dependent uptake of inorganic phosphate by the intracellular malaria parasite. Nature 2006, 443, 582–585. [Google Scholar] [CrossRef]
- Krishna, S.; Woodrow, C.; Webb, R.; Penny, J.; Takeyasu, K.; Kimura, M.; East, J.M. Expression and functional characterization of a Plasmodium falciparum Ca2+-ATPase (PfATP4) belonging to a subclass unique to apicomplexan organisms. J. Biol. Chem. 2001, 276, 10782–10787. [Google Scholar] [CrossRef] [Green Version]
- Rottmann, M.; McNamara, C.; Yeung, B.K.; Lee, M.C.S.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D.M.; Dharia, N.V.; Tan, J.; et al. Spiroindolones, a potent compound class for the treatment of malaria. Science 2010, 329, 1175–1180. [Google Scholar] [CrossRef] [Green Version]
- Spillman, N.J.; Kirk, K. The malaria parasite cation ATPase PfATP4 and its role in the mechanism of action of a new arsenal of antimalarial drugs. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Spillman, N.J.; Allen, R.J.; McNamara, C.W.; Yeung, B.K.; Winzeler, E.A.; Diagana, T.T.; Kirk, K. Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 2013, 13, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Díaz, M.B.; Ebert, D.; Salinas, Y.; Pradhan, A.; Lehane, A.M.; Myrand-Lapierre, M.E.; O’Loughlin, K.G.; Shackleford, D.M.; De Almeida, M.J.; Carrillo, A.K.; et al. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc. Natl. Acad. Sci. USA 2014, 111, E5455–E5462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flannery, E.L.; McNamara Case, W.; Kim, S.W.; Kato, T.S.; Li, F.; Teng, C.H.; Kerstin Gagaring, K.; Manary, M.J.; Rachel Barboa, R.; Meister, S.; et al. Mutations in the P Type cation-transporter ATPase 4, PfATP4, mediate resistance to both aminopyrazole and spiroindolone antimalarials. ACS Chem. Biol. 2015, 10, 413–420. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Pukrittayakamee, S.; Phyo, A.P.; Rueangweerayut, R.; Nosten, F.; Jittamala, P.; Jeeyapant, A.; Jain, J.P.; Lefèvre, G.; Li, R.; et al. Spiroindolone KAE609 for Falciparum and Vivax malaria. N. Engl. J. Med. 2014, 2014. 371, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Suwanarusk, R.; Malleret, B.; Cooke, B.M.; Nosten, F.; Lau, Y.L.; Dao, M.; Lim, C.T.; Renia, L.; Tan, K.S.W.; et al. A Basis for rapid clearance of circulating ring-stage malaria parasites by the spiroindolone KAE609. J. Infect. Dis. 2016, 213, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Rosling, J.E.O.; Ridgway, M.C.; Summers, R.L.; Kirk, K.; Lehane, A.M. Biochemical characterization and chemical inhibition of PfATP4-associated Na+-ATPase activity in Plasmodium falciparum membranes. J. Biol. Chem. 2018, 293, 13327–13337. [Google Scholar] [CrossRef] [Green Version]
- Dennis, A.S.M.; Lehane, A.M.; Ridgway, M.C.; Holleran, J.P.; Kirk, K. Cell swelling induced by the antimalarial KAE609 (Cipargamin) and other PfATP4-associated antimalarials. Antimicrob. Agents Chemother. 2018, 62, e00087-18. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, A.B.; Morrisey, J.M.; Zhang, Z.; Das, S.; Daly, T.M.; Otto, T.D.; Spillman, N.J.; Wyvratt, M.; Siegl, P.; Marfurt, J.; et al. Pyrazoleamide compounds are potent antimalarials that target Na+ homeostasis in intraerythrocytic Plasmodium falciparum. Nat. Commun. 2014, 5, 5521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Bhatanagar, S.; Morrisey, J.M.; Daly, T.M.; Burns, J.M., Jr.; Coppens, I.; Vaidya, A.B. Na+ Influx induced by new antimalarials causes rapid alterations in the cholesterol content and morphology of Plasmodium falciparum. PLoS Pathog. 2016, 12, e1005647. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, S.A.; Zoleko-Manego, R.; Renner, K.C.; Schmitt, E.K.; Mombo-Ngoma, G.; Grobusch, M.P. The early preclinical and clinical development of cipargamin (KAE609), a novel antimalarial compound. Travel Med. Infect. Dis. 2020, 101765. [Google Scholar] [CrossRef]
- Leong, F.J.; Li, R.; Jain, J.P.; Lefèvre, G.; Magnusson, B.; Diagana, T.T.; Pertel, P. A first-in-human randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study of novel antimalarial Spiroindolone KAE609 (Cipargamin) to assess its safety, tolerability, and pharmacokinetics in healthy adult volunteers. Antimicrob. Agents Chemother. 2014, 58, 6209–6214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, D.S.; Jain, J.P.; Kangas, M.; Lefèvre, G.; Machineni, S.; Griffin, P.; Lickliter, J. Open-label, single-dose, parallel-group study in healthy volunteers to determine the drug-drug interaction potential between KAE609 (cipargamin) and piperaquine. Antimicrob. Agents Chemother. 2015, 59, 3493–3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huskey, S.E.; Zhu, C.Q.; Fredenhagen, A.; Kühnöl, J.; Luneau, A.; Jian, Z.; Yang, Z.; Miao, Z.; Yang, F.; Jain, J.P.; et al. 2KAE609 (Cipargamin), a new spiroindolone agent for the treatment of malaria: Evaluation of the absorption, distribution, metabolism, and excretion of a single oral 300-mg dose of [14C]KAE609 in healthy male subjects. Drug Metab. Dispos. 2016, 44, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Hien, T.T.; White, N.J.; Thuy-Nhien, N.T.; Hoa, N.T.; Thuan, P.D.; Tarning, J.; Nosten, F.; Magnusson, B.; Jain, J.P.; Hamed, K. Estimation of the in vivo MIC of cipargamin in uncomplicated Plasmodium falciparum malaria. Antimicrob. Agents Chemother. 2017, 61, e01940-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novartis Clinical Trials Results CKAE609X2202. An Open Label, Single Dose Study to Assess Efficacy, Safety, Tolerability and Pharmacokinetics of KAE609 in Adult Patients with Acute, Uncomplicated Plasmodium falciparum Malaria Mono-Infection. Available online: https://www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=14013 (accessed on 1 September 2020).
- Novartis Clinical Trials Results CKAE609A2109. A Phase I interventional, Sequential, Single-Site Study to Characterize the Effectiveness of Oral KAE609 in Reducing Assexual and Sexual Blood-Stage P. falciparum Following Inoculation in Healthy Volunteers and Subsequent Infectivity to Mosquitoes. Available online: https://www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=17484 (accessed on 1 September 2020).
- Chughlay, M.F.; Akakpo, S.; Odedra, A.; Odedra, A.; Csermak-Renner, K.; Djeriou, E.; Winnips, C.; Leboulleux, D.; Gaur, A.H.; Shanks, G.D.; et al. Liver enzyme elevations in Plasmodium falciparum volunteer infection studies: Findings and recommendations. Am. J. Trop. Med. Hyg. 2020, 103, 378–393. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Fomovska, A.; Muench, S.; Lai, B.S.; Mui, E.; McLeod, R. Spiroindolone that inhibits PfATPase4 is a potent, cidal inhibitor of Toxoplasma gondii tachyzoites in vitro and in vivo. Antimicrob. Agents Chemother. 2014, 58, 1789–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagini, G.A.; Lloyd, D.; Kirk, K.; Edwards, M.R. The membrane potential of Giardia intestinalis. FEMS Microbiol. Lett. 2000, 192, 153–157. [Google Scholar] [CrossRef]
- Biagini, G.A.; Knodler, L.A.; Saliba, K.J.; Kirk, K.; Edwards, M.R. Na+-dependent pH regulation by the amitochondriate protozoan parasite Giardia intestinalis. J. Biol. Chem. 2001, 276, 29157–29162. [Google Scholar] [CrossRef] [Green Version]
- Bakker-Grunwald, T.; Löhden, U.; Trissl, D. Effects of cytochalasin B on Na+ content and cell volume of Entamoeba histolytica. Biochim. Biophys. Acta 1985, 815, 170–174. [Google Scholar] [CrossRef]
- Bakker-Grunwald, T.; Keller, F.; Trissl, D. Effects of amiloride on Na+ content and pinocytosis in Entamoeba histolytica. Biochim. Biophys. Acta 1986, 854, 265–269. [Google Scholar] [CrossRef]
- Siddiqui, R.; Roberts, S.K.; Ong, T.Y.Y.; Mungroo, M.R.; Anwar, A.; Khan, N.A. Novel insights into the potential role of ion transport in sensory perception in Acanthamoeba. Parasit. Vectors 2019, 12, 538. [Google Scholar] [CrossRef] [PubMed]
- Lefurgey, A.; Gannon, M.; Blum, J.; Ingram, P. Leishmania donovani amastigotes mobilize organic and inorganic osmolytes during regulatory volume decrease. J. Eukaryot. Microbiol. 2005, 52, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Russo-Abrahão, T.; Alves-Bezerra, M.; Majerowicz, D.; Freitas-Mesquita, A.L.; Dick, C.F.; Gondim, K.C.; Meyer-Fernandes, J.R. Transport of inorganic phosphate in Leishmania infantum and compensatory regulation at low inorganic phosphate concentration. Biochim. Biophys. Acta 2013, 1830, 2683–2689. [Google Scholar] [CrossRef] [PubMed]

| Author | Phase | Target | Population | Healthy/Infected | Reference |
|---|---|---|---|---|---|
| NJ White | 2 | PfATP4 Na+-ATPase | Adults | Uncomplicated P. vivax or P. falciparum malaria | [47] |
| FJ Leong | 1 | PfATP4 Na+-ATPase | Male adults | Healthy | [54] |
| DS Stein | 1 | Male adults | Healthy | [55] | |
| SW Huskey | 1 | Adults | Healthy | [56] | |
| TT Hien | 2a | Male adults | P. falciparum malaria | [57] | |
| Study CKAE609X2202 | 2 | Adults | P. falciparum malaria | [58] | |
| Study CKAE609A2109 | 1 | Adults | Human challenged model-induced P. falciparum malaria in healthy adults | [59] | |
| MF Chuglay CNCT0025L3086 | 2 | Adults | [60] | ||
| SAM Bouwman | The authors searched for preclinical studies and clinical trials using cipargamin | [53] | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dick, C.F.; Meyer-Fernandes, J.R.; Vieyra, A. The Functioning of Na+-ATPases from Protozoan Parasites: Are These Pumps Targets for Antiparasitic Drugs? Cells 2020, 9, 2225. https://doi.org/10.3390/cells9102225
Dick CF, Meyer-Fernandes JR, Vieyra A. The Functioning of Na+-ATPases from Protozoan Parasites: Are These Pumps Targets for Antiparasitic Drugs? Cells. 2020; 9(10):2225. https://doi.org/10.3390/cells9102225
Chicago/Turabian StyleDick, Claudia F., José Roberto Meyer-Fernandes, and Adalberto Vieyra. 2020. "The Functioning of Na+-ATPases from Protozoan Parasites: Are These Pumps Targets for Antiparasitic Drugs?" Cells 9, no. 10: 2225. https://doi.org/10.3390/cells9102225
APA StyleDick, C. F., Meyer-Fernandes, J. R., & Vieyra, A. (2020). The Functioning of Na+-ATPases from Protozoan Parasites: Are These Pumps Targets for Antiparasitic Drugs? Cells, 9(10), 2225. https://doi.org/10.3390/cells9102225








