Long-Term Low-Dose Delta-9-Tetrahydrocannbinol (THC) Administration to Simian Immunodeficiency Virus (SIV) Infected Rhesus Macaques Stimulates the Release of Bioactive Blood Extracellular Vesicles (EVs) that Induce Divergent Structural Adaptations and Signaling Cues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care, Ethics and Experimental Procedures
2.2. Blood Sample Collection
2.3. Cells and Chemical Reagents
2.4. Isolation of Blood Extracellular Vesicles (BEVs)
2.5. Nanoparticle Tracking Analysis (NTA)
2.6. Western Blot Analysis of EV Cargo and Cellular Gene Expression
2.7. Viability Assay
2.8. Internalization of BEVs by Monocytes
2.9. Cell Spreading and Adhesion Assays
2.10. Analysis of Cytoskeletal Dynamics
2.11. Statistical Analysis
3. Results
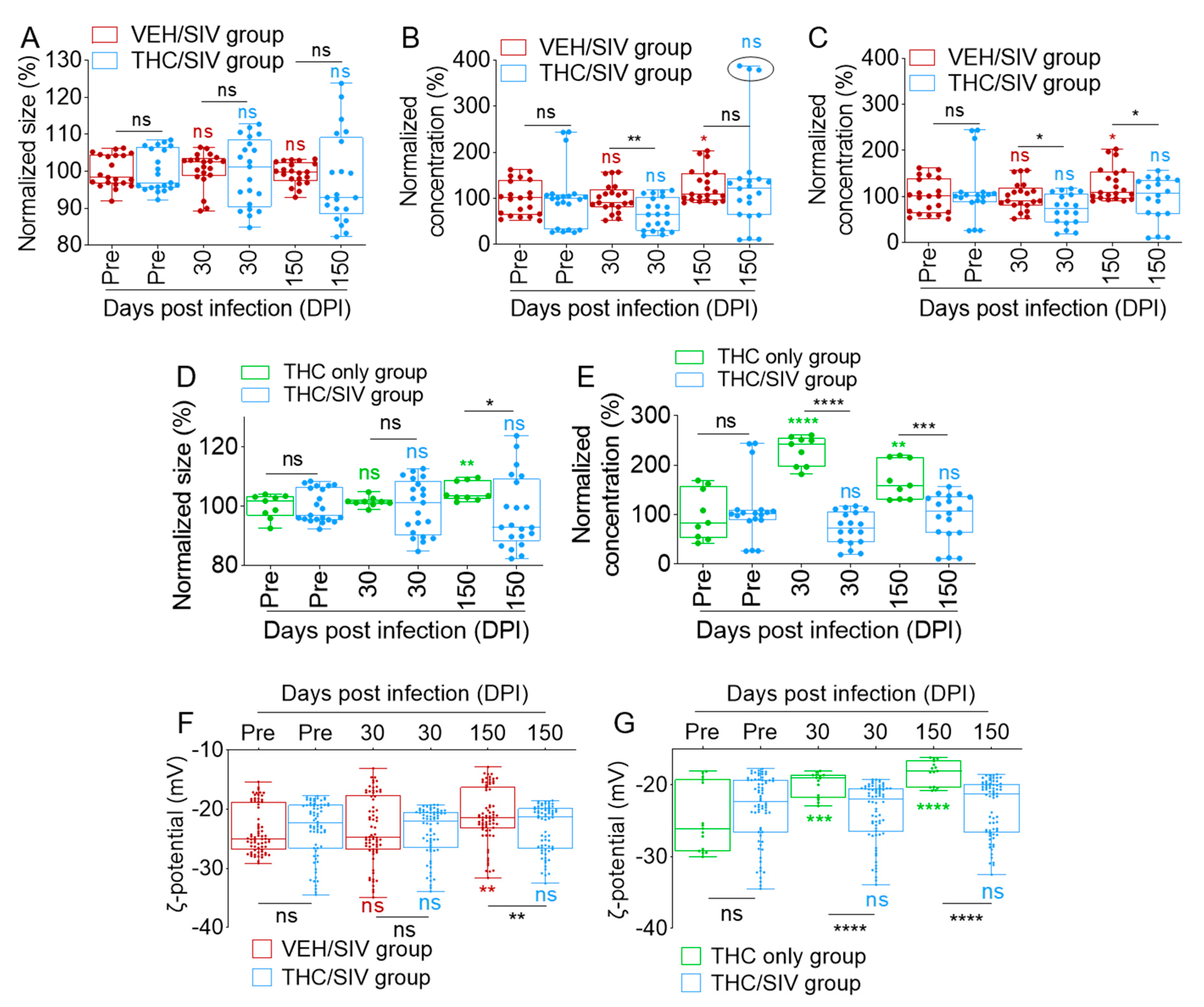
3.1. VEH/SIV and THC/SIV Rhesus Macaques Have Similar Blood Plasma Spectra
3.2. Long-Term Low-Dose THC Treatment Decreased BEV Concentration in SIV-Infected RMs
3.3. Infection Regulates the Levels of BEV-Associated Tetraspanins as Well as Other Protein Complexes
3.4. Human U937 Monocyte-Like Cells Tolerate VEH/SIV and THC/SIV BEVs
3.5. VEH/SIV and THC/SIV BEVs are Internalized by Human U937 Monocyte-Like Cells and the BEVs Modulate Cellular Gene Expression
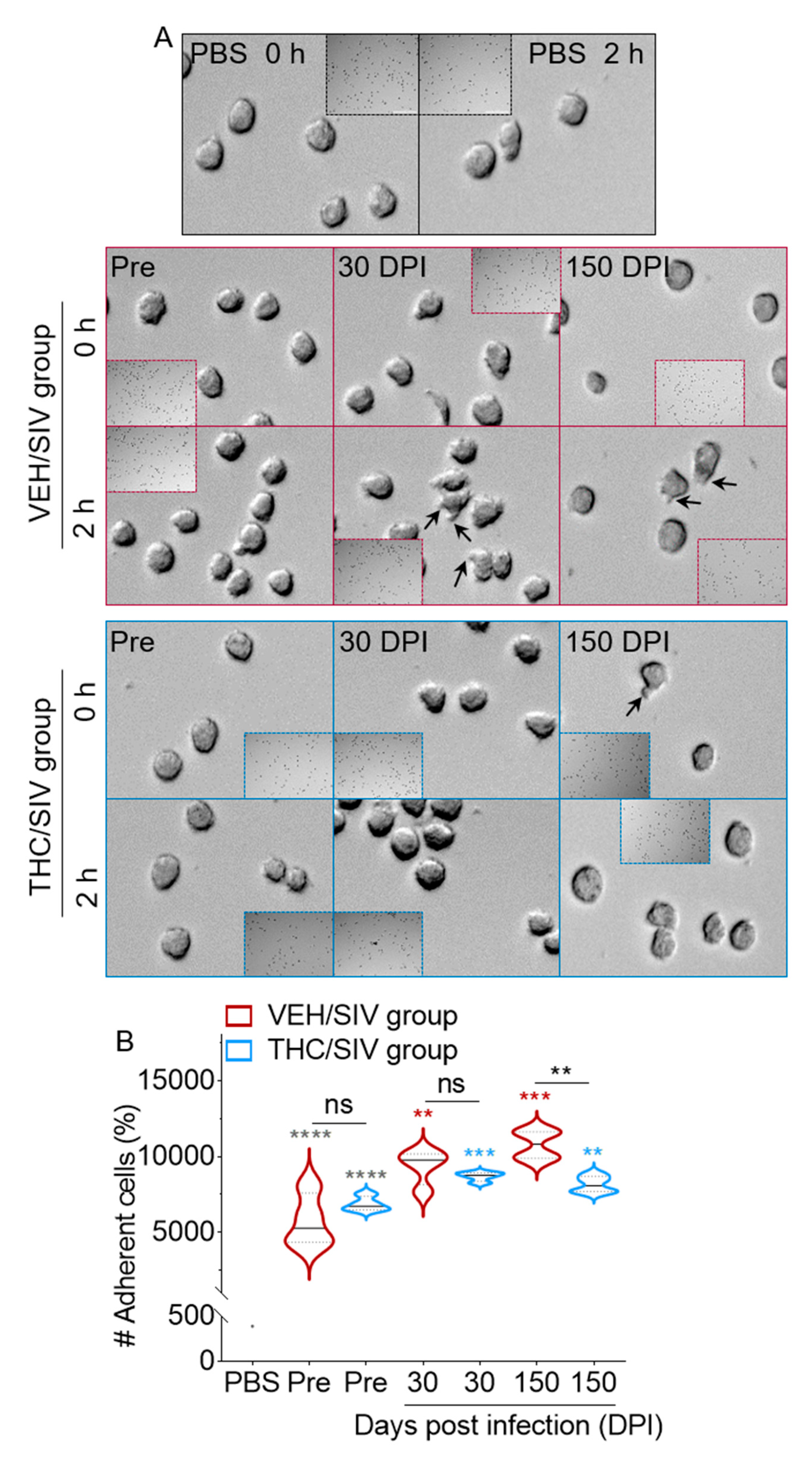
3.6. VEH/SIV BEVs Potentiate BEV-Mediated Human U937 Monocyte-Like Cell Spreading and Adhesion to Type I Collagen While THC/SIV BEVs dampen the effect
3.7. VEH/SIV and THC/SIV BEVs Induce Distinct Cytoskeletal Changes in Human U937 Monocyte-Like Cells
3.8. VEH/SIV and THC/SIV BEVs Mediate Divergent Signaling in Human U937 Monocyte-Like Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arévalo-Martín, A.; Vela, J.M.; Molina-Holgado, E.; Borrell, J.; Guaza, C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J. Neurosci. 2003, 23, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, B.; Talebi, S.; Toro, D.; Gonzalez, K.; Menoscal, J.P.; Shaw, R.; Hassen, G.W. Hyperthermia and severe rhabdomyolysis from synthetic cannabinoids. Am. J. Emerg. Med. 2016, 34, 121.E1–121.E2. [Google Scholar] [CrossRef] [PubMed]
- Rawls, S.M.; Tallarida, R.J.; Kon, D.A.; Geller, E.B.; Adler, M.W. GABAA receptors modulate cannabinoid-evoked hypothermia. Pharmacol. Biochem. Behav. 2004, 78, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.V.; Del Bel, E.A.; Guimarães, F.S. Cannabidiol attenuates catalepsy induced by distinct pharmacological mechanisms via 5-HT1A receptor activation in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 43–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonego, A.B.; Gomes, F.V.; Del Bel, E.A.; Guimaraes, F.S. Cannabidiol attenuates haloperidol-induced catalepsy and c-Fos protein expression in the dorsolateral striatum via 5-HT1A receptors in mice. Behav. Brain Res. 2016, 309, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zurier, R.B.; Burstein, S.H. Cannabinoids, inflammation, and fibrosis. Faseb J. 2016, 30, 3682–3689. [Google Scholar] [CrossRef] [Green Version]
- Romero-Zerbo, S.Y.; García-Fernández, M.; Espinosa-Jiménez, V.; Pozo-Morales, M.; Escamilla-Sánchez, A.; Sánchez-Salido, L.; Lara, E.; Cobo-Vuilleumier, N.; Rafacho, A.; Olveira, G.; et al. The atypical cannabinoid Abn-CBD reduces inflammation and protects liver, pancreas, and adipose tissue in a mouse model of prediabetes and non-alcoholic fatty liver disease. Front. Endocrinol. 2020, 11, 103. [Google Scholar] [CrossRef]
- Chandra, L.C.; Kumar, V.; Torben, W.; Vande Stouwe, C.; Winsauer, P.; Amedee, A.; Molina, P.E.; Mohan, M. Chronic administration of Δ9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute simian immunodeficiency virus infection of rhesus macaques. J. Virol. 2015, 89, 1168–1181. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Torben, W.; Kenway, C.S.; Schiro, F.R.; Mohan, M. Longitudinal examination of the intestinal lamina propria cellular compartment of simian immunodeficiency virus-infected rhesus macaques provides broader and deeper insights into the link between aberrant microRNA expression and persistent immune activation. J. Virol. 2016, 90, 5003–5019. [Google Scholar]
- Kumar, V.; Torben, W.; Mansfield, J.; Alvarez, X.; Vande Stouwe, C.; Li, J.; Byrareddy, S.N.; Didier, P.J.; Pahar, B.; Molina, P.E.; et al. Cannabinoid attenuation of intestinal inflammation in chronic SIV-infected rhesus macaques involves T cell modulation and differential expression of micro-RNAs and pro-inflammatory genes. Front. Immunol. 2019, 10, 914. [Google Scholar] [CrossRef]
- Molina, P.E.; Amedee, A.M.; Lecapitaine, N.J.; Zabaleta, J.; Mohan, M.; Winsauer, P.J.; Vande Stouwe, C.; Mcgoey, R.R.; Auten, M.W.; Lamotte, L.; et al. Modulation of gut-specific mechanisms by chronic Δ (9)-tetrahydrocannabinol administration in male rhesus macaques infected with simian immunodeficiency virus: A systems biology analysis. Aids Res. Hum. Retrovir. 2014, 30, 567–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, L.; Song, K.; Vande Stouwe, C.; Hollenbach, A.; Amedee, A.; Mohan, M.; Winsauer, P.; Molina, P. Δ9-Tetrahydrocannabinol (Δ9-THC) promotes neuroimmune-modulatory microRNA profile in striatum of simian immunodeficiency virus (SIV)-infected macaques. J. Neuroimmune Pharmacol. 2016, 11, 192–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergeria, C.L.; Dolan, S.B.; Johnson, M.W.; Campbell, C.M.; Dunn, K.E. Evaluating the co-use of opioids and cannabis for pain among current users using hypothetical purchase tasks. J. Psychopharmacol. (Oxf. U.K.) 2020, 34, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N.A. Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef]
- Baban, B.; Khodadadi, H.; Vaibhav, K.; Marchetti, C.; Riccardi, C.; Mozaffari, M.S. Regulation of innate lymphoid cells in acute kidney injury: Crosstalk between cannabidiol and GILZ. J. Immunol. Res. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Greco, L.; Russo, V.; Rapino, C.; Germanio, C.D.; Fezza, F.; Bernabò, N.; Berardinelli, P.; Peserico, A.; Fazio, D.; Maccarrone, M.; et al. Characterization of endocannabinoid system and interleukin profiles in ovine AEC: Cannabinoid receptors type-1 and type-2 as key effectors of pro-inflammatory response. Cells 2020, 9, 1008. [Google Scholar] [CrossRef] [Green Version]
- Turcotte, C.; Archambault, A.S.; Dumais, É.; Martin, C.; Blanchet, M.R.; Bissonnette, E.; Ohashi, N.; Yamamoto, K.; Itoh, T.; Laviolette, M.; et al. Endocannabinoid hydrolysis inhibition unmasks that unsaturated fatty acids induce a robust biosynthesis of 2-arachidonoyl-glycerol and its congeners in human myeloid leukocytes. Faseb J. 2020, 34, 4253–4265. [Google Scholar] [CrossRef] [Green Version]
- Schatz, A.R.; Lee, M.; Condie, R.B.; Pulaski, J.T.; Kaminski, N.E. Cannabinoid receptors CB1 and CB2: A characterization of expression and adenylate cyclase modulation within the immune system. Toxicol. Appl. Pharmacol. 1997, 142, 278–287. [Google Scholar] [CrossRef]
- Haney, M.; Gunderson, E.W.; Rabkin, J.; Hart, C.L.; Vosburg, S.K.; Comer, S.D.; Foltin, R.W. Dronabinol and marijuana in HIV-positive marijuana smokers: Caloric intake, mood, and sleep. J. Acquir. Immune Defic. Syndr. 2007, 45, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Riggs, P.K.; Vaida, F.; Rossi, S.S.; Sorkin, L.S.; Gouaux, B.; Grant, I.; Ellis, R.J. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012, 1431, 46–52. [Google Scholar] [CrossRef]
- Kader, R.; Govender, R.; Seedat, S.; Koch, J.R.; Parry, C. Understanding the impact of hazardous and harmful use of alcohol and/or other drugs on ARV adherence and disease progression. PLoS ONE 2015, 10, e0125088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, A.M.; Heverling, H.; Pham, P.A.; Stolbach, A. A review of the toxicity of HIV medications. J. Am. Coll. Med. Toxicol. 2014, 10, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidot, D.C.; Lerner, B.; Gonzalez, R. Cannabis use, medication management and adherence among persons living with HIV. Aids Beh. 2017, 21, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.E.; Winsauer, P.; Zhang, P.; Walker, E.; Birke, L.; Amedee, A.; Stouwe, C.V.; Troxclair, D.; Mcgoey, R.; Varner, K.; et al. Cannabinoid administration attenuates the progression of simian immunodeficiency virus. Aids Res. Hum. Retrovir. 2011, 27, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Winsauer, P.J.; Molina, P.E.; Amedee, A.M.; Filipeanu, C.M.; Mcgoey, R.R.; Troxclair, D.A.; Walker, E.M.; Birke, L.L.; Stouwe, C.V.; Howard, J.M.; et al. Tolerance to chronic delta-9-tetrahydrocannabinol (Delta(9)-THC) in rhesus macaques infected with simian immunodeficiency virus. Exp. Clin. Psychopharmacol. 2011, 19, 154–172. [Google Scholar] [CrossRef] [Green Version]
- Milloy, M.J.; Marshall, B.; Kerr, T.; Richardson, L.; Hogg, R.; Guillemi, S.; Montaner, J.S.; Wood, E. High-intensity cannabis use associated with lower plasma human immunodeficiency virus-1 RNA viral load among recently infected people who use injection drugs. Drug Alcohol Rev. 2015, 34, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, M.D.; Crawford, R.B.; Henriquez, J.E.; Aldhamen, Y.A.; Gulick, P.; Amalfitano, A.; Kaminski, N.E. HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-gamma-inducible protein 10 levels compared with nonusing HIV patients. Aids 2018, 32, 419–429. [Google Scholar]
- Pertwee, R.G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010, 17, 1360–1381. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, E.D.; Dutra, R.C. Cannabinoid receptors as therapeutic targets for autoimmune diseases: Where do we stand? Drug Discov. Today 2019, 24, 1845–1853. [Google Scholar] [CrossRef]
- Purohit, V.; Rapaka, R.S.; Rutter, J. Cannabinoid receptor-2 and HIV-associated neurocognitive disorders. J. Neuroimmune Pharmacol. 2014, 9, 447–453. [Google Scholar] [CrossRef]
- Raborn, E.S.; Jamerson, M.; Marciano-Cabral, F.; Cabral, G.A. Cannabinoid inhibits HIV-1 Tat-stimulated adhesion of human monocyte-like cells to extracellular matrix proteins. Life Sci. 2014, 104, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laczkovics, C.; Kothgassner, O.D.; Felnhofer, A.; Klier, C.M. Cannabidiol treatment in an adolescent with multiple substance abuse, social anxiety and depression. Neuropsychiatrie 2020, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Kaddour, H.; Lyu, Y.; Welch, J.L.; Paromov, V.; Mandape, S.N.; Sakhare, S.S.; Pandhare, J.; Stapleton, J.T.; Pratap, S.; Dash, C.; et al. Proteomics profiling of autologous blood and semen exosomes from HIV-infected and uninfected individuals reveals compositional and functional variabilities. Mol. Cell Proteom. Mcp 2020, 19, 78–100. [Google Scholar] [CrossRef]
- Madison, M.N.; Jones, P.H.; Okeoma, C.M. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology 2015, 482, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Madison, M.N.; Roller, R.J.; Okeoma, C.M. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 2014, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Welch, J.L.; Kaddour, H.; Schlievert, P.M.; Stapleton, J.T.; Okeoma, C.M. Semen exosomes promote transcriptional silencing of HIV-1 by disrupting NF-kB/Sp1/Tat circuitry. J. Virol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Welch, J.L.; Kaddour, H.; Winchester, L.; Fletcher, C.V.; Stapleton, J.T.; Okeoma, C.M. Semen extracellular vesicles from HIV-1-Infected individuals inhibit HIV-1 replication in vitro, and extracellular vesicles carry antiretroviral drugs in vivo. J. Acquir. Immune Defic. Syndr. 2020, 83, 90–98. [Google Scholar] [CrossRef]
- Welch, J.L.; Kaufman, T.M.; Stapleton, J.T.; Okeoma, C.M. Semen exosomes inhibit HIV infection and HIV-induced proinflammatory cytokine production independent of the activation state of primary lymphocytes. Febs Lett. 2020, 594, 695–709. [Google Scholar] [CrossRef]
- Welch, J.L.; Madison, M.N.; Margolick, J.B.; Galvin, S.; Gupta, P.; Martinez-Maza, O.; Dash, C.; Okeoma, C.M. Effect of prolonged freezing of semen on exosome recovery and biologic activity. Sci. Rep. 2017, 7, 45034. [Google Scholar] [CrossRef] [Green Version]
- Welch, J.L.; Stapleton, J.T.; Okeoma, C.M. Vehicles of intercellular communication: Exosomes and HIV-1. J. Gen. Virol 2019, 100, 350. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kaddour, K.; Steven, K.; Panzner, T.D.; Nadia, S.; Eun-Young, K.; Jeremy, M.; Heather, M.; Martinez-Maza, O.; Joseph, B.; et al. Human immunodeficiency virus (HIV) infection and use of illicit substances promote secretion of semen exosomes that enhance monocyte adhesion and induce actin reorganization and chemotactic migration. Cells 2019, 8, 1027. [Google Scholar] [CrossRef] [Green Version]
- Madison, M.N.; Okeoma, C.M. Exosomes: Implications in HIV-1 pathogenesis. Viruses 2015, 7, 4093–4118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madison, M.N.; Welch, J.L.; Okeoma, C.M. Isolation of exosomes from semen for in vitro uptake and HIV-1 infection assays. Bio. Protoc. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Simons, M.; Raposo, G. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Lotvall, J.; Valadi, H. Cell to cell signalling via exosomes through esRNA. Cell Adhes. Migra. 2007, 1, 156–158. [Google Scholar] [CrossRef]
- Admyre, C.; Grunewald, J.; Thyberg, J.; Gripenback, S.; Tornling, G.; Eklund, A.; Scheynius, A.; Gabrielsson, S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur. Respir. J. 2003, 22, 578–583. [Google Scholar] [CrossRef]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filen, J.J.; Lahesmaa, R.; Norman, M.; Neve, E.P.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef]
- Baum, M.K.; Rafie, C.; Lai, S.; Sales, S.; Page, B.; Campa, A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J. Acquir. Immune Defic. Syndr. 2009, 50, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Caby, M.P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunolog. 2005, 17, 879–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanisamy, V.; Sharma, S.; Deshpande, A.; Zhou, H.; Gimzewski, J.; Wong, D.T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE 2010, 5, e8577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.A.; Daniel, R. Human vaginal fluid contains exosomes that have an inhibitory effect on an early step of the HIV-1 life cycle. Aids 2016, 30, 2611–2616. [Google Scholar] [CrossRef]
- Vojtech, L.; Woo, S.; Hughes, S.; Levy, C.; Ballweber, L.; Sauteraud, R.P.; Strobl, J.; Westerberg, K.; Gottardo, R.; Tewari, M.; et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014, 42, 7290–7304. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Yao, H.; Chaudhuri, A.D.; Duan, M.; Yelamanchili, S.V.; Wen, H.; Cheney, P.D.; Fox, H.S.; Buch, S. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death Dis. 2012, 3, e381. [Google Scholar] [CrossRef] [Green Version]
- Dominy, S.S.; Brown, J.N.; Ryder, M.I.; Gritsenko, M.; Jacobs, J.M.; Smith, R.D. Proteomic analysis of saliva in HIV-positive heroin addicts reveals proteins correlated with cognition. PLoS ONE 2014, 9, e89366. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, C.; Zhou, Y.; Luo, C.; Ou, J.; Li, J.; Mo, Z. Expression of microRNAs in the serum exosomes of methamphetamine-dependent rats vs. ketamine-dependent rats. Exp. Ther. Med. 2018, 15, 3369–3375. [Google Scholar] [CrossRef]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. Fems Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Kim, O.Y.; Gho, Y.S. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014, 47, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Mcnamara, R.P.; Costantini, L.M.; Myers, T.A.; Schouest, B.; Maness, N.J.; Griffith, J.D.; Damania, B.A.; Maclean, A.G.; Dittmer, D.P. Nef secretion into extracellular vesicles or exosomes is conserved across human and simian immunodeficiency viruses. MBio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Kaddour, H.; Lyu, Y.; Shouman, N.; Mohan, M.; Okeoma, C.M. Development of novel high-resolution size-guided turbidimetry-enabled particle purification liquid chromatography (PPLC): Extracellular vesicles and membraneless condensates in focus. Int. J. Mol. Sci. 2020, 21, 5361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Böker, K.O.; Lemus-Diaz, N.; Rinaldi Ferreira, R.; Schiller, L.; Schneider, S.; Gruber, J. The impact of the CD9 tetraspanin on lentivirus infectivity and exosome secretion. Mol. Ther.J. Am. Soc. Gene Ther. 2018, 26, 634–647. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Mó, M.; Tejedor, R.; Rousselle, P.; Sánchez -Madrid, F. Tetraspanins in intercellular adhesion of polarized epithelial cells: Spatial and functional relationship to integrins and cadherins. J. Cell. Sci. 2001, 114, 577–587. [Google Scholar]
- Cha, B.H.; Shin, S.R.; Leijten, J.; Li, Y.C.; Singh, S.; Liu, J.C.; Annabi, N.; Abdi, R.; Dokmeci, M.R.; Vrana, N.E.; et al. Integrin-mediated interactions control macrophage polarization in 3D hydrogels. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Kaji, K.; Oda, S.; Miyazaki, S.; Kudo, A. Infertility of CD9-deficient mouse eggs is reversed by mouse CD9, human CD9, or mouse CD81; polyadenylated mRNA injection developed for molecular analysis of sperm-egg fusion. Dev. Biol. 2002, 247, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Kaji, K.; Oda, S.; Shikano, T.; Ohnuki, T.; Uematsu, Y.; Sakagami, J.; Tada, N.; Miyazaki, S.; Kudo, A. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat. Genet. 2000, 24, 279–282. [Google Scholar] [CrossRef]
- Przewoźniak, M.; Czaplicka, I.; Czerwińska, A.M.; Markowska-Zagrajek, A.; Moraczewski, J.; Stremińska, W.; Jańczyk-Ilach, K.; Ciemerych, M.A.; Brzoska, E. Adhesion proteins—an impact on skeletal myoblast differentiation. PLoS ONE 2013, 8, e61760. [Google Scholar] [CrossRef] [Green Version]
- Gordón-Alonso, M.; Yañez-Mó, M.; Barreiro, O.; Alvarez, S.; Muñoz-Fernández, M.A.; Valenzuela-Fernández, A.; Sánchez-Madrid, F. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. J. Immunol. 2006, 177, 5129–5137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Kodys, K.; Babcock, G.J.; Szabo, G. CD81/CD9 tetraspanins aid plasmacytoid dendritic cells in recognition of hepatitis C virus-infected cells and induction of interferon-alpha. Hepatology 2013, 58, 940–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, Y.; Tachibana, I.; Miyado, K.; Kobayashi, M.; Miyazaki, T.; Funakoshi, T.; Kimura, H.; Yamane, H.; Saito, Y.; Goto, H.; et al. Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J. Cell. Biol. 2003, 161, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeringer, E.; Barta, T.; Schageman, J.; Cheng, A.; Vlassov, A.V. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef]
- Tippett, E.; Cameron, P.U.; Marsh, M.; Crowe, S.M. Characterization of tetraspanins CD9, CD53, CD63, and CD81 in monocytes and macrophages in HIV-1 infection. J. Leukoc. Biol. 2013, 93, 913–920. [Google Scholar] [CrossRef]
- Badolato, R.; Sozzani, S.; Malacarne, F.; Bresciani, S.; Fiorini, M.; Borsatti, A.; Albertini, A.; Mantovani, A.; Ugazio, A.G.; Notarangelo, L.D. Monocytes from Wiskott-Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J. Immunol. 1998, 161, 1026–1033. [Google Scholar]
- Meconi, S.; Jacomo, V.; Boquet, P.; Raoult, D.; Mege, J.L.; Capo, C. Coxiella burnetii induces reorganization of the actin cytoskeleton in human monocytes. Infect. Immun. 1998, 66, 5527–5533. [Google Scholar] [CrossRef] [Green Version]
- Otte, A.; Mandel, K.; Reinstrom, G.; Hass, R. Abolished adherence alters signaling pathways in phorbol ester-induced human U937 cells. Cell Commun. Signal. Ccs 2011, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Recher, C.; Ysebaert, L.; Beyne-Rauzy, O.; Mansat-De Mas, V.; Ruidavets, J.B.; Cariven, P.; Demur, C.; Payrastre, B.; Laurent, G.; Racaud-Sultan, C. Expression of focal adhesion kinase in acute myeloid leukemia is associated with enhanced blast migration, increased cellularity, and poor prognosis. Cancer Res. 2004, 64, 3191–3197. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Chen, Q.; Lai, X.; Zhu, C.; Chen, C.; Zhao, X.; Deng, R.; Xu, M.; Yuan, H.; Wang, Y.; et al. SUMOylation of Grb2 enhances the ERK activity by increasing its binding with Sos1. Mol. Cancer 2014, 13, 95. [Google Scholar] [CrossRef] [Green Version]
- Honma, M.; Higuchi, O.; Shirakata, M.; Yasuda, T.; Shibuya, H.; Iemura, S.; Natsume, T.; Yamanashi, Y. Dok-3 sequesters Grb2 and inhibits the Ras-Erk pathway downstream of protein-tyrosine kinases. Genes Cells 2006, 11, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Yin, X.M.; Dong, Z. Inhibition of Bid-induced apoptosis by Bcl-2 tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J. Biol. Chem. 2003, 278, 16992–16999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Kosgodage, U.S.; Mould, R.; Henley, A.B.; Nunn, A.V.; Guy, G.W.; Thomas, E.L.; Inal, J.M.; Bell, J.D.; Lange, S. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front. Pharmacol. 2018, 9, 889. [Google Scholar] [CrossRef] [Green Version]
- Jolly, C.; Mitar, I.; Sattentau, Q.J. Requirement for an intact T-cell actin and tubulin cytoskeleton for efficient assembly and spread of human immunodeficiency virus type 1. J. Virol. 2007, 81, 5547–5560. [Google Scholar] [CrossRef] [Green Version]
- Gouin, E.; Welch, M.D.; Cossart, P. Actin-based motility of intracellular pathogens. Curr. Opin. Microbiol. 2005, 8, 35–45. [Google Scholar] [CrossRef]
- Ospina Stella, A.; Turville, S. All-round manipulation of the actin cytoskeleton by HIV. Viruses 2018, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Low, H.; Mukhamedova, N.; Cui, H.L.; Mcsharry, B.P.; Avdic, S.; Hoang, A.; Ditiatkovski, M.; Liu, Y.; Fu, Y.; Meikle, P.J.; et al. Cytomegalovirus restructures lipid rafts via a US28/CDC42-mediated pathway, enhancing cholesterol efflux from host cells. Cell Rep. 2016, 16, 186–200. [Google Scholar] [CrossRef] [Green Version]
- Chichili, G.R.; Rodgers, W. Cytoskeleton-membrane interactions in membrane raft structure. Cell. Mole. Life Sci. Cmls 2009, 66, 2319–2328. [Google Scholar] [CrossRef] [Green Version]
- Mukhamedova, N.; Hoang, A.; Dragoljevic, D.; Dubrovsky, L.; Pushkarsky, T.; Low, H.; Ditiatkovski, M.; Fu, Y.; Ohkawa, R.; Meikle, P.J.; et al. Exosomes containing HIV protein Nef reorganize lipid rafts potentiating inflammatory response in bystander cells. PLoS Pathog. 2019, 15, e1007907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Baumgartner, M. Filopodia and membrane blebs drive efficient matrix invasion of macrophages transformed by the intracellular parasite Theileria annulata. PLoS ONE 2013, 8, e75577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafrenie, R.M.; Wahl, L.M.; Epstein, J.S.; Hewlett, I.K.; Yamada, K.M.; Dhawan, S. HIV-1-Tat protein promotes chemotaxis and invasive behavior by monocytes. J. Immunol. 1996, 157, 974–977. [Google Scholar] [PubMed]
- Campbell, J.H.; Hearps, A.C.; Martin, G.E.; Williams, K.C.; Crowe, S.M. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS 2014, 28, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Buch, S.J. Cannabinoid receptor 2 activation: A means to prevent monocyte-endothelium engagement. Am. J. Pathol. 2013, 183, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Ley, K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 2008, 18, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlaepfer, D.D.; Hanks, S.K.; Hunter, T.; Van Der Geer, P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 1994, 372, 786–791. [Google Scholar] [CrossRef]
- Morris, M.A.; Laverick, L.; Wei, W.; Davis, A.M.; O’neill, S.; Wood, L.; Wright, J.; Dawson, C.W.; Young, L.S. The EBV-encoded oncoprotein, LMP1, induces an epithelial-to-mesenchymal transition (EMT) via its CTAR1 domain through integrin-mediated ERK-MAPK signaling. Cancers 2018, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Yee, K.L.; Weaver, V.M.; Hammer, D.A. Integrin-mediated signalling through the MAP-kinase pathway. Iet Syst. Biol. 2008, 2, 8–15. [Google Scholar] [CrossRef]
- Clark, E.A.; King, W.G.; Brugge, J.S.; Symons, M.; Hynes, R.O. Integrin-mediated signals regulated by members of the rho family of GTPases. J. Cell. Biol. 1998, 142, 573–586. [Google Scholar] [CrossRef] [Green Version]
- Parsons, J.T. Integrin-mediated signalling: Regulation by protein tyrosine kinases and small GTP-binding proteins. Curr. Opin. Cell Biol. 1996, 8, 146–152. [Google Scholar] [CrossRef]
- Subramani, D.; Alahari, S.K. Integrin-mediated function of Rab GTPases in cancer progression. Mol. Cancer 2010, 9, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Tien, J.; Pirone, D.M.; Gray, D.S.; Bhadriraju, K.; Chen, C.S. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA 2003, 100, 1484–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Buckner, C.M.; Luers, A.J.; Calderon, T.M.; Eugenin, E.A.; Berman, J.W. Neuroimmunity and the blood-brain barrier: Molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. J. Neuroimmune Pharmacol. 2006, 1, 160–181. [Google Scholar] [CrossRef] [Green Version]
- Roberts, T.K.; Buckner, C.M.; Berman, J.W. Leukocyte transmigration across the blood-brain barrier: Perspectives on neuroAIDS. Front. Biosci. (Landmark Ed.) 2010, 15, 478–536. [Google Scholar] [CrossRef] [Green Version]
- Kuo, J.C. Mechanotransduction at focal adhesions: Integrating cytoskeletal mechanics in migrating cells. J. Cell. Mol. Med. 2013, 17, 704–712. [Google Scholar] [CrossRef]
- Roca-Cusachs, P.; Del Rio, A.; Puklin-Faucher, E.; Gauthier, N.C.; Biais, N.; Sheetz, M.P. Integrin-dependent force transmission to the extracellular matrix by alpha-actinin triggers adhesion maturation. Proc. Natl. Acad. Sci. USA 2013, 110, E1361–E1370. [Google Scholar] [CrossRef] [Green Version]
- Roca-Cusachs, P.; Gauthier, N.C.; Del Rio, A.; Sheetz, M.P. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc. Natl. Acad. Sci. USA 2009, 106, 16245–16250. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Peng, N.; Zhuang, H.; Zhang, D.; Wang, Y.; Hua, Z.C. Heat shock proteins HSP70 and MRJ cooperatively regulate cell adhesion and migration through urokinase receptor. Bmc Cancer 2014, 14, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevchenko, M.A.; Troyanova, N.I.; Servuli, E.A.; Bolkhovitina, E.L.; Fedorina, A.S.; Sapozhnikov, A.M. Study of Immunomodulatory Effects of Extracellular HSP70 in a Mouse Model of Allergic Airway Inflammation. Biochem. (Mosc.) 2016, 81, 1384–1395. [Google Scholar] [CrossRef] [PubMed]





| Animal ID | SIV Inoculum | Duration of Infection | Plasma Viral Loads 106/mL at 6 Months Post-SIV | Opportunistic Infections |
|---|---|---|---|---|
| Vehicle-treated SIV-infected rhesus macaques | ||||
| IH96 | SIVmac251 | 180 | 0.1 | ND |
| HV48 | SIVmac251 | 150 | 4 | ND |
| IN24 | SIVmac251 | 180 | 9.4 | ND |
| JC81 | SIVmac251 | 180 | 0.38 | ND |
| JH47 | SIVmac251 | 180 | 2 | ND |
| JR36 | SIVmac251 | 180 | 0.5 | ND |
| IV95 | SIVmac251 | 180 | 0.02 | ND |
| Delta-9-tetrahydrocannabinol-treated SIV-infected rhesus macaques | ||||
| IA83 | SIVmac251 | 180 | 1.5 | ND |
| IH69 | SIVmac251 | 180 | 0.06 | ND |
| HI09 | SIVmac251 | 180 | 0.01 | ND |
| JB82 | SIVmac251 | 180 | 7.7 | ND |
| IA04 | SIVmac251 | 150 | 0.66 | ND |
| JI45 | SIVmac251 | 180 | 3 | ND |
| JC85 | SIVmac251 | 180 | 0.02 | ND |
| Delta-9-tetrahydrocannabinol-treated SIV-uninfected rhesus macaques | ||||
| HN79 | NA | NA | NA | NA |
| HN39 | NA | NA | NA | NA |
| HI78 | NA | NA | NA | NA |
| Chemicals | Company | City, State, Country | CatLog Number |
|---|---|---|---|
| Roswell Park Memorial Institute (RPMI) 1640 | Corning | Corning, NY, USA | 10-040-CV |
| Fetal bovine serum (FBS) | Atlanta Biologicals | Flowery Branch, GA, USA | S11150 |
| Penicillin-streptomycin | Corning | Corning, NY, USA | 30-002-CI |
| Amphotericin B | Corning | Corning, NY, USA | 30-003-CF |
| Sodium pyruvate | Corning | Corning, NY, USA | 25-005-CI |
| l-glutamate | Corning | Corning, NY, USA | 25030081 |
| 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) | Research Products International | Mt Prospect, IL, USA | 30TY40 |
| Type I collagen, bovine | Corning | Corning, NY, USA | 354231 |
| 10× DPBS | Corning | Corning, NY, USA | 20-031-CV |
| Bradford reagent | Bio-Rad | Hercules, CA, USA | 5000006 |
| Trypan blue | Life Technologies | Carlsbad, CA, USA | 15250061 |
| SYTO RNASelect stain | Thermofisher | Grand Island, NY, USA | S32703 |
| AlexaFluor 594 Phalloidin | Thermofisher | Grand Island, NY, USA | A12381 |
| Triton X-100 | Sigma | St. Louis, MO, USA | T8532 |
| Paraformaldehyde (PFA) | Sigma | St. Louis, MO, USA | P6148 |
| NucBlue™ Live ReadyProbes™ reagent | Thermo Fisher Scientific | Waltham, MA, USA | R37605 |
| CD63 (H5C6) | Developmental Studies Hybridoma Bank (DSHB) | Iowa City, IA, USA | P08962 |
| CD9 (602.29 cl. 11) | Developmental Studies Hybridoma Bank (DSHB) | Iowa City, IA, USA | P21926 |
| CD81 | Novus Biologicals | Centennial, CO, USA | SN206-01 |
| HSP70 | R&D systems | Minneapolis, MN, USA | AF1663 |
| β-actin | Proteintech | Rosemont, IL, USA | 60008-1-Ig |
| Integrin β1 (D2E5) | Cell Signaling | Beverly, MA, USA | 9699 |
| Integrin α5 (D7B7G) | Cell Signaling | Beverly, MA, USA | 98204 |
| Phospho-FAK (Tyr397) (D20B1) | Cell Signaling | Beverly, MA, USA | 8556 |
| FAK | Cell Signaling | Beverly, MA, USA | 3285 |
| Phosphor-ERK1/2 (pMAPK) (Thr202/Tyr204) (197G2) | Cell Signaling | Beverly, MA, USA | 4377 |
| P44/42 MAPK (ERK1/2) (137F5) | Cell Signaling | Beverly, MA, USA | 4695 |
| Cleaved caspase 3 (cCas3) (Asp175) (5A1E) | Cell Signaling | Beverly, MA, USA | 9664 |
| Caspase 3 (Cas3) (D3R6Y) | Cell Signaling | Beverly, MA, USA | 14220 |
| Bid/tBid (human specific) | Cell Signaling | Beverly, MA, USA | 2002 |
| Ras (27H5) | Cell Signaling | Beverly, MA, USA | 3339 |
| Grb2 | Cell Signaling | Beverly, MA, USA | 3972 |
| IRDye® 800CW donkey anti-mouse IgG (H + L) | LI-COR | Lincoln, NE, USA | 926-32212 |
| IRDye® 800CW donkey anti-rabbit IgG (H + L) | LI-COR | Lincoln, NE, USA | 926-32213 |
| IRDye® 680RD donkey anti-mouse IgG (H + L) | LI-COR | Lincoln, NE, USA | 926-68072 |
| Exosome spin columns | Thermofisher | Grand Island, NY, USA | 4484449 |
| 96-well glass-bottom plate | Cellvis | Mountain View, CA, USA | P96-1.5P |
| PVDF membrane | Bio-Rad | Hercules, CA, USA | 1620177 |
| Sephadex G-50 fine beads | GE-Healthcare | Pittsburgh, PA, USA | 17004201 |
| Econo-column | Bio-Rad | Hercules, CA, USA | 7374721 |
| Synergy-H1 microplate reader | BioTek | Winooski, VT, USA | - |
| ZetaView PMX 110 | Particle Metrix | Mebane, NC, USA | - |
| Lionheart FX automated microscope | BioTek | Winooski, VT, USA | - |
| Luna-II automated cell counter | Logos Biosystems | Annandale, VA, USA | - |
| Odyssey infrared imaging system (LI-COR) | LI-COR | Lincoln, NE, USA | - |
| BEV Group | Concentration (µg) | Cell Viability (%) | p Value |
|---|---|---|---|
| VEH/SIV | 20 | 91.2 | 0.0133 |
| THC/SIV | 20 | 91.6 | 0.0186 |
| VEH/SIV | 40 | 78.7 | <0.0001 |
| THC/SIV | 40 | 81.2 | 0.0002 |
| VEH/SIV | 100 | 79.7 | 0.0005 |
| THC/SIV | 100 | 81.0 | 0.0010 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Y.; Kopcho, S.; Mohan, M.; Okeoma, C.M. Long-Term Low-Dose Delta-9-Tetrahydrocannbinol (THC) Administration to Simian Immunodeficiency Virus (SIV) Infected Rhesus Macaques Stimulates the Release of Bioactive Blood Extracellular Vesicles (EVs) that Induce Divergent Structural Adaptations and Signaling Cues. Cells 2020, 9, 2243. https://doi.org/10.3390/cells9102243
Lyu Y, Kopcho S, Mohan M, Okeoma CM. Long-Term Low-Dose Delta-9-Tetrahydrocannbinol (THC) Administration to Simian Immunodeficiency Virus (SIV) Infected Rhesus Macaques Stimulates the Release of Bioactive Blood Extracellular Vesicles (EVs) that Induce Divergent Structural Adaptations and Signaling Cues. Cells. 2020; 9(10):2243. https://doi.org/10.3390/cells9102243
Chicago/Turabian StyleLyu, Yuan, Steven Kopcho, Mahesh Mohan, and Chioma M. Okeoma. 2020. "Long-Term Low-Dose Delta-9-Tetrahydrocannbinol (THC) Administration to Simian Immunodeficiency Virus (SIV) Infected Rhesus Macaques Stimulates the Release of Bioactive Blood Extracellular Vesicles (EVs) that Induce Divergent Structural Adaptations and Signaling Cues" Cells 9, no. 10: 2243. https://doi.org/10.3390/cells9102243






