Involvement of Actin and Actin-Binding Proteins in Carcinogenesis
Abstract
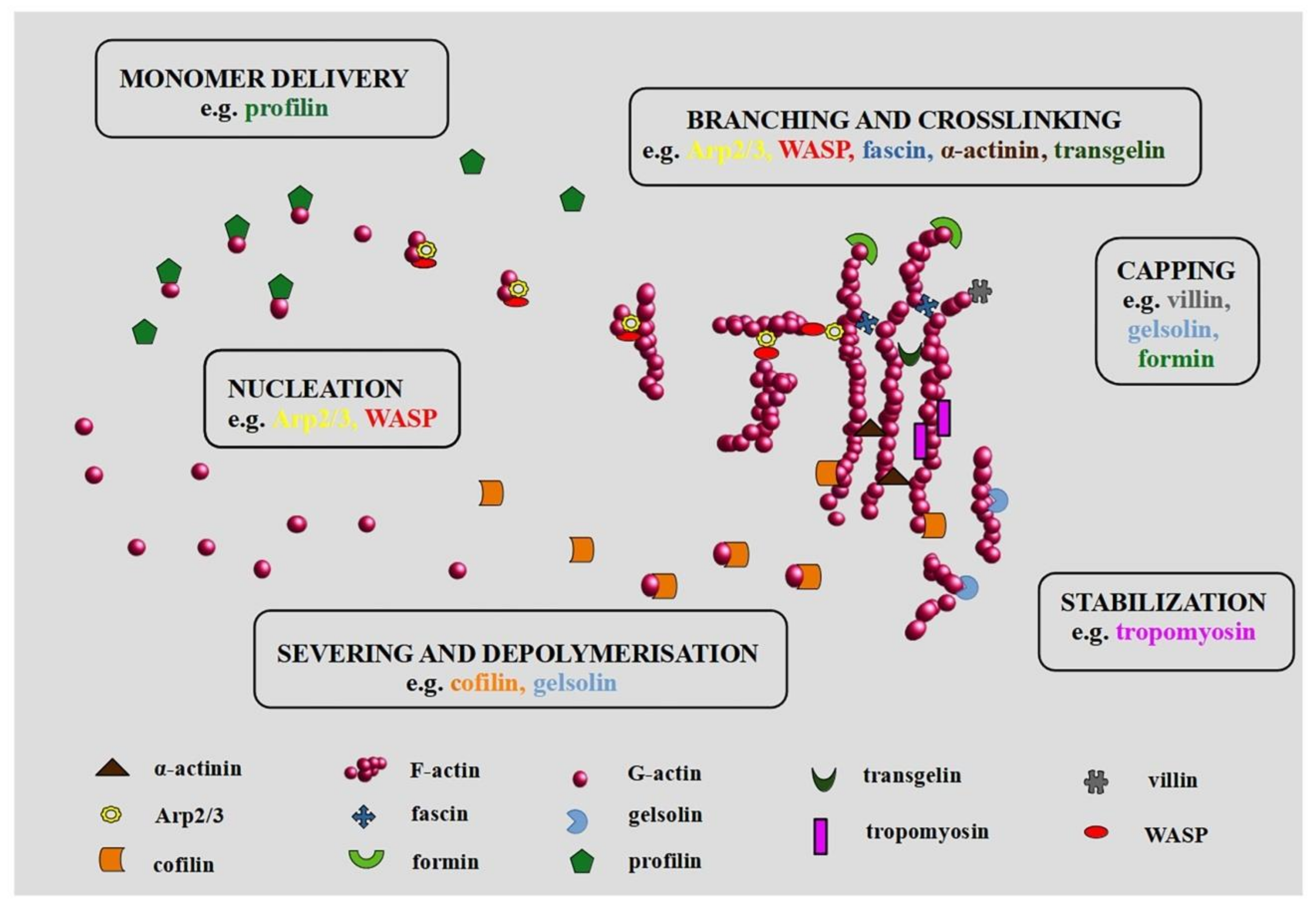
:1. Introduction
2. Cytoskeleton, Its Structure, Function, and Regulation
3. Actin and ABPs in Carcinogenesis
Actin in the Nucleus
4. The Involvement of Actin and ABPs in Cancer Progression (EMT and Metastasis)
5. Role of Actin in Angiogenesis and Vasculogenic Mimicry
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Taniguchi, H.; Fukagai, K.; Okamuro, S.; Kobayashi, A. Inhibitory Mechanism of FAT4 Gene Expression in Response to Actin Dynamics during Src-Induced Carcinogenesis. PLoS ONE 2015, 10, e0118336. [Google Scholar] [CrossRef] [Green Version]
- Izdebska, M.; Zielińska, W.; Grzanka, D.; Gagat, M. The Role of Actin Dynamics and Actin-Binding Proteins Expression in Epithelial-to-Mesenchymal Transition and Its Association with Cancer Progression and Evaluation of Possible Therapeutic Targets. BioMed Res. Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Remedios, C.G.; Chhabra, D.; Kekic, M.; Dedova, I.V.; Tsubakihara, M.; Berry, D.A.; Nosworthy, N.J. Actin Binding Proteins: Regulation of Cytoskeletal Microfilaments. Physiol. Rev. 2003, 83, 433–473. [Google Scholar] [CrossRef] [PubMed]
- Winder, S.J.; Ayscough, K.R. Actin-binding proteins. J. Cell Sci. 2005, 118, 651–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzanka, D.; Marszałek, A.; Izdebska, M.; Gackowska, L.; Szczepanski, M.A.; Grzanka, D. Actin Cytoskeleton Reorganization Correlates with Cofilin Nuclear Expression and Ultrastructural Changes in CHO AA8 Cell Line after Apoptosis and Mitotic Catastrophe Induction by Doxorubicin. Ultrastruct. Pathol. 2011, 35, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelleher, J.F.; Atkinson, S.J.; Pollard, T.D. Sequences, structural models, and cellular localization of the actin-related proteins Arp2 and Arp3 from Acanthamoeba. J. Cell Biol. 1995, 131, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Higgs, H.N.; Pollard, T.D. Regulation of actin filament network formation through ARP2/3 complex: Activation by a diverse array of proteins. Annu. Rev. Biochem. 2001, 70, 649–676. [Google Scholar] [CrossRef]
- Mullins, R.D.; Heuser, J.A.; Pollard, T.D. The interaction of Arp2/3 complex with actin: Nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA 1998, 95, 6181–6186. [Google Scholar] [CrossRef] [Green Version]
- Padrick, S.B.; Doolittle, L.K.; Brautigam, C.A.; King, D.S.; Rosen, M.K. Arp2/3 complex is bound and activated by two WASP proteins. Proc. Natl. Acad. Sci. USA 2011, 108, E472–E479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, A.M.; Karginov, A.V.; Kinley, A.W.; Weed, S.A.; Li, Y.; Parsons, J.; Cooper, J.A. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 2001, 11, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Dawes, A.T.; Ermentrout, G.B.; Cytrynbaum, E.N.; Edelstein-Keshet, L. Actin filament branching and protrusion velocity in a simple 1D model of a motile cell. J. Theor. Biol. 2006, 242, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Suraneni, P.; Rubinstein, B.; Unruh, J.R.; Durnin, M.; Hanein, D.; Li, R. The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J. Cell Biol. 2012, 197, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Suarez, C.; Kovar, D.R. Internetwork competition for monomers governs actin cytoskeleton organization. Nat. Rev. Mol. Cell Biol. 2016, 17, 799–810. [Google Scholar] [CrossRef] [Green Version]
- Pernier, J.; Shekhar, S.; Jegou, A.; Guichard, B.; Carlier, M.-F. Profilin Interaction with Actin Filament Barbed End Controls Dynamic Instability, Capping, Branching, and Motility. Dev. Cell 2016, 36, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Leyrat, C.; Grimes, J.M.; Robinson, R.C. Structural basis of thymosin- 4/profilin exchange leading to actin filament polymerization. Proc. Natl. Acad. Sci. USA 2014, 111, E4596–E4605. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Kerleau, M.; Suzuki, E.L.; Wioland, H.; Jouet, S.; Guichard, B.; Lenz, M.; Romet-Lemonne, G.; Jegou, A. Modulation of formin processivity by profilin and mechanical tension. eLife 2018, 7, e34176. [Google Scholar] [CrossRef]
- Dominguez, R. The WH2 Domain and Actin Nucleation: Necessary but Insufficient. Trends Biochem. Sci. 2016, 41, 478–490. [Google Scholar] [CrossRef] [Green Version]
- Gauvin, T.J.; Fukui, J.; Peterson, J.R.; Higgs, H.N. Isoform-Selective Chemical Inhibition of mDia-Mediated Actin Assembly. Biochemistry 2009, 48, 9327–9329. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Ando, Y.; Yasuda, S.; Hosoya, H.; Watanabe, N.; Ishizaki, T.; Narumiya, S. mDia2 Induces the Actin Scaffold for the Contractile Ring and Stabilizes Its Position during Cytokinesis in NIH 3T3 Cells. Mol. Biol. Cell 2008, 19, 2328–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Zhang, J.; Ahmad, S.; Rozier, L.; Yu, H.; Deng, H.; Mao, Y. Aurora B Regulates Formin mDia3 in Achieving Metaphase Chromosome Alignment. Dev. Cell 2011, 20, 342–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, W.I.; Ahmed, S. mDia1-3 in mammalian filopodia. Commun. Integr. Biol. 2012, 5, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Colón-Franco, J.M.; Gomez, T.S.; Billadeau, D.D. Dynamic remodeling of the actin cytoskeleton by FMNL1γ is required for structural maintenance of the Golgi complex. J. Cell Sci. 2011, 124, 3118–3126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardberg, M.; Kaipio, K.; Lehtinen, L.; Mikkonen, P.; Heuser, V.D.; Talvinen, K.; Iljin, K.; Kampf, C.; Uhlen, M.; Grenman, R.; et al. FHOD1, a Formin Upregulated in Epithelial-Mesenchymal Transition, Participates in Cancer Cell Migration and Invasion. PLoS ONE 2013, 8, e74923. [Google Scholar] [CrossRef] [Green Version]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef] [Green Version]
- Dopie, J.; Skarp, K.-P.; Rajakylä, E.K.; Tanhuanpää, K.; Vartiainen, M.K. Active maintenance of nuclear actin by importin 9 supports transcription. Proc. Natl. Acad. Sci. USA 2012, 109, E544–E552. [Google Scholar] [CrossRef] [Green Version]
- Wioland, H.; Jegou, A.; Romet-Lemonne, G. Quantitative Variations with pH of Actin Depolymerizing Factor/Cofilin’s Multiple Actions on Actin Filaments. Biochemistry 2018, 58, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Huang, J.; Liu, B.; Xing, B.; Bordeleau, F.; Reinhart-King, C.A.; Li, W.; Zhang, J.J.; Huang, X.-Y. Improving fascin inhibitors to block tumor cell migration and metastasis. Mol. Oncol. 2016, 10, 966–980. [Google Scholar] [CrossRef] [Green Version]
- Khurana, S.; George, S.P. The role of actin bundling proteins in the assembly of filopodia in epithelial cells. Cell Adhes. Migr. 2011, 5, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Chiang, T.-S.; Wu, H.-F.; Lee, F.-J.S. ADP-ribosylation factor–like 4C binding to filamin-A modulates filopodium formation and cell migration. Mol. Biol. Cell 2017, 28, 3013–3028. [Google Scholar] [CrossRef]
- Winkelman, J.D.; Suarez, C.; Hocky, G.M.; Harker, A.J.; Morganthaler, A.N.; Christensen, J.R.; Voth, G.A.; Bartles, J.R.; Kovar, D.R. Fascin- and α-Actinin-Bundled Networks Contain Intrinsic Structural Features that Drive Protein Sorting. Curr. Biol. 2016, 26, 2697–2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.-K.; Han, S.; Xing, B.; Huang, J.; Liu, B.; Bordeleau, F.; Reinhart-King, C.A.; Zhang, J.J.; Huang, X.-Y. Targeted inhibition of fascin function blocks tumour invasion and metastatic colonization. Nat. Commun. 2015, 6, 7465. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, J.J.; Huang, X.-Y. Anti-Metastasis Fascin Inhibitors Decrease the Growth of Specific Subtypes of Cancers. Cancers 2020, 12, 2287. [Google Scholar] [CrossRef]
- US National Library of Medicine. ClinicalTrials.gov. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03199586 (accessed on 17 September 2020).
- Kumar, N.; Zhao, P.; Tomar, A.; Galea, C.A.; Khurana, S. Association of Villin with Phosphatidylinositol 4,5-Bisphosphate Regulates the Actin Cytoskeleton. J. Boil. Chem. 2003, 279, 3096–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; George, S.P.; Roy, S.; Pham, E.; Esmaeilniakooshkghazi, A.; Khurana, S. Both the anti- and pro-apoptotic functions of villin regulate cell turnover and intestinal homeostasis. Sci. Rep. 2016, 6, 35491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, S.D.; Mullins, R.D. VASP is a processive actin polymerase that requires monomeric actin for barbed end association. J. Cell Biol. 2010, 191, 571–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, A.J. Rho family proteins and regulation of the actin cytoskeleton. Prog. Mol. Subcell. Biol. 1999, 22, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Padrick, S.B.; Rosen, M.K. Physical mechanisms of signal integration by WASP family proteins. Annu. Rev. Biochem. 2010, 79, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Borek, D.; Padrick, S.B.; Gomez, T.S.; Metlagel, Z.; Ismail, A.M.; Umetani, J.; Billadeau, D.D.; Otwinowski, Z.; Rosen, M.K. Structure and control of the actin regulatory WAVE complex. Nat. Cell Biol. 2010, 468, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Delorme, V.; Machacek, M.; DerMardirossian, C.; Anderson, K.L.; Wittmann, T.; Hanein, D.; Waterman, C.M.; Danuser, G.; Bokoch, G.M. Cofilin Activity Downstream of Pak1 Regulates Cell Protrusion Efficiency by Organizing Lamellipodium and Lamella Actin Networks. Dev. Cell 2007, 13, 646–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, I.; Jeon, B.T.; Jeong, E.A.; Kim, E.-J.; Kang, D.; Lee, J.S.; Jeong, B.G.; Kim, J.H.; Choi, B.H.; Lee, J.E.; et al. Pak1/LIMK1/Cofilin Pathway Contributes to Tumor Migration and Invasion in Human Non-Small Cell Lung Carcinomas and Cell Lines. Korean J. Physiol. Pharmacol. 2012, 16, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurita, S.; Gunji, E.; Ohashi, K.; Mizuno, K. Actin filaments-stabilizing and -bundling activities of cofilin-phosphatase Slingshot-1. Genes Cells 2007, 12, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Lammers, M.; Rose, R.; Scrima, A.; Wittinghofer, A. The regulation of mDia1 by autoinhibition and its release by Rho•GTP. EMBO J. 2005, 24, 4176–4187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, J.S.; Powell, B.C.; Cheney, R.E. A Millennial Myosin Census. Mol. Biol. Cell 2001, 12, 780–794. [Google Scholar] [CrossRef] [Green Version]
- Merritt, R.C.; Manor, U.; Salles, F.T.; Grati, M.; Dose, A.C.; Unrath, W.C.; Quintero, O.A.; Yengo, C.M.; Kachar, B. Myosin IIIB Uses an Actin-Binding Motif in Its Espin-1 Cargo to Reach the Tips of Actin Protrusions. Curr. Biol. 2012, 22, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Alieva, N.O.; Efremov, A.K.; Hu, S.; Oh, D.; Chen, Z.; Natarajan, M.; Ong, H.T.; Jegou, A.; Romet-Lemonne, G.; Groves, J.T.; et al. Myosin IIA and formin dependent mechanosensitivity of filopodia adhesion. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Lordier, L.; Mazzi, S.; Chang, Y.; Lapierre, V.; Larghero, J.; Debili, N.; Raslova, H.; Vainchenker, W. Activity of nonmuscle myosin II isoforms determines localization at the cleavage furrow of megakaryocytes. Blood 2016, 128, 3137–3145. [Google Scholar] [CrossRef] [Green Version]
- De Lanerolle, P. Nuclear actin and myosins at a glance. J. Cell Sci. 2012, 125, 4945–4949. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; George, S.P.; Srinivasan, K.; Patnaik, S.; Khurana, S. Actin reorganization as the molecular basis for the regulation of apoptosis in gastrointestinal epithelial cells. Cell Death Differ. 2012, 19, 1514–1524. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Klionsky, D.J. Regulation of JMY’s actin nucleation activity by TTC5/STRAP and LC3 during autophagy. Autophagy 2019, 15, 373–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purvanov, V.; Holst, M.; Khan, J.; Baarlink, C.; Grosse, R. G-protein-coupled receptor signaling and polarized actin dynamics drive cell-in-cell invasion. eLife 2014, 3, e02786. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-D.; Chen, Y.-B.; Zhao, J.-J.; Zhang, X.-F.; Zhu, G.-C.; Weng, D.-S.; Pan, K.; Lv, L.; Pan, Q.-Z.; Jiang, S.-S.; et al. TES functions as a Mena-dependent tumor suppressor in gastric cancer carcinogenesis and metastasis. Cancer Commun. 2019, 39, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Cao, L.; Xiao, L.; Song, J.-X.; Zhang, Y.-J.; Zheng, P.; Zheng, S.-G. Hypoxia induces actin cytoskeleton remodeling by regulating the binding of CAPZA1 to F-actin via PIP2 to drive EMT in hepatocellular carcinoma. Cancer Lett. 2019, 448, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.-Y.; Ru, Y.; Sun, J.-T.; Gao, S.-G.; Wang, Y.-F.; Wang, L.-D.; Feng, X.-S. Expression of CDX2 and villin in gastric cardiac intestinal metaplasia and the relation with gastric cardiac carcinogenesis. Asian Pac. J. Cancer Prev. 2012, 13, 247–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Yang, X.; Chen, C.; Liu, B.; Ren, B.; Wang, L.; Zhao, K.; Yu, S.; Ming, H. Expression of the Arp2/3 complex in human gliomas and its role in the migration and invasion of glioma cells. Oncol. Rep. 2013, 30, 2127–2136. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Kim, S.; An, H.-T.; Ko, J. α-Actinin-4 regulates cancer stem cell properties and chemoresistance in cervical cancer. Carcinog. 2019, 41, 940–949. [Google Scholar] [CrossRef]
- Tian, Z.-Q.; Shi, J.-W.; Wang, X.-R.; Li, Z.; Wang, G.-Y. New cancer suppressor gene for colorectal adenocarcinoma: Filamin A. World J. Gastroenterol. 2015, 21, 2199–2205. [Google Scholar] [CrossRef]
- Hao, R.; Liu, Y.; Du, Q.; Liu, L.; Chen, S.; You, H.; Lu, T. Transgelin-2 expression in breast cancer and its relationships with clinicopathological features and patient outcome. Breast Cancer 2019, 26, 776–783. [Google Scholar] [CrossRef]
- Tanaka, H.; Shirkoohi, R.; Nakagawa, K.; Qiao, H.; Fujita, H.; Okada, F.; Hamada, J.-I.; Kuzumaki, S.; Takimoto, M.; Kuzumaki, N. siRNA gelsolin knockdown induces epithelial-mesenchymal transition with a cadherin switch in human mammary epithelial cells. Int. J. Cancer 2006, 118, 1680–1691. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.Y.; Whited, J.L. Parallels between wound healing, epimorphic regeneration and solid tumors. Development 2020, 147, dev181636. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Sanna, V.; Ahmad, N.; Sechi, M.; Mukhtar, H. Resveratrol nanoformulation for cancer prevention and therapy. Ann. N. Y. Acad. Sci. 2015, 1348, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Belin, B.J.; Lee, T.; Mullins, R.D. DNA damage induces nuclear actin filament assembly by Formin -2 and Spire-½ that promotes efficient DNA repair. Elife 2015, 4, e07735. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, Y. Functions of nuclear actin-binding proteins in human cancer. Oncol. Lett. 2017, 15, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Grzanka, D.; Grzanka, D.; Orlikowska, M. Fluorescence and ultrastructural localization of actin distribution patterns in the nucleus of HL-60 and K-562 cell lines treated with cytostatic drugs. Oncol. Rep. 2004, 11, 765–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izdebska, M.; Gagat, M.; Grzanka, D.; Grzanka, A. Ultrastructural localization of F-actin using phalloidin and quantum dots in HL-60 promyelocytic leukemia cell line after cell death induction by arsenic trioxide. Acta Histochem. 2013, 115, 487–495. [Google Scholar] [CrossRef]
- Stüven, T.; Hartmann, E.; Görlich, D. Exportin 6: A novel nuclear export receptor that is specific for profilin·actin complexes. EMBO J. 2003, 22, 5928–5940. [Google Scholar] [CrossRef] [Green Version]
- Rando, O.J.; Zhao, K.; Janmey, P.; Crabtree, G.R. Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proc. Natl. Acad. Sci. USA 2002, 99, 2824–2829. [Google Scholar] [CrossRef] [Green Version]
- Willhoft, O.; Wigley, D.B. INO80 and SWR1 complexes: The non-identical twins of chromatin remodelling. Curr. Opin. Struct. Biol. 2020, 61, 50–58. [Google Scholar] [CrossRef]
- Hu, P.; Wu, S.; Hernandez, N. A role for -actin in RNA polymerase III transcription. Genes Dev. 2004, 18, 3010–3015. [Google Scholar] [CrossRef] [Green Version]
- Yoo, Y.; Wu, X.; Guan, J.-L. A Novel Role of the Actin-nucleating Arp2/3 Complex in the Regulation of RNA Polymerase II-dependent Transcription. J. Boil. Chem. 2007, 282, 7616–7623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baarlink, C.; Plessner, M.; Sherrard, A.; Morita, K.; Misu, S.; Virant, D.; Kleinschnitz, E.-M.; Harniman, R.; Alibhai, M.; Baumeister, S.; et al. A transient pool of nuclear F-actin at mitotic exit controls chromatin organization. Nat. Cell Biol. 2017, 19, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Obrdlik, A.; Percipalle, P. The F-actin severing protein cofilin-1 is required for RNA polymerase II transcription elongation. Nucleus 2011, 2, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, S.K.; Claudianos, C.; Campbell, H.D. Evolution of the gelsolin family of actin-binding proteins as novel transcriptional coactivators. BioEssays 2005, 27, 388–396. [Google Scholar] [CrossRef] [PubMed]
- King, S.J.; Nowak, K.; Suryavanshi, N.; Holt, I.; Shanahan, C.M.; Ridley, A.J. Nesprin-1 and nesprin-2 regulate endothelial cell shape and migration. Cytoskeleton 2014, 71, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Sur-Erdem, I.; Hussain, M.S.; Asif, M.; Pınarbası, N.; Aksu, A.C.; Noegel, A.A. Nesprin-1 impact on tumorigenic cell phenotypes. Mol. Biol. Rep. 2019, 47, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, M.; Fusco, J.P.; Segura, V.; Lozano, M.D.; Minucci, S.; Echeveste, J.I.; Gurpide, A.; Andueza, M.; Melero, I.; Sanmamed, M.F.; et al. Identification of mutations associated with acquired resistance to sunitinib in renal cell cancer. Int. J. Cancer 2019, 145, 1991–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Femi, O.F. Genetic alterations and PIK3CA gene mutations and amplifications analysis in cervical cancer by racial groups in the United States. Int. J. Health Sci. 2018, 12, 28–32. [Google Scholar]
- Galamb, O.; Kalmár, A.; Barták, B.K.; Patai Árpád, V.; Leiszter, K.; Peterfia, B.; Wichmann, B.; Valcz, G.; Veres, G.; Tulassay, Z.; et al. Aging related methylation influences the gene expression of key control genes in colorectal cancer and adenoma. World J. Gastroenterol. 2016, 22, 10325–10340. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Hieda, M.; Yokoyama, Y.; Nishioka, Y.; Yoshidome, K.; Tsujimoto, M.; Matsuura, N. Global loss of a nuclear lamina component, lamin A/C, and LINC complex components SUN1, SUN2, and nesprin-2 in breast cancer. Cancer Med. 2015, 4, 1547–1557. [Google Scholar] [CrossRef]
- Kelkar, P.; Walter, A.; Papadopoulos, S.; Mroß, C.; Munck, M.; Peche, V.S.; A Noegel, A. Nesprin-2 mediated nuclear trafficking and its clinical implications. Nucleus 2015, 6, 479–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patnaik, S.; George, S.P.; Pham, E.; Roy, S.; Singh, K.; Figueroa, M.; Khurana, S. By moonlighting in the nucleus, villin regulates epithelial plasticity. Mol. Biol. Cell 2016, 27, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Altintas, S.; Bayrak, M.; Altintas, Y. Prognostic Value of CDX2 and Villin Expression in Advanced Stage Colorectal Carcinoma. J. Coll. Physicians Surg. Pak. 2019, 29, 1057–1061. [Google Scholar] [CrossRef]
- Bruun, J.; Sveen, A.; Barros, R.; Eide, P.W.; Eilertsen, I.; Kolberg, M.; Pellinen, T.; David, L.; Svindland, A.; Kallioniemi, O.-P.; et al. Prognostic, predictive, and pharmacogenomic assessments of CDX2 refine stratification of colorectal cancer. Mol. Oncol. 2018, 12, 1639–1655. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Brakebusch, C. Senescence regulation by nuclear N-WASP: A role in cancer? Oncoscience 2019, 6, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Schrank, B.R.; Aparicio, T.; Li, Y.; Chang, W.; Chait, B.T.; Gundersen, G.G.; Gottesman, M.E.; Gautier, J. Nuclear ARP2/3 drives DNA break clustering for homology-directed repair. Nat. Cell Biol. 2018, 559, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, N.V.; Almuzzaini, B.; Kritikou, J.S.; Baptista, M.A.P.; Oliveira, M.M.; Keszei, M.; Snapper, S.B.; Percipalle, P.; Westerberg, L.S. Nuclear Wiskott–Aldrich syndrome protein co-regulates T cell factor 1-mediated transcription in T cells. Genome Med. 2017, 9, 91. [Google Scholar] [CrossRef]
- Li, H.; Petersen, S.; Mariscal, A.G.; Brakebusch, C. Negative Regulation of p53-Induced Senescence by N-WASP Is Crucial for DMBA/TPA-Induced Skin Tumor Formation. Cancer Res. 2019, 79, 2167–2181. [Google Scholar] [CrossRef] [Green Version]
- Lubeseder-Martellato, C.; Alexandrow, K.; Hidalgo-Sastre, A.; Heid, I.; Boos, S.L.; Briel, T.; Schmid, R.M.; Siveke, J.T. Oncogenic KRas-induced Increase in Fluid-phase Endocytosis is Dependent on N-WASP and is Required for the Formation of Pancreatic Preneoplastic Lesions. EBioMedicine 2017, 15, 90–99. [Google Scholar] [CrossRef] [Green Version]
- An, H.-T.; Yoo, S.; Ko, J. α-Actinin-4 induces the epithelial-to-mesenchymal transition and tumorigenesis via regulation of Snail expression and β-catenin stabilization in cervical cancer. Oncogene 2016, 35, 5893–5904. [Google Scholar] [CrossRef]
- Grzanka, D.; Izdebska, M.; Klimaszewska-Wiśniewska, A.; Gagat, M. The alterations in SATB1 and nuclear F-actin expression affect apoptotic response of the MCF-7 cells to geldanamycin. Folia Histochem. et Cytobiol. 2015, 53, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.; Wei, W.; Wu, Z.; Wang, J.; Ding, X.; Sheng, Y.; Han, Y.; Wu, Q. ARPC2 promotes breast cancer proliferation and metastasis. Oncol. Rep. 2019, 41, 3189–3200. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.-Q.; Zhang, T.-P.; Zhao, W.-J.; Liu, Z.-W.; You, L.; Zhou, L.; Guo, J.-C.; Zhao, Y. Filamin A: Insights into its Exact Role in Cancers. Pathol. Oncol. Res. 2015, 22, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; López-Camacho, C.; Tang, J.-Y.; Mendoza-Villanueva, D.; Maya-Mendoza, A.; Jackson, D.A.; Shore, P. Cytoskeletal protein filamin A is a nucleolar protein that suppresses ribosomal RNA gene transcription. Proc. Natl. Acad. Sci. USA 2012, 109, 1524–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Miller, R.T.; Freter, C.E. Signaling regulation and role of filamin A cleavage in Ca2+-stimulated migration of androgen receptor-deficient prostate cancer cells. Oncotarget 2016, 8, 3840–3853. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, M.; Bai, G.; Li, X.; Sun, Z.; Yang, J.; Wang, L.; Sun, J. Filamin A inhibits tumor progression through regulating BRCA1 expression in human breast cancer. Oncol. Lett. 2018, 16, 6261–6266. [Google Scholar] [CrossRef] [PubMed]
- Burgio, E.; Migliore, L. Towards a systemic paradigm in carcinogenesis: Linking epigenetics and genetics. Mol. Biol. Rep. 2014, 42, 777–790. [Google Scholar] [CrossRef]
- Sun, B.; Fang, Y.; Li, Z.; Chen, Z.; Xiang, J. Role of cellular cytoskeleton in epithelial-mesenchymal transition process during cancer progression. Biomed. Rep. 2015, 3, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Argenzio, E.; Klarenbeek, J.; Kedziora, K.M.; Nahidiazar, L.; Isogai, T.; Perrakis, A.; Jalink, K.; Moolenaar, W.H.; Innocenti, M. Profilin binding couples chloride intracellular channel protein CLIC4 to RhoA-mDia2 signaling and filopodium formation. J. Biol. Chem. 2018, 293, 19161–19176. [Google Scholar] [CrossRef] [Green Version]
- Shankar, J.; Nabi, I.R. Actin Cytoskeleton Regulation of Epithelial Mesenchymal Transition in Metastatic Cancer Cells. PLoS ONE 2015, 10, e0119954. [Google Scholar] [CrossRef] [Green Version]
- Iwai, S.; Yonekawa, A.; Harada, C.; Hamada, M.; Katagiri, W.; Nakazawa, M.; Yura, Y. Involvement of the Wnt-β-catenin pathway in invasion and migration of oral squamous carcinoma cells. Int. J. Oncol. 2010, 37, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Steffen, A.; Ladwein, M.; Dimchev, G.; Hein, A.; Schwenkmezger, L.; Arens, S.; Ladwein, K.I.; Holleboom, J.M.; Schur, F.; Small, J.V.; et al. Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J. Cell Sci. 2013, 126, 4572–4588. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Lee, Y.; Yoon, Y.J.; Kim, C.; Park, S.; Kim, S.; Kim, N.D.; Han, D.C.; Kwon, B.-M. Pimozide suppresses cancer cell migration and tumor metastasis through binding to ARPC2, a subunit of the Arp2/3 complex. Cancer Sci. 2019, 110, 3788–3801. [Google Scholar] [CrossRef]
- Qi, H.; Fu, X.; Li, Y.; Pang, X.; Chen, S.; Zhu, X.; Li, F.; Tan, W. SATB1 promotes epithelial-mesenchymal transition and metastasis in prostate cancer. Oncol. Lett. 2017, 13, 2577–2582. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Zhou, H.; Wang, X.; Liu, Z. Silencing SATB1 with siRNA inhibits the proliferation and invasion of small cell lung cancer cells. Cancer Cell Int. 2013, 13, 8. [Google Scholar] [CrossRef] [Green Version]
- Han, H.-J.; Russo, J.; Kohwi, Y.; Kohwi-Shigematsu, T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nat. Cell Biol. 2008, 452, 187–193. [Google Scholar] [CrossRef]
- Nodin, B.; Hedner, C.; Uhlén, M.; Jirström, K. Expression of the global regulator SATB1 is an independent factor of poor prognosis in high grade epithelial ovarian cancer. J. Ovarian Res. 2012, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Frömberg, A.; Rabe, M.; Aigner, A. Multiple effects of the special AT-rich binding protein 1 (SATB1) in colon carcinoma. Int. J. Cancer 2014, 135, 2537–2546. [Google Scholar] [CrossRef]
- Tu, W.; Luo, M.; Wang, Z.; Yan, W.; Xia, Y.; Deng, H.; He, J.; Han, P.; Tian, D. Upregulation of SATB1 promotes tumor growth and metastasis in liver cancer. Liver Int. 2012, 32, 1064–1078. [Google Scholar] [CrossRef]
- Lee, M.-H.; Kundu, J.K.; Chae, J.-I.; Shim, J.-H. Targeting ROCK/LIMK/cofilin signaling pathway in cancer. Arch. Pharmacal Res. 2019, 42, 481–491. [Google Scholar] [CrossRef]
- Collazo, J.; Zhu, B.; Larkin, S.; Martin, S.K.; Pu, H.; Horbinski, C.; Koochekpour, S.; Kyprianou, N. Cofilin drives cell-invasive and metastatic responses to TGF-β in prostate cancer. Cancer Res. 2014, 74, 2362–2373. [Google Scholar] [CrossRef] [Green Version]
- Bracalente, C.; Rinflerch, A.R.; Ibañez, I.L.; García, F.M.; Volonteri, V.; Galimberti, G.N.; Klamt, F.; Durán, H. Cofilin-1 levels and intracellular localization are associated with melanoma prognosis in a cohort of patients. Oncotarget 2018, 9, 24097–24108. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Tao, L.; Jin, F.; Gu, H.; Dai, X.; Ni, T.; Feng, J.; Ding, Y.; Xiao, W.; Qian, Y.; et al. Cofilin 1 induces the epithelial-mesenchymal transition of gastric cancer cells by promoting cytoskeletal rearrangement. Oncotarget 2017, 8, 39131–39142. [Google Scholar] [CrossRef] [Green Version]
- Hensley, P.; Zetter, D.; Horbinski, C.M.; Strup, S.E.; Kyprianou, N. Association of epithelial-mesenchymal transition and nuclear cofilin with advanced urothelial cancer. Hum. Pathol. 2016, 57, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.G.; Lu, Y.F.; Zhang, J.; Hu, W.N. Filamin A regulates MMP-9 expression and suppresses prostate cancer cell migration and invasion. Tumor Biol. 2014, 35, 3819–3826. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, T.; Gao, D.; Zhang, Y.; Zhao, Q.; Liu, S.; Su, T.; Bernier, M.; Zhao, R. Filamin A expression correlates with proliferation and invasive properties of human metastatic melanoma tumors: Implications for survival in patients. J. Cancer Res. Clin. Oncol. 2014, 140, 1913–1926. [Google Scholar] [CrossRef]
- Ji, Y.; Jiang, J.; Huang, L.; Feng, W.; Zhang, Z.; Jin, L.; Xing, X. Sperm-associated antigen 4 (SPAG4) as a new cancer marker interacts with Nesprin3 to regulate cell migration in lung carcinoma. Oncol. Rep. 2018, 40, 783–792. [Google Scholar] [CrossRef] [Green Version]
- Kovac, B.; Mäkelä, T.P.; Vallenius, T. Increased α-actinin-1 destabilizes E-cadherin-based adhesions and associates with poor prognosis in basal-like breast cancer. PLoS ONE 2018, 13, e0196986. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Li, J.-J.; Deng, T.; Li, B.-H.; Geng, P.-L.; Zeng, X.-T. Actinin-4 as a Diagnostic Biomarker in Serum of Breast Cancer Patients. Med. Sci. Monit. 2019, 25, 3298–3302. [Google Scholar] [CrossRef]
- Liu, X.; Chu, K.-M. α-Actinin-4 promotes metastasis in gastric cancer. Lab. Investig. 2017, 97, 1084–1094. [Google Scholar] [CrossRef] [Green Version]
- Ubelmann, F.; Chamaillard, M.; El-Marjou, F.; Simon, A.; Netter, J.; Vignjevic, D.M.; Nichols, B.L.; Quezada-Calvillo, R.; Grandjean, T.; Louvard, D.; et al. Enterocyte loss of polarity and gut wound healing rely upon the F-actin–severing function of villin. Proc. Natl. Acad. Sci. USA 2013, 110, E1380–E1389. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Zhang, Y.; Wu, L.; Xie, W.; Li, L.; Yuan, Y.; Chen, Y.; Lin, Y.; He, X. Elevated transgelin/TNS1 expression is a potential biomarker in human colorectal cancer. Oncotarget 2017, 9, 1107–1113. [Google Scholar] [CrossRef] [Green Version]
- Hurst, V.; Shimada, K.; Gasser, S.M. Nuclear Actin and Actin-Binding Proteins in DNA Repair. Trends Cell Boil. 2019, 29, 462–476. [Google Scholar] [CrossRef] [Green Version]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [Green Version]
- Fong, G.-H.; Rossant, J.; Gertsenstein, M.; Breitman, M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nat. Cell Biol. 1995, 376, 66–70. [Google Scholar] [CrossRef]
- Shalaby, F.; Rossant, J.; Yamaguchi, T.P.; Gertsenstein, M.; Wu, X.-F.; Breitman, M.L.; Schuh, A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nat. Cell Biol. 1995, 376, 62–66. [Google Scholar] [CrossRef]
- Cudmore, M.J.; Hewett, P.W.; Ahmad, S.; Wang, K.-Q.; Cai, M.; Al-Ani, B.; Fujisawa, T.; Ma, B.; Sissaoui, S.; Ramma, W.; et al. The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat. Commun. 2012, 3, 972. [Google Scholar] [CrossRef] [Green Version]
- Stoletov, K.V.; Terman, B.I. Nck and Crk mediate distinct VEGF induced signaling pathways that serve overlapping functions in endothelial cell migration. Exp. Cell Res. 2004, 295, 258–268. [Google Scholar] [CrossRef]
- Gong, C.; Stoletov, K.V.; Terman, B.I. VEGF treatment induces signaling pathways that regulate both actin polymerization and depolymerization. Angiogenesis 2004, 7, 313–322. [Google Scholar] [CrossRef]
- Rohatgi, R.; Ho, H.-Y.H.; Kirschner, M.W. Mechanism of N-Wasp Activation by Cdc42 and Phosphatidylinositol 4,5-Bisphosphate. J. Cell Biol. 2000, 150, 1299–1310. [Google Scholar] [CrossRef] [Green Version]
- Barry, D.M.; Xu, K.; Meadows, S.M.; Zheng, Y.; Norden, P.R.; Davis, G.E.; Cleaver, O. Cdc42 is required for cytoskeletal support of endothelial cell adhesion during blood vessel formation in mice. Development 2015, 142, 3058–3070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamalice, L.; Houle, F.; Jourdan, G.; Huot, J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene 2004, 23, 434–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Stoel, M.; Schimmel, L.; Nawaz, K.; Van Stalborch, A.-M.; De Haan, A.; Klaus-Bergmann, A.; Valent, E.T.; Koenis, D.S.; Amerongen, G.P.V.N.; De Vries, C.J.; et al. DLC1 is a direct target of activated YAP/TAZ that drives collective migration and sprouting angiogenesis. J. Cell Sci. 2020, 133, jcs239947. [Google Scholar] [CrossRef] [PubMed]
- Elaimy, A.L.; Mercurio, A.M. Convergence of VEGF and YAP/TAZ signaling: Implications for angiogenesis and cancer biology. Sci. Signal. 2018, 11, eaau1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kim, Y.H.; Kim, J.; Park, D.Y.; Bae, H.; Lee, D.-H.; Kim, K.H.; Hong, S.P.; Jang, S.P.; Kubota, Y.; et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Investig. 2017, 127, 3441–3461. [Google Scholar] [CrossRef]
- Fujii, Y.; Hirahara, N.; Kaji, S.; Taniura, T.; Hyakudomi, R.; Yamamoto, T.; Tajima, Y. Bevacizumab-induced intestinal perforation in a patient with inoperable breast cancer: A case report and review of the literature. J. Med. Case Rep. 2018, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Del Valle-Pérez, B.; Martínez, V.G.; Lacasa-Salavert, C.; Figueras, A.; Shapiro, S.S.; Takafuta, T.; Casanovas, O.; Capellá, G.; Ventura, F.; Viñals, F. Filamin B Plays a Key Role in Vascular Endothelial Growth Factor-induced Endothelial Cell Motility through Its Interaction with Rac-1 and Vav-2. J. Boil. Chem. 2010, 285, 10748–10760. [Google Scholar] [CrossRef] [Green Version]
- Gagat, M.; Grzanka, D.; Izdebska, M.; Grzanka, D. Effect of L-homocysteine on endothelial cell-cell junctions following F-actin stabilization through tropomyosin-1 overexpression. Int. J. Mol. Med. 2013, 32, 115–129. [Google Scholar] [CrossRef]
- Gagat, M.; Grzanka, D.; Izdebska, M.; Sroka, W.D.; Marszałł, M.P.; Grzanka, D. Tropomyosin-1 protects endothelial cell–cell junctions against cigarette smoke extract through F-actin stabilization in EA.hy926 cell line. Acta Histochem. 2014, 116, 606–618. [Google Scholar] [CrossRef]
- Fernández-Cortés, M.; Delgado-Bellido, D.; Oliver, F.J. Vasculogenic Mimicry: Become an Endothelial Cell “But Not So Much” . Front. Oncol. 2019, 9, 803. [Google Scholar] [CrossRef] [Green Version]
- Valdivia, A.; Mingo, G.; Aldana, V.; Pinto, M.P.; Ramirez, M.; Retamal, C.; Gonzalez, A.; Nualart, F.; Corvalan, A.H.; Owen, G.I. Fact or Fiction, It Is Time for a Verdict on Vasculogenic Mimicry? Front. Oncol. 2019, 9, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.P.; Liao, Y.D.; Mai, D.M.; Xie, P.; Qiang, Y.Y.; Zheng, L.S.; Wang, M.Y.; Mei, Y.; Meng, D.F.; Xu, L.; et al. Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: A meta-analysis. Angiogenesis 2016, 19, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.; Dong, H.; Liu, Q.; Zhao, T.; Yao, F.; Xu, Y.; Chen, B.; Zheng, X.; Wu, Y.; Jin, F.; et al. ALDH1 Expression and Vasculogenic Mimicry Are Positively Associated with Poor Prognosis in Patients with Breast Cancer. Cell. Physiol. Biochem. 2018, 49, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Zhao, X.; Liu, T.; Zhang, Y.; Sun, R.; Dong, X.; Liu, F.; Zhao, N.; Zhang, D.; Wu, L.; et al. LOXL2 promotes vasculogenic mimicry and tumour aggressiveness in hepatocellular carcinoma. J. Cell. Mol. Med. 2018, 23, 1363–1374. [Google Scholar] [CrossRef] [Green Version]
- Hori, A.; Shimoda, M.; Naoi, Y.; Kagara, N.; Tanei, T.; Miyake, T.; Shimazu, K.; Kim, S.J.; Noguchi, S. Vasculogenic mimicry is associated with trastuzumab resistance of HER2-positive breast cancer. Breast Cancer Res. 2019, 21, 1–18. [Google Scholar] [CrossRef]
- Fu, D.; He, X.; Yang, S.; Xu, W.; Lin, T.; Feng, X. Zoledronic acid inhibits vasculogenic mimicry in murine osteosarcoma cell line in vitro. BMC Musculoskelet. Disord. 2011, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Maes, H.; Van Eygen, S.; Krysko, D.V.; Vandenabeele, P.; Nys, K.; Rillaerts, K.; Garg, A.D.; Verfaillie, T.; Agostinis, P. BNIP3 supports melanoma cell migration and vasculogenic mimicry by orchestrating the actin cytoskeleton. Cell Death Dis. 2014, 5, e1127. [Google Scholar] [CrossRef] [Green Version]
- Aikins, A.R.; Kim, M.; Raymundo, B.; Kim, C.-W. Featured Article: Downregulation of transgelin blocks interleukin-8 utilization and suppresses vasculogenic mimicry in breast cancer cells. Exp. Boil. Med. 2017, 242, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Skruber, K.; Warp, P.V.; Shklyarov, R.; Thomas, J.D.; Swanson, M.S.; Henty-Ridilla, J.L.; Read, T.-A.; Vitriol, E.A. Arp2/3 and Mena/VASP Require Profilin 1 for Actin Network Assembly at the Leading Edge. Curr. Biol. 2020, 30, 2651–2664. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2019, 77, 1745–1770. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izdebska, M.; Zielińska, W.; Hałas-Wiśniewska, M.; Grzanka, A. Involvement of Actin and Actin-Binding Proteins in Carcinogenesis. Cells 2020, 9, 2245. https://doi.org/10.3390/cells9102245
Izdebska M, Zielińska W, Hałas-Wiśniewska M, Grzanka A. Involvement of Actin and Actin-Binding Proteins in Carcinogenesis. Cells. 2020; 9(10):2245. https://doi.org/10.3390/cells9102245
Chicago/Turabian StyleIzdebska, Magdalena, Wioletta Zielińska, Marta Hałas-Wiśniewska, and Alina Grzanka. 2020. "Involvement of Actin and Actin-Binding Proteins in Carcinogenesis" Cells 9, no. 10: 2245. https://doi.org/10.3390/cells9102245
APA StyleIzdebska, M., Zielińska, W., Hałas-Wiśniewska, M., & Grzanka, A. (2020). Involvement of Actin and Actin-Binding Proteins in Carcinogenesis. Cells, 9(10), 2245. https://doi.org/10.3390/cells9102245







