Syncytia in Fungi
Abstract
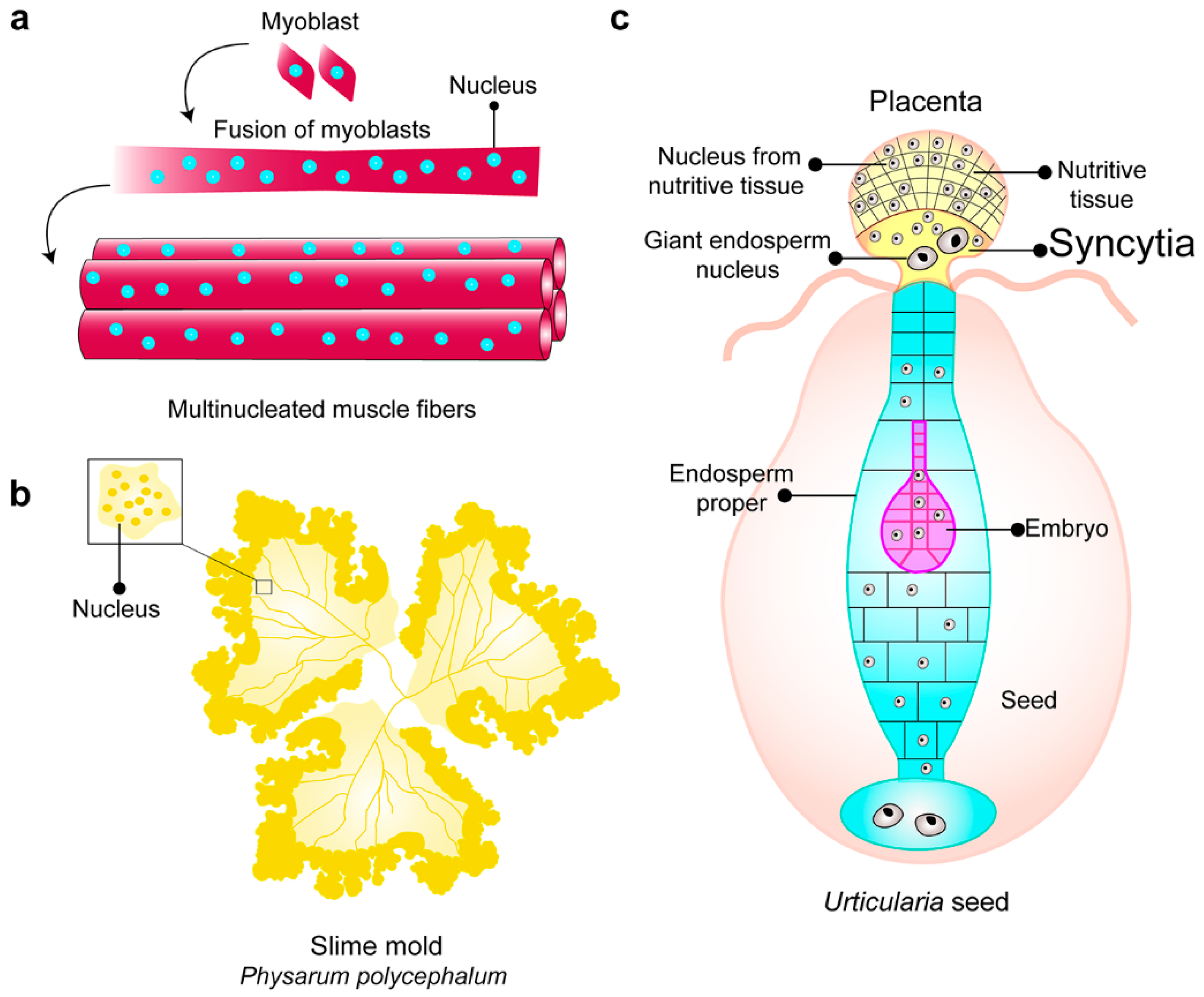
1. Syncytia
2. Differentiation within Fungal Syncytia
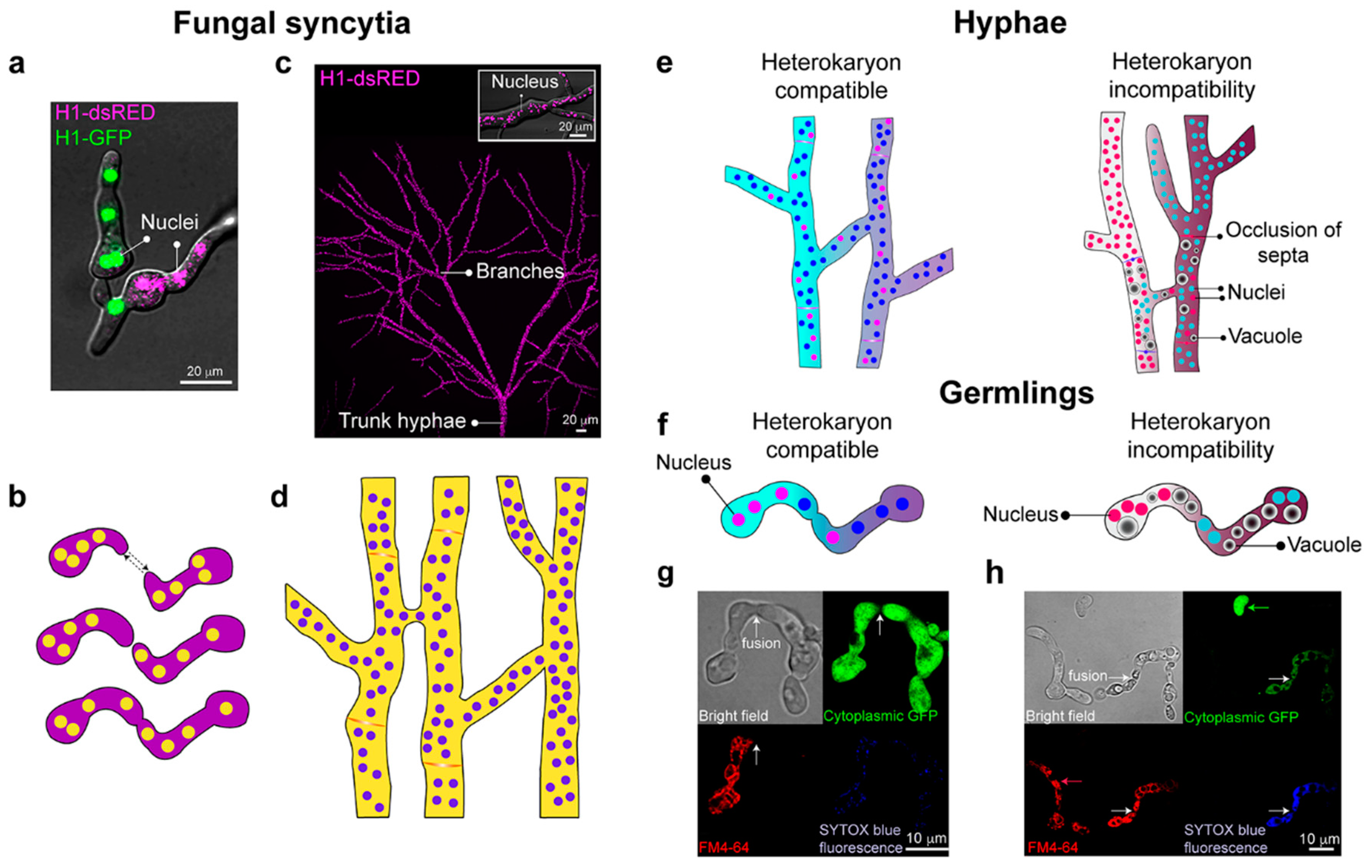
2.1. Hyphal Architecture
2.2. Colony Aging
2.3. Heterogeneity between Hyphal Compartments
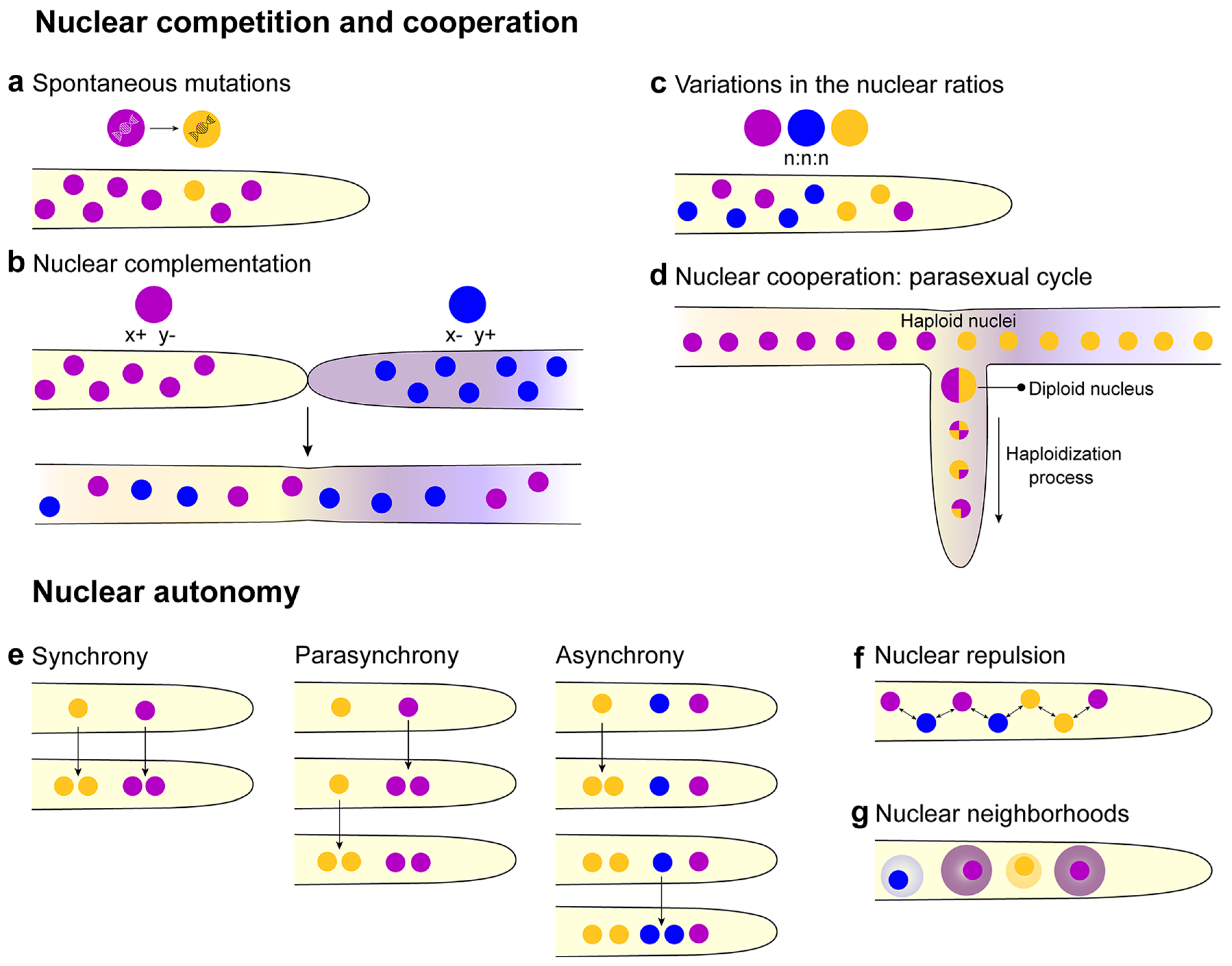
2.4. Nuclear Competition and Cooperation
2.5. Nuclear Autonomy
2.6. Mitochondrial Autonomy
3. Conclusion and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Zeldovich, V.B.; Clausen, C.H.; Bradford, E.; Fletcher, D.A.; Maltepe, E.; Robbins, J.R.; Bakardjiev, A.I. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog. 2013, 9, e1003821. [Google Scholar] [CrossRef] [PubMed]
- Abmayr, S.M.; Zhuang, S.; Geisbrecht, E.R. Myoblast fusion in Drosophila. Methods Mol. Biol. 2008, 475, 75–97. [Google Scholar] [PubMed]
- Scheven, B.A.; Haas, E.W.K.-D.; Wassenaar, A.M.; Nijweide, P.J. Differentiation kinetics of osteoclasts in the periosteum of embryonic bones in vivo and in vitro. Anat. Rec. 1986, 214, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.W.; Jarlfors, U. Plasmodial ultrastructure of the myxomycete Physarum polycephalum. Tissue Cell 1972, 4, 15–36. [Google Scholar] [CrossRef]
- Guttes, E.; Guttes, S.; Rusch, H.P. Morphological observations on growth and differentation of Physarum polycephalum grown in pure culture. Dev. Biol. 1961, 3, 588–614. [Google Scholar] [CrossRef]
- Plachno, B.J.; Swiatek, P. Syncytia in plants: Cell fusion in endosperm-placental syncytium formation in Utricularia (Lentibulariaceae). Protoplasma 2011, 248, 425–435. [Google Scholar] [CrossRef]
- Laundon, D.; Chrismas, N.; Wheeler, G.; Cunliffe, M. Chytrid rhizoid morphogenesis resembles hyphal development in multicellular fungi and is adaptive to resource availability. Proc. Biol. Sci. 2020, 287, 20200433. [Google Scholar] [CrossRef]
- Płachno, B.J.; Swiątek, P.; Sas-Nowosielska, H.; Kozieradzka-Kiszkurno, M. Organisation of the endosperm and endosperm-placenta syncytia in bladderworts (Utricularia, Lentibulariaceae) with emphasis on the microtubule arrangement. Protoplasma 2013, 250, 863–873. [Google Scholar] [CrossRef]
- Roca, G.M.; Read, N.D.; Wheals, A.E. Conidial anastomosis tubes in filamentous fungi. FEMS Microbiol. Lett. 2005, 249, 191–198. [Google Scholar] [CrossRef]
- Hickey, P.C.; Jacobson, D.; Read, N.D.; Glass, N.L. Live-cell imaging of vegetative hyphal fusion in Neurospora crassa. Fungal Genet. Biol. 2002, 37, 109–119. [Google Scholar] [CrossRef]
- Glass, N.L.; Fleißner, A. Re-wiring the network: Understanding the mechanism and function of anastomosis in filamentous ascomycete fungi. In The Mycota; Kues, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 123–139. [Google Scholar]
- Rayner, A.D.M. Interconnectedness and Individualism in Fungal Mycelia; Cambridge University Press: Cambridge, UK, 1996; pp. 193–232. [Google Scholar]
- Fischer, M.S.; Glass, N.L. Communicate and fuse: How filamentous fungi establish and maintain an interconnected mycelial network. Front. Microbiol. 2019, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Herzog, S.; Schumann, M.R.; Fleissner, A. Cell fusion in Neurospora crassa. Curr. Opin. Microbiol. 2015, 28, 53–59. [Google Scholar] [CrossRef]
- Goryachev, A.B.; Lichius, A.; Wright, G.D.; Read, N.D. Excitable behavior can explain the “ping-pong” mode of communication between cells using the same chemoattractant. Bioessays 2012, 34, 259–266. [Google Scholar] [CrossRef]
- Fleissner, A.; Leeder, A.C.; Roca, M.G.; Read, N.D.; Glass, N.L. Oscillatory recruitment of signaling proteins to cell tips promotes coordinated behavior during cell fusion. Proc. Nat. Acad. Sci. USA 2009, 106, 19387–19392. [Google Scholar] [CrossRef]
- Simonin, A.; Palma-Guerrero, J.; Fricker, M.; Glass, N.L. Physiological significance of network organization in fungi. Eukaryot. Cell 2012, 11, 1345–1352. [Google Scholar] [CrossRef]
- Glass, N.L.; Dementhon, K. Non-self recognition and programmed cell death in filamentous fungi. Curr. Opin. Microbiol. 2006, 9, 553–558. [Google Scholar] [CrossRef]
- Bastiaans, E.; Debets, A.J.; Aanen, D.K. Experimental demonstration of the benefits of somatic fusion and the consequences for allorecognition. Evolution 2015, 69, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Leeder, A.C.; Jonkers, W.; Li, J.; Glass, N.L. Germination and early colony establishment in Neurospora crassa requires a MAP kinase regulatory network. Genetics 2013, 195, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; Glass, N.L.; Pringle, A. Cooperation among germinating spores facilitates the growth of the fungus, Neurospora crassa. Biol. Lett. 2012, 8, 419–422. [Google Scholar] [CrossRef][Green Version]
- Roper, M.; Simonin, A.; Hickey, P.C.; Leeder, A.; Glass, N.L. Nuclear dynamics in a fungal chimera. Proc. Natl. Acad. Sci. USA 2013, 110, 12875–12880. [Google Scholar] [CrossRef] [PubMed]
- Heaton, L.L.M.; Jones, N.S.; Fricker, M.D. A mechanistic explanation of the transition to simple multicellularity in fungi. Nat. Commun. 2020, 11, 2594. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R. Nuclear behavior in fungal hyphae. FEMS Microbiol. Lett. 2005, 249, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Pontecorvo, G. The parasexual cycle in fungi. Annu. Rev. Microbiol. 1956, 10, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F. Fungal vegetative compatibility. Annu. Rev. Phytopathol. 1993, 31, 127–150. [Google Scholar] [CrossRef]
- Bastiaans, E.; Aanen, D.K.; Debets, A.J.; Hoekstra, R.F.; Lestrade, B.; Maas, M.F. Regular bottlenecks and restrictions to somatic fusion prevent the accumulation of mitochondrial defects in Neurospora. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130448. [Google Scholar] [CrossRef][Green Version]
- Debets, F.; Yang, X.; Griffiths, A.J. Vegetative incompatibility in Neurospora: Its effect on horizontal transfer of mitochondrial plasmids and senescence in natural populations. Curr. Genet. 1994, 26, 113–119. [Google Scholar] [CrossRef]
- Caten, C.E. Vegetative incompatibility and cytoplasmic infection in fungi. J. Gen. Microbiol. 1972, 72, 221–229. [Google Scholar] [CrossRef]
- Kinsey, J.A. Tad, a LINE-like transposable element of Neurospora, can transpose between nuclei in heterokaryons. Genetics 1990, 126, 317–323. [Google Scholar]
- Debets, A.J.M.; Griffiths, A.J.F. Polymorphism of het-genes prevents resource plundering in Neurospora crassa. Mycol. Res. 1998, 102, 1343–1349. [Google Scholar] [CrossRef]
- Saupe, S.J. Molecular genetics of heterokaryon incompatibility in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 2000, 64, 489–502. [Google Scholar] [CrossRef]
- Goncalves, A.P.; Heller, J.; Daskalov, A.; Videira, A.; Glass, N.L. Regulated forms of cell death in fungi. Front. Microbiol. 2017, 8, 1837. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Jacobson, D.J.; Shiu, P.K. The genetics of hyphal fusion and vegetative incompatibility in filamentous ascomycete fungi. Annu. Rev. Genet. 2000, 34, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, A.; Mitchell, P.S.; Sandstrom, A.; Vance, R.E.; Glass, N.L. Molecular characterization of a fungal gasdermin-like protein. Proc. Natl. Acad. Sci. USA 2020, 117, 18600–18607. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Zhao, J.; Rosenfield, G.; Kowbel, D.J.; Gladieux, P.; Glass, N.L. Characterization of greenbeard genes involved in long-distance kind discrimination in a microbial eukaryote. PLoS Biol. 2016, 14, e1002431. [Google Scholar] [CrossRef]
- Goncalves, A.P.; Heller, J.; Span, E.A.; Rosenfield, G.; Do, H.P.; Palma-Guerrero, J.; Requena, N.; Marletta, M.A.; Glass, N.L. Allorecognition upon fungal cell-cell contact determines social cooperation and impacts the acquisition of multicellularity. Curr. Biol. 2019, 29, 3006–3017.e3003. [Google Scholar] [CrossRef]
- Daskalov, A.; Gladieux, P.; Heller, J.; Glass, N.L. Programmed cell death in Neurospora crassa is controlled by the allorecognition determinant rcd-1. Genetics 2019, 213, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Clave, C.; Gladieux, P.; Saupe, S.J.; Glass, N.L. NLR surveillance of essential SEC-9 SNARE proteins induces programmed cell death upon allorecognition in filamentous fungi. Proc. Natl. Acad. Sci. USA 2018, 115, E2292–E2301. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, A.; Heller, J.; Herzog, S.; Fleissner, A.; Glass, N.L. Molecular mechanisms regulating cell fusion and heterokaryon formation in filamentous fungi. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Goncalves, A.P.; Glass, N.L. Fungal social barriers: To fuse, or not to fuse, that is the question. Commun. Integr. Biol. 2020, 13, 39–42. [Google Scholar] [CrossRef]
- Muirhead, C.A.; Glass, N.L.; Slatkin, M. Multilocus self-recognition systems in fungi as a cause of trans-species polymorphism. Genetics 2002, 161, 633–641. [Google Scholar]
- Steele, G.C.; Trinci, A.P. Morphology and growth kinetics of hyphae of differentiated and undifferentiated mycelia of Neurospora crassa. J. Gen. Microbiol. 1975, 91, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Bistis, G.N.; Perkins, D.D.; Read, N.D. Different cell types in Neurospora crassa. Fungal Genet. Newslett. 2003, 50, 17–19. [Google Scholar] [CrossRef][Green Version]
- Lew, R.R. Mass flow and pressure-driven hyphal extension in Neurospora crassa. Microbiology 2005, 151, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.D. Hyphal branching in filamentous fungi. Dev. Biol. 2019, 451, 35–39. [Google Scholar] [CrossRef]
- McLean, K.M.; Prosser, J.I. Development of vegetative mycelium during colony growth of Neurospora crassa. Trans. Br. Mycol. Soc. 1987, 88, 489–495. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Ishikawa, E.; Shoji, J.Y.; Nakano, H.; Kitamoto, K. Septum-directed secretion in the filamentous fungus Aspergillus oryzae. Mol. Microbiol. 2011, 81, 40–55. [Google Scholar] [CrossRef]
- Bleichrodt, R.J.; van Veluw, G.J.; Recter, B.; Maruyama, J.; Kitamoto, K.; Wosten, H.A. Hyphal heterogeneity in Aspergillus oryzae is the result of dynamic closure of septa by Woronin bodies. Mol. Microbiol. 2012, 86, 1334–1344. [Google Scholar] [CrossRef]
- Bleichrodt, R.J.; Vinck, A.; Read, N.D.; Wosten, H.A. Selective transport between heterogeneous hyphal compartments via the plasma membrane lining septal walls of Aspergillus niger. Fungal Genet. Biol. 2015, 82, 193–200. [Google Scholar] [CrossRef]
- Tegelaar, M.; Wosten, H.A.B. Functional distinction of hyphal compartments. Sci. Rep. 2017, 7, 6039. [Google Scholar] [CrossRef]
- Schmieder, S.S.; Stanley, C.E.; Rzepiela, A.; van Swaay, D.; Sabotic, J.; Norrelykke, S.F.; de Mello, A.J.; Aebi, M.; Kunzler, M. Bidirectional propagation of signals and nutrients in fungal networks via specialized hyphae. Curr. Biol. 2019, 29, 217–228.e214. [Google Scholar] [CrossRef]
- Moore, D. Tissue Formation. In The Growing Fungus; Gow, N.A.R., Gadd, G.M., Eds.; Chapman & Hall: London, UK, 1994; pp. 423–465. [Google Scholar]
- Watkinson, S.C.; Boddy, L.; Money, N.P. The Fungi, 3rd ed.; Academic Press: Cambridge, MA, USA, 2016; p. 466. [Google Scholar]
- Yafetto, L. The structure of mycelial cords and rhizomorphs in fungi: A mini review. Mycosphere 2018, 9, 984–998. [Google Scholar] [CrossRef]
- Watkinson, S. Growth of rhizmorphs, mycelial strands, coremia and sclerotia. In Fungal Walls and Hyphal Growth; Burnett, J.H., Trinci, A.P.J., Eds.; Cambridge University Press: Cambridge, UK, 1979; pp. 93–113. [Google Scholar]
- Anderson, J.B.; Bruhn, J.N.; Kasimer, D.; Wang, H.; Rodrigue, N.; Smith, M.L. Clonal evolution and genome stability in a 2500-year-old fungal individual. Proc. Biol. Sci. 2018, 285, 20182233. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.E.; Gladfelter, A.S. Nuclear autonomy in multinucleate fungi. Curr. Opin. Microbiol. 2015, 28, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Pieuchot, L.; Lai, J.; Loh, R.A.; Leong, F.Y.; Chiam, K.H.; Stajich, J.; Jedd, G. Cellular subcompartments through cytoplasmic streaming. Dev. Cell. 2015, 34, 410–420. [Google Scholar] [CrossRef]
- Lai, J.; Koh, C.H.; Tjota, M.; Pieuchot, L.; Raman, V.; Chandrababu, K.B.; Yang, D.; Wong, L.; Jedd, G. Intrinsically disordered proteins aggregate at fungal cell-to-cell channels and regulate intercellular connectivity. Proc. Natl. Acad. Sci. USA 2012, 109, 15781–15786. [Google Scholar] [CrossRef]
- Nitsche, B.M.; Burggraaf-van Welzen, A.M.; Lamers, G.; Meyer, V.; Ram, A.F. Autophagy promotes survival in aging submerged cultures of the filamentous fungus Aspergillus niger. Appl. Microbiol. Biotechnol. 2013, 97, 8205–8218. [Google Scholar] [CrossRef]
- Anderson, C.A.; Roberts, S.; Zhang, H.; Kelly, C.M.; Kendall, A.; Lee, C.; Gerstenberger, J.; Koenig, A.B.; Kabeche, R.; Gladfelter, A.S. Ploidy variation in multinucleate cells changes under stress. Mol. Biol. Cell 2015, 26, 1129–1140. [Google Scholar] [CrossRef]
- Roper, M.; Lee, C.; Hickey, P.C.; Gladfelter, A.S. Life as a moving fluid: Fate of cytoplasmic macromolecules in dynamic fungal syncytia. Curr. Opin. Microbiol. 2015, 26, 116–122. [Google Scholar] [CrossRef]
- Zacchetti, B.; Wosten, H.A.B.; Claessen, D. Multiscale heterogeneity in filamentous microbes. Biotechnol. Adv. 2018, 36, 2138–2149. [Google Scholar] [CrossRef]
- Teichert, I.; Wolff, G.; Kuck, U.; Nowrousian, M. Combining laser microdissection and RNA-seq to chart the transcriptional landscape of fungal development. BMC Genom. 2012, 13, 511. [Google Scholar] [CrossRef]
- de Bekker, C.; van Veluw, G.J.; Vinck, A.; Wiebenga, L.A.; Wosten, H.A. Heterogeneity of Aspergillus niger microcolonies in liquid shaken cultures. Appl. Environ. Microbiol. 2011, 77, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Vinck, A.; Terlou, M.; Pestman, W.R.; Martens, E.P.; Ram, A.F.; van den Hondel, C.A.M.J.J.; Wosten, H.A.B. Hyphal differentiation in the exploring mycelium of Aspergillus niger. Mol. Microbiol. 2005, 58, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, T.; Glass, N.L. Dissecting colony development of Neurospora crassa using mRNA profiling and comparative genomics approaches. Eukaryot. Cell 2008, 7, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.M.; de Vries, R.P.; Conesa, A.; de Bekker, C.; Talon, M.; Menke, H.H.; van Peij, N.N.; Wosten, H.A. Spatial differentiation in the vegetative mycelium of Aspergillus niger. Eukaryot. Cell 2007, 6, 2311–2322. [Google Scholar] [CrossRef]
- Caten, C.E.; Jinks, J.L. Heterokaryosis: Its significance in wild homothallic ascomycetes and fungi imperfecti. Trans. Br. Mycol. Soc. 1966, 49, 81–93. [Google Scholar] [CrossRef]
- Strom, N.B.; Bushley, K.E. Two genomes are better than one: History, genetics, and biotechnological applications of fungal heterokaryons. Fungal Biol. Biotechnol. 2016, 3, 4. [Google Scholar] [CrossRef]
- Gehrmann, T.; Pelkmans, J.F.; Ohm, R.A.; Vos, A.M.; Sonnenberg, A.S.M.; Baars, J.J.P.; Wosten, H.A.B.; Reinders, M.J.T.; Abeel, T. Nucleus-specific expression in the multinuclear mushroom-forming fungus Agaricus bisporus reveals different nuclear regulatory programs. Proc. Natl. Acad. Sci. USA 2018, 115, 4429–4434. [Google Scholar] [CrossRef]
- Kafer, E. An 8-chromosome map of Aspergillus nidulans. Adv. Genet. 1958, 9, 105–145. [Google Scholar]
- McGuire, I.C.; Davis, J.E.; Double, M.L.; MacDonald, W.L.; Rauscher, J.T.; McCawley, S.; Milgroom, M.G. Heterokaryon formation and parasexual recombination between vegetatively incompatible lineages in a population of the chestnut blight fungus, Cryphonectria parasitica. Mol. Ecol. 2005, 14, 3657–3669. [Google Scholar] [CrossRef]
- Tsai, H.J.; Nelliat, A. A double-edged sword: Aneuploidy is a prevalent strategy in fungal adaptation. Genes 2019, 10, 787. [Google Scholar] [CrossRef]
- Miao, V.P.; Covert, S.F.; VanEtten, H.D. A fungal gene for antibiotic resistance on a dispensable (“B”) chromosome. Science 1991, 254, 1773–1776. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Rep, M.; Kistler, H.C. The genomic organization of plant pathogenicity in Fusarium species. Curr. Opin. Plant Biol. 2010, 13, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Beerens, B.; Bosch, M.; Linmans, J.; Rep, M. Nuclear dynamics and genetic rearrangement in heterokaryotic colonies of Fusarium oxysporum. Fungal Genet. Biol. 2016, 91, 20–31. [Google Scholar] [CrossRef]
- Zakharov, I.A.; Yarovoy, B.P. Cytoduction as a new tool in studying the cytoplasmic heredity in yeast. Mol. Cell. Biochem. 1977, 14, 15–18. [Google Scholar] [CrossRef]
- Mela, A.P.; Momany, M. Internuclear diffusion of histone H1 within cellular compartments of Aspergillus nidulans. PLoS ONE 2018, 13, e0201828. [Google Scholar] [CrossRef]
- Roca, M.G.; Kuo, H.C.; Lichius, A.; Freitag, M.; Read, N.D. Nuclear dynamics, mitosis, and the cytoskeleton during the early stages of colony initiation in Neurospora crassa. Eukaryot. Cell 2010, 9, 1171–1183. [Google Scholar] [CrossRef]
- Jinks, J.L. Heterokaryosis; a system of adaption in wild fungi. Proc. R. Soc. Lond. B Biol. Sci. 1952, 140, 83–99. [Google Scholar]
- Davis, R.H. Adaptation in pantothenate-requiring Neurospora. II. Nuclear competition during adaptation. Am. J. Bot. 1960, 47, 648–654. [Google Scholar] [CrossRef]
- James, T.Y.; Stenlid, J.; Olson, A.; Johannesson, H. Evolutionary significance of imbalanced nuclear ratios within heterokaryons of the basidiomycete fungus Heterobasidion parviporum. Evolution 2008, 62, 2279–2296. [Google Scholar] [CrossRef]
- Ramsdale, M.; Rayner, A.D.M. Imbalanced nuclear ratios, post-germination mortality and phenotype-genotype relationships in allopatrically-derived heterokaryons of Heterobasidion annosum. New Phytol. 1996, 133, 303–319. [Google Scholar] [CrossRef]
- James, T.Y.; Johansson, S.B.; Johannesson, H. Trikaryon formation and nuclear selection in pairings between heterokaryons and homokaryons of the root rot pathogen Heterobasidion parviporum. Mycol. Res. 2009, 113, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Zeyl, C.; DeVisser, J.A. Estimates of the rate and distribution of fitness effects of spontaneous mutation in Saccharomyces cerevisiae. Genetics 2001, 157, 53–61. [Google Scholar] [PubMed]
- Bastiaans, E.; Debets, A.J.; Aanen, D.K. Experimental evolution reveals that high relatedness protects multicellular cooperation from cheaters. Nat. Commun. 2016, 7, 11435. [Google Scholar] [CrossRef] [PubMed]
- Buss, L.W. Somatic cell parasitism and the evolution of somatic tissue compatibility. Proc. Natl. Acad. Sci. USA 1982, 79, 5337–5341. [Google Scholar] [CrossRef]
- Samils, N.; Oliva, J.; Johannesson, H. Nuclear interactions in a heterokaryon: Insight from the model Neurospora tetrasperma. Proc. Biol. Sci. 2014, 281. [Google Scholar] [CrossRef]
- Freitag, M.; Hickey, P.C.; Raju, N.B.; Selker, E.U.; Read, N.D. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 2004, 41, 897–910. [Google Scholar] [CrossRef]
- Gladfelter, A.S. Nuclear anarchy: Asynchronous mitosis in multinucleated fungal hyphae. Curr. Opin. Microbiol. 2006, 9, 547–552. [Google Scholar] [CrossRef]
- Rosenberger, R.F.; Kessel, M. Synchrony of nuclear replication in individual hyphae of Aspergillus nidulans. J. Bacteriol. 1967, 94, 1464–1469. [Google Scholar] [CrossRef]
- Clutterbuck, A.J. Synchronous nuclear division and septation in Aspergillus nidulans. J. Gen. Microbiol. 1970, 60, 133–135. [Google Scholar] [CrossRef][Green Version]
- Anderson, C.A.; Eser, U.; Korndorf, T.; Borsuk, M.E.; Skotheim, J.M.; Gladfelter, A.S. Nuclear repulsion enables division autonomy in a single cytoplasm. Curr. Biol. 2013, 23, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Han, T.W.; Kato, M.; Xie, S.; Wu, L.C.; Mirzaei, H.; Pei, J.; Chen, M.; Xie, Y.; Allen, J.; Xiao, G.; et al. Cell-free formation of RNA granules: Bound RNAs identify features and components of cellular assemblies. Cell 2012, 149, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Han, T.W.; Xie, S.; Shi, K.; Du, X.; Wu, L.C.; Mirzaei, H.; Goldsmith, E.J.; Longgood, J.; Pei, J.; et al. Cell-free formation of RNA granules: Low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012, 149, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Banjade, S.; Cheng, H.C.; Kim, S.; Chen, B.; Guo, L.; Llaguno, M.; Hollingsworth, J.V.; King, D.S.; Banani, S.F.; et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 2012, 483, 336–340. [Google Scholar] [CrossRef]
- Lee, C.; Occhipinti, P.; Gladfelter, A.S. PolyQ-dependent RNA-protein assemblies control symmetry breaking. J. Cell Biol. 2015, 208, 533–544. [Google Scholar] [CrossRef]
- Lee, C.; Zhang, H.; Baker, A.E.; Occhipinti, P.; Borsuk, M.E.; Gladfelter, A.S. Protein aggregation behavior regulates cyclin transcript localization and cell-cycle control. Dev. Cell 2013, 25, 572–584. [Google Scholar] [CrossRef]
- Dundon, S.E.; Chang, S.S.; Kumar, A.; Occhipinti, P.; Shroff, H.; Roper, M.; Gladfelter, A.S. Clustered nuclei maintain autonomy and nucleocytoplasmic ratio control in a syncytium. Mol. Biol. Cell 2016, 27, 2000–2007. [Google Scholar] [CrossRef]
- Langdon, E.M.; Qiu, Y.; Ghanbari Niaki, A.; McLaughlin, G.A.; Weidmann, C.A.; Gerbich, T.M.; Smith, J.A.; Crutchley, J.M.; Termini, C.M.; Weeks, K.M.; et al. mRNA structure determines specificity of a polyQ-driven phase separation. Science 2018, 360, 922–927. [Google Scholar] [CrossRef]
- Plamann, M.; Minke, P.F.; Tinsley, J.H.; Bruno, K.S. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J. Cell Biol. 1994, 127, 139–149. [Google Scholar] [CrossRef]
- Xiang, X.; Beckwith, S.M.; Morris, N.R. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 1994, 91, 2100–2104. [Google Scholar] [CrossRef]
- Alberti-Segui, C.; Dietrich, F.; Altmann-Johl, R.; Hoepfner, D.; Philippsen, P. Cytoplasmic dynein is required to oppose the force that moves nuclei towards the hyphal tip in the filamentous ascomycete Ashbya gossypii. J. Cell Sci. 2001, 114, 975–986. [Google Scholar] [PubMed]
- Sachsenmaier, W.; Remy, U.; Plattner-Schobel, R. Initiation of synchronous mitosis in Physarum polycephalum. A model of the control of cell division in eukariots. Exp. Cell Res. 1972, 73, 41–48. [Google Scholar] [CrossRef]
- Dynesen, J.; Nielsen, J. Branching is coordinated with mitosis in growing hyphae of Aspergillus nidulans. Fungal Genet. Biol. 2003, 40, 15–24. [Google Scholar] [CrossRef]
- Neumann, F.R.; Nurse, P. Nuclear size control in fission yeast. J. Cell Biol. 2007, 179, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Kume, K.; Cantwell, H.; Neumann, F.R.; Jones, A.W.; Snijders, A.P.; Nurse, P. A systematic genomic screen implicates nucleocytoplasmic transport and membrane growth in nuclear size control. PLoS Genet. 2017, 13, e1006767. [Google Scholar] [CrossRef]
- Roberts, S.E.; Gladfelter, A.S. Nuclear dynamics and cell growth in fungi. In Growth, Differentiation and Sexuality; Wendland, J., Ed.; Springer: Cham, Switzerland, 2016; pp. 27–46. [Google Scholar]
- May, G.; Taylor, J.W. Patterns of mating and mitochondrial DNA inheritance in the agaric Basidiomycete Coprinus cinereus. Genetics 1988, 118, 213–220. [Google Scholar]
- Lee, S.B.; Taylor, J.W. Uniparental inheritance and replacement of mitochondrial DNA in Neurospora tetrasperma. Genetics 1993, 134, 1063–1075. [Google Scholar]
- Daubois, L.; Beaudet, D.; Hijri, M.; de la Providencia, I. Independent mitochondrial and nuclear exchanges arising in Rhizophagus irregularis crossed-isolates support the presence of a mitochondrial segregation mechanism. BMC Microbiol. 2016, 16, 11. [Google Scholar] [CrossRef]
- Aanen, D.K.; Kuyper, T.W.; Debets, A.J.; Hoekstra, R.F. The evolution of non-reciprocal nuclear exchange in mushrooms as a consequence of genomic conflict. Proc. Biol. Sci. 2004, 271, 1235–1241. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mela, A.P.; Rico-Ramírez, A.M.; Glass, N.L. Syncytia in Fungi. Cells 2020, 9, 2255. https://doi.org/10.3390/cells9102255
Mela AP, Rico-Ramírez AM, Glass NL. Syncytia in Fungi. Cells. 2020; 9(10):2255. https://doi.org/10.3390/cells9102255
Chicago/Turabian StyleMela, Alexander P., Adriana M. Rico-Ramírez, and N. Louise Glass. 2020. "Syncytia in Fungi" Cells 9, no. 10: 2255. https://doi.org/10.3390/cells9102255
APA StyleMela, A. P., Rico-Ramírez, A. M., & Glass, N. L. (2020). Syncytia in Fungi. Cells, 9(10), 2255. https://doi.org/10.3390/cells9102255





