Functional Foods: An Approach to Modulate Molecular Mechanisms of Alzheimer’s Disease
Abstract
:1. Introduction
2. Nutraceuticals: Overview
2.1. Classification of Nutraceuticals
- (i)
- Classification of nutraceuticals based on origin: vegetable, animal and microbial.Not only foods from plant or animal origin are sources of nutraceuticals, but also bacteria. In some cases, the identification of the source is not immediate; for example, conjugated linoleic acid, which is essential to human nutrition, is mainly found in animal food such as beef and dairy products, but only because it is produced by the bacteria present in the rumen of the cows. Many nutraceuticals have a high conserved biochemical structure between species and therefore can be found in both animal and vegetable or can be a microbe’s by-product. This applies to choline and phosphatidylcholine, for example.
- (ii)
- Classification of nutraceuticals based on the mechanism of actionThe classification according to the mechanism of action is widely used by doctors, nutritionists, dietitians and, generally speaking, by those who provide people with useful indications to improve their health. Hence, who deals with cardiovascular diseases will be more interested in those compounds improving the lipid profile and with anti-inflammatory action, as well as who deals with oncology will be more interested in knowing those compounds having anticancer properties.
- (iii)
- Classification of nutraceuticals based on their chemistry natureBased on the chemical nature of the different compounds, consistently with [52], nutraceuticals can be classified as:
- (1)
- Dietary fibers: substances of vegetable origin present in foods that are not metabolized in the large digestive tract and increase volume of the intestinal content. Chemically, dietary fiber means carbohydrate polymers with a degree of polymerization not lower than 3, which are neither digested nor absorbed in the small intestine. Examples include fruit, barley, oats, lignin, cellulose, pectin, etc. Generous intake of these fibers by diet is associated with a low risk of cardiovascular disease, hypertension, diabetes, obesity, colon cancer and gastrointestinal disorders.
- (2)
- Probiotics: probiotics are food components consisting of live microbes that have many beneficial effects on the human body. Diets rich in probiotics, prepared naturally or by industrial fermentation processes, have shown positive effects due to improvement of the intestinal microbiota.
- (3)
- Prebiotics: unlike probiotics, they are not live organisms. They are short-chain polysaccharides that have unique chemical structures-in particular fructose-based oligosaccharides that exist naturally in food or are added in the food-that are not digested by humans, but still have many useful effects on our body. In the colon, the structure and performance of the microbiota change, thus influencing the growth and action of specific bacteria improving the health of the host. Prebiotics represent the nourishment of probiotics and stimulate their activity in the gastro-intestinal tract. Examples of these foods are the roots of chicory, banana, tomato, allium, beans, etc.
- (4)
- Polyunsaturated fatty acids: omega 3 fatty acids, e.g., α-linolenic acid, eicosapentaenoic acid and docosahexaenoic acid, are present in oily fish, flax seeds, soybeans, etc. or omega 6 fatty acids, e.g., α-linoleic acid and arachidonic acid, are found in corn, safflower, sunflower and soybean, etc.
- (5)
- Antioxidant vitamins: vitamin C, vitamin E and carotenoids. These vitamins are abundant in many fruits and vegetables. Regular intake of them helps to prevent a range of diseases.
- (6)
- Polyphenols: they are phytochemicals produced by plants to protect against photosynthetic stress and reactive oxygen species. Among the others, flavonoids, anthocyanins and phenolic acids, found in a variety of foods, have anti-inflammatory and antioxidant properties.
- (7)
- Spices: esoteric food additives used to improve the sensory quality of food. Most of the spices are terpenes and other components of essential oils. Minimal amount of diet spices has antioxidant, chemopreventive, anti-mutagenic, anti-inflammatory and immune effects.
3. Alzheimer: Molecular Aspects and Causes of the Disease
- remember (both short and long term) and recognize,
- speak and write,
- make decisions and solve problems,
- interpret sensory input,
- orientate and juggle yourself the world.
3.1. β-Amyloid and Tau Proteins
- In the brain without cognitive impairment there can be Aβ and tau deposition.
- Clinical diagnosis of AD does not involve the status of Aβ and tau in the brain.
- Size and enlargement of the plaques are not related to cognitive impairment.
- Amyloid in the brain is not a warning sign of dementia.
3.2. Apoptosis
3.3. Mitochondrial Dysfunction and Oxidative Stress
3.4. Microbiota and Diet
- i.
- microbiota cooperates with the host to maintain optimal health status of the individual;
- ii.
- nutrition influences the intestinal microbiota and the risk to develop AD.
4. Nutraceuticals for Neuroprotection and Treatment of Alzheimer’s Disease
- oxidative stress and mitochondrial dysfunction,
- Aβ and tau toxicity and aggregation,
- neuronal damage and apoptosis,
- memory loss and cognitive decline.
4.1. Nutraceutical Compounds against Oxidative Stress and Mitochondrial Dysfunction
4.1.1. Genistein
4.1.2. Resveratrol (RSV)
4.1.3. Curcumin
4.1.4. Carotenoid
4.1.5. Lycopene
4.1.6. Extra Virgin Olive Oil (EVOO)
4.2. Nutraceutical Compounds against Aβ and Tau Toxicity and Aggregation
4.2.1. Genistein
4.2.2. RSV
4.2.3. Curcumin
4.2.4. (−)Epigallocatechin-3-Gallate
4.2.5. Lycopene
4.2.6. EVOO
4.2.7. Coconut Oil
4.3. Nutraceutical Compounds against Neuronal Damage and Apoptosis
4.3.1. Genistein
4.3.2. RSV
4.3.3. Lycopene
4.3.4. EVOO
4.4. Nutraceutical Compounds against Memory Loss and Cognitive Decline
4.4.1. Genistein
4.4.2. RSV
4.4.3. Curcuma Longa
4.4.4. Lycopene
4.4.5. Ginkgo Biloba
4.4.6. EVOO
5. Nutrients Modulation of Gut Microbiota as Therapeutic Strategy for the Treatment of Alzheimer’s Disease
5.1. Prebiotics and Probiotics
5.2. Polyunsaturated Fatty Acids (PUFAs)
5.3. Polyphenols
6. Bioactive Compound Actions on Epigenetic Mechanisms in Alzheimer’s Disease
6.1. Impact of Dietary Factors on DNA Methylation
6.2. Impact of Dietary Factors on Histone Post-Translational Modification
6.3. Impact of Dietary Factors on Microrna Regulating Action
7. The Research Continues
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full name |
| AD | Alzheimer’s disease |
| AGE | aged garlic extract |
| ANT | adenine nucleotide translocator |
| APP | β-Amyloid precursor protein |
| Aβ | β-amyloid peptide |
| BACE | Beta-Site APP-Cleaving Enzyme |
| BBB | blood–brain barrier |
| CGCs | cerebellum granule cells |
| CNS | central nervous system |
| CUR | Curcumin |
| DHA | docosahexaenoic acid |
| DNMT | DNA methyl transferase |
| DZN | Daidzein |
| EC | epicatechin |
| EGCG | (−)epigallocatechin-3-gallate |
| EPA | eicosapentaenoicacid |
| ETs | ellagitannins |
| EVOO | extravirgin olive oil |
| FOXO | forkhead O |
| GEN | Genistein |
| GSH | reduced glutathione |
| GSK | glycogen synthase kinase-3β |
| HAT | acetyltransferase |
| HCY | homocysteine |
| HDAC | histone deacetylase |
| LOAD | late-onset AD |
| LYC | Lycopene |
| MCI | Mild Cognitive Impairment |
| MD | Mediterranean diet |
| MDA | malondialdehyde |
| MGBaxis | microbiota-gut-brain axis |
| mtDNA | mitochondrial DNA |
| NFT | neurofibrillary tangles |
| NGF | Nerve growth factor |
| OLE | oleuropein |
| OMO | Morinda officinalis |
| OXPHOS | oxidative phosphorylation |
| PSEN | presenilin |
| PUFA | polyunsaturated fatty acids |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RSV | resveratrol |
| SAH | S-adenosylhomocystein |
| SAM | S-adenosylmethionine. |
References
- Bergamini, E. Nutraceuticals: A valuable aid to be used cautiously. G. Gerontol. 2010, 58, 255–258. [Google Scholar]
- Bekris, L.M.; Yu, C.E.; Bird, T.D.; Tsuang, D.W. Genetics of Alzheimer Disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.H.; Marioni, R.E.; Harris, S.E.; Deary, I.J. Brain age and other bodily ‘ages’: Implications for neuropsychiatry. Mol. Psychiatry 2019, 24, 266–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, G.W.; Ercoli, L.M.; Silverman, D.H.; Huang, S.C.; Komo, S.; Bookheimer, S.Y.; Lavretsky, H.; Miller, K.; Siddarth, P.; Rasgon, N.L.; et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 6037–6042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.C. Living too long: The current focus of medical research on increasing the quantity, rather than the quality, of life is damaging our health and harming the economy. EMBO Rep. 2015, 16, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Bush, A.I.; Ceccarelli, A.; Cooper, J.; de Jager, C.A.; Erickson, K.I.; Fraser, G.; Kesler, S.; Levin, S.M.; Lucey, B.; et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol. Aging 2014, 35, S74–S78. [Google Scholar] [CrossRef] [Green Version]
- Cremonini, A.L.; Caffa, I.; Cea, M.; Nencioni, A.; Odetti, P.; Monacelli, F. Nutrients in the Prevention of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2019, 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Amini, Y.; Saif, N.; Greer, C.; Hristov, H.; Isaacson, R. The Role of Nutrition in Individualized Alzheimer’s Risk Reduction. Curr. Nutr. Rep. 2020, 9, 55–63. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant Therapies for Neuroprotection-A Review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef] [Green Version]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell. Longev. 2019, 2019, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subash, S.; Essa, M.M.; Al-Adawi, S.; Memon, M.A.; Manivasagam, T.; Akbar, M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural. Regen. Res. 2014, 9, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.M.; Romeiro, C.F.R.; Rodrigues, C.A.; Cerqueira, A.R.L.; Monteiro, M.C. Mitochondrial Dysfunction and Alpha-Lipoic Acid: Beneficial or Harmful in Alzheimer’s Disease? Oxid. Med. Cell. Longev. 2019, 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Cascella, M.; Bimonte, S.; Muzio, M.R.; Schiavone, V.; Cuomo, A. The efficacy of Epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer’s disease: An overview of pre-clinical studies and translational perspectives in clinical practice. Infect. Agent Cancer 2017, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef] [Green Version]
- Colizzi, C. The protective effects of polyphenols on Alzheimer’s disease: A systematic review. Alzheimers. Dement. 2018, 5, 184–196. [Google Scholar] [CrossRef]
- Moretti, R.; Peinkhofer, C. B Vitamins and Fatty Acids: What Do They Share with Small Vessel Disease-Related Dementia? Int. J. Mol. Sci. 2019, 20, 5797. [Google Scholar] [CrossRef] [Green Version]
- Mazzanti, G.; Di Giacomo, S. Curcumin and Resveratrol in the Management of Cognitive Disorders: What Is the Clinical Evidence? Molecules 2016, 21, 1243. [Google Scholar] [CrossRef] [Green Version]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Reale, M.; Costantini, E.; Jagarlapoodi, S.; Khan, H.; Belwal, T.; Cichelli, A. Relationship of Wine Consumption with Alzheimer’s Disease. Nutrients 2020, 12, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Rochina, J.M.; Aguilar, M.A.; Hu Yang, I. Improvement of Main Cognitive Functions in Patients With Alzheimer’s Disease After Treatment With Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimers Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Mediterranean and MIND Diets Containing Olive Biophenols Reduces the Prevalence of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFelice, S.L. FIM Rationale and Proposed Guidelines for the Nutraceutical Research & Education Act-NREA. Foundation for Innovation in Medicine. Available online: https://fimdefelice.org/fim-rationale-and-proposed-guidelines-for-the-nutraceutical-research-education-act-nrea/ (accessed on 10 November 2002).
- Kalra, E.K. Nutraceutical-Definition and Introduction. AAPS PharmSci. 2003, 5, E25. [Google Scholar] [CrossRef] [Green Version]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Lelario, F.; Scrano, L.; De Franchi, S.; Bonomo, M.G.; Salzano, G.; Milan, S.; Milella, L.; Bufo, S.A. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chem. Biol. Technol. Agric. 2018, 5, 13. [Google Scholar] [CrossRef]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef] [Green Version]
- Franzago, M.; Santurbano, D.; Vitacolonna, E.; Stuppia, L. Genes and Diet in the Prevention of Chronic Diseases in Future Generations. Int. J. Mol. Sci. 2020, 21, 2633. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Tech. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanna, B.; Mishra, A. Metabolites Unravel Nutraceutical Potential of Edible Seaweeds: An Emerging Source of Functional Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef] [Green Version]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Cerdà, B.; Tomàs-Barberàn, F.; Espìn, J.C. Metabolism of chemopreventive and antioxidant ellagitannins from strawberries, raspberries, walnuts and oak-aged wines in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomàs-Barberàn, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Cerdà, B.; Espìn, J.C.; Parra, A.; Martìnez, P.; Tomàs-Barberàn, F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolized into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef]
- Kaur, S. Free radicals and antioxidant (nutraceuticals). Book to human health. Int. J. Nat. Product Sci. 2012, 1, 175. [Google Scholar]
- Dutta, S.; Ali, K.M.; Dash, S.K.; Giri, B. Role of nutraceuticals on health promotion and disease prevention: A review. J. Drug Deliv. Ther. 2018, 8, 42–47. [Google Scholar] [CrossRef]
- Song, H.; Cui, J.; Mossine, V.V.; Greenlief, C.M.; Fritsche, K.; Sun, G.Y.; Gu, Z. Bioactive components from garlic on brain resiliency against neuroinflammation and neurodegeneration (Review). Exp. Ther. Med. 2020, 19, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Zeisel, S.H. Regulation of “Nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef] [PubMed]
- Merriam-Webster Online Dictionary; Merriam-Webster Inc.: Springfield, MA, USA, 2015; Available online: https://www.merriam-webster.com/ (accessed on 22 September 2020).
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F. Nutraceuticals, Functional Foods and Dietary Supplements in Health and Disease. J. Food Drug Anal. 2012, 20 (Suppl. 1), 226–230. [Google Scholar] [CrossRef]
- Sawicka, B.; Ziarati, P.; Krochmal-Marczak, B.; Skiba, D. Nutraceuticals in food and pharmacy. A Review. Agron. Sci. 2020, 74, 7–31. [Google Scholar] [CrossRef]
- Pandey, N.; Meena, R.P.; Rai, S.K.; Pandey-Roy, S. Medicinal plants derived nutraceuticals: A re-emerging health aid. Int. J. Pharma Bio Sci. 2011, 2, 419–441. [Google Scholar]
- Wildman, R.E. (Ed.) Handbook of Nutraceuticals and Functional Foods; CRC Press: Boca Raton, FL, USA, 2016; Available online: https://books.google.com/books?hl=zh-CN&lr=&id=ej8qBgAAQBAJ&oi=fnd&pg=PP1&dq=Handbook+of+Nutraceuticals+and+Functional+Foods&ots=f5c7g95KKq&sig=klOJzfoXi9TqUnRSvywK3SvT9Q0#v=onepage&q=Handbook%20of%20Nutraceuticals%20and%20Functional%20Foods&f=false.
- Prakash, D.; Gupta, C.; Sharma, G. Importance of phytochemicals in nutraceuticals. J. Chin. Med. Res. Develop. 2012, 1, 70–78. [Google Scholar]
- Ghani, U.; Naeem, M.; Rafeeq, H.; Imtiaz, U.; Amjad, A.; Ullah, S.; Rehman, A.; Qasim, F. A Novel Approach towards Nutraceuticals and Biomedical Applications. Sch Int. J. Biochem. 2019, 2, 245–252. [Google Scholar] [CrossRef]
- Ruchi, S.; Amanjot, K.; Sourav, T.; Keerti, B.; Sujit, B. Role of nutraceuticals in health care: A review. Int. J. Green Pharm. 2017, 11, S385–S394. [Google Scholar]
- Asghar, A.; Randhawa, M.A.; Masood, M.M.; Abdullah, M.; Irshad, M.A. Nutraceutical formulation strategies to enhance the bioavailability and efficiency: An overview. In Role of Materials Science in Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 329–352. [Google Scholar] [CrossRef]
- Zaki, N.M. Progress and problems in nutraceuticals delivery. J. Bioeq. Bioavail. 2014, 6, 75–77. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Marziano, M.; Rungratanawanich, W.; Memo, M.; Uberti, D. Nutrition and AGE-ing: Focusing on Alzheimer’s disease. Oxid. Med. Cell. Longev. 2017, 2017, 10. [Google Scholar] [CrossRef]
- Nicolia, V.; Lucarelli, M.; Fuso, A. Environment, epigenetics and neurodegeneration: Focus on nutrition in Alzheimer’s disease. Exp. Gerontol. 2015, 68, 8–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Checler, F. Presenilins: Multifunctional Proteins Involved in Alzheimer’s Disease Pathology. IUBMB Life 1999, 48, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Bird, T.D. Genetic aspects of Alzheimer disease. Genet. Med. 2008, 10, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms, and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Chen, Y.; Mok, K.Y.; Kwok, T.C.Y.; Mok, V.C.T.; Guo, Q.; Ip, F.C.; Chen, Y.; Mullapudi, N.; Alzheimer’s Disease Neuroimaging Initiative; et al. Non-coding variability at the APOE locus contributes to the Alzheimer’s risk. Nat. Commun. 2019, 10, 3310. [Google Scholar] [CrossRef] [Green Version]
- Kodali, M.; Parihar, V.K.; Hattiangady, B.; Mishra, V.; Shuai, B.; Shetty, A.K. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature, and reduced glial activation. Sci Rep. 2015, 28, 8075. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S. Selective Neuronal Death in Neurodegenerative Diseases: The Ongoing Mystery. Yale J. Biol. Med. 2019, 92, 695–705. [Google Scholar]
- Yamin, G.; Ono, K.; Inayathullah, M.; Teplow, D.B. Amyloid β-Protein Assembly as a Therapeutic Target of Alzheimer’s Disease. Curr. Pharm. Des. 2008, 14, 3231–3246. [Google Scholar] [CrossRef] [PubMed]
- Poddar, J.; Pradhan, M.; Ganguly, G.; Chakrabarti, S. Biochemical deficits and cognitive decline in brain aging: Intervention by dietary supplements. J. Chem. Neuroanat. 2019, 95, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the β-Amyloid Peptide. J. Alzh. Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid βeta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, K.M.; Kennedy, K.M.; Park, D.C. Beta-amyloid deposition and the aging brain. Neuropsychol. Rev. 2009, 19, 436–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, C.C.; Villemagne, V.L. Brain amyloid imaging. J. Nucl. Med. 2011, 52, 1733–1740. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Aizenstein, H.J.; Nebes, R.D.; Saxton, J.A.; Price, J.C.; Mathis, C.A.; Tsopelas, N.D.; Ziolko, S.K.; James, J.A.; Snitz, B.E.; Houck, P.R.; et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008, 65, 1509–1517. [Google Scholar] [CrossRef]
- Huang, Y.R.; Liu, R.T. The Toxicity and Polymorphism of β-Amyloid Oligomers. Int. J. Mol. Sci. 2020, 21, 4477. [Google Scholar] [CrossRef]
- Creegan, R.; Hunt, W.; McManus, A.; Rainey-Smith, S.R. Diet, nutrients and metabolism: Cogs in the wheel driving Alzheimer’s disease pathology? Br. J. Nutr. 2015, 113, 1499–1517. [Google Scholar] [CrossRef] [Green Version]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Substantiation for the Use of Curcumin during the Development of Neurodegeneration after Brain Ischemia. Int. J. Mol. Sci. 2020, 21, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ittner, A.; Ittner, L.M. Dendritic Tau in Alzheimer’s Disease. Neuron 2018, 99, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Kabir, M.T.; Rahman, M.S.; Behl, T.; Jeandet, P.; Ashraf, G.M.; Najda, A.; Bin-Jumah, M.N.; El-Seedi, H.R.; Abdel-Daim, M.M. Revisiting the Amyloid Cascade Hypothesis: From Anti-Aβ Therapeutics to Auspicious New Ways for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 5858. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Rottkamp, C.A.; Nunomura, A.; Raina, A.K.; Perry, G. Oxidative stress in Alzheimer’s disease. Biochim. Biophys. Acta 2000, 1502, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adhes. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Perluigi, M.; Sultana, R.; Cenini, G.; Di Domenico, F.; Memo, M.; Pierce, W.M.; Coccia, R.; Butterfield, D.A. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer’s disease: Role of lipid peroxidation in Alzheimer’s disease pathogenesis. Proteomics Clin. Appl. 2009, 3, 682–693. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.P.; Clark, I.A.; Vissel, B. Questions concerning the role of amyloid-β in the definition, aetiology and diagnosis of Alzheimer’s disease. Acta Neuropathol. 2018, 136, 663–689. [Google Scholar] [CrossRef] [Green Version]
- Frankish, H.; Horton, R. Prevention and management of dementia: A priority for public health. Lancet 2017, 390, 2614–2615. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Ballard, C.; Lang, I. Alcohol and dementia: A complex relationship with potential for dementia prevention. Lancet Public Health 2018, 3, e103–e104. [Google Scholar] [CrossRef] [Green Version]
- Launer, L.L. Blood Pressure Control as an Intervention to Prevent Dementia. Lancet Neurol. 2019, 18, 906–908. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.R.; Hong, C.J.; Kim, J.Y.; Kim, E.K.; Sun, W.; Yu, S.W. Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol. Brain 2016, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef] [PubMed]
- Calissano, P.; Ciotti, M.T.; Galli, C.; Mercanti, D.; Dus, L.; Canu, N.; Barbato, C.; Vitolo, O.V.; Atlante, A.; Gagliardi, S. The Role of IGF-I in Cerebellar Granule Cell Survival and Terminal Differentiation. In IGFs in the Nervous System; Müller, E.E., Ed.; Springer: Berlin, Germany, 1998; pp. 60–71. Available online: https://link.springer.com/chapter/10.1007/978-88-470-2246-1_5.
- Calissano, P.; Matrone, C.; Amadoro, G. Apoptosis and in vitro Alzheimer disease neuronal models. Commun. Integr. Biol. 2009, 2, 163–169. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.R.; Galli, C.; Ciotti, M.T.; Calissano, P. Induction of Apoptosis in Cerebellar Granule Neurons by Low Potassium: Inhibition of Death by Insulin-Like Growth Factor I and cAMP. Proc. Natl. Acad. Sci. USA 1993, 90, 10989–10993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, D.M.A. The neuropathology of Alzheimer’s disease: A review with pathogenetic, aetiological and therapeutic considerations. Mech. Ageing 1985, 31, 213–255. [Google Scholar] [CrossRef]
- Hyman, B.T.; Van Hoesen, G.W.; Damasio, A.R. Memory-related neural systems in Alzheimer’s disease: An anatomic study. Neurology 1990, 40, 1721–1730. [Google Scholar] [CrossRef]
- Tait, S.W.; Ichim, G.; Green, D.R. Die another way – non-apoptotic mechanisms of cell death. J. Cell Sci. 2014, 127, 2135–2144. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Diff. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Hartman, M.L. Non-Apoptotic Cell Death Signaling Pathways in Melanoma. Int. J. Mol. Sci. 2020, 21, 2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, G.; Mazziotta, J.; Collins, M.; Baxter, L.; Phelps, M.; Mandelkern, M.; Kaplan, A.; La, R.A.; Adamson, C.; Chang, L. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 1995, 273, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Sorbi, S.; Bird, E.D.; Blass, J.P. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann. Neurol. 1983, 13, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F.; Besnard, A.M. Thiamine-dependent enzyme changes in temporal cortex of patients with Alzheimer’s disease. Metab. Brain Dis. 1990, 5, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacoma, F.; Bettendorff, L.; Grisar, T.; Kish, S.J. Brain thiamine, its phosphate esters, and its metabolizing enzymes in Alzheimer’s disease. Ann. Neurol. 1996, 39, 585–591. [Google Scholar] [CrossRef]
- Bubber, P.; Haroutunian, V.; Fisch, G.; Blass, J.P.; Gibson, G.E. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Ann. Neurol. 2005, 57, 695–703. [Google Scholar] [CrossRef]
- Maurer, I.; Zierz, S.; Möller, H.J. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol. Aging 2000, 21, 455–462. [Google Scholar] [CrossRef]
- Parker, W.D.; Filley, C.M.; Parks, J.K. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology 1990, 40, 1302–1303. [Google Scholar] [CrossRef]
- Lezi, E.; Swerdlow, R.H. Mitochondria in Neurodegeneration. Adv. Exp. Med. Biol. 2012, 942, 269–286. [Google Scholar] [CrossRef] [Green Version]
- Atlante, A.; de Bari, L.; Bobba, A.; Amadoro, G. A disease with a sweet tooth: Exploring the Warburg effect in Alzheimer’s disease. Biogerontology 2017, 18, 301–319. [Google Scholar] [CrossRef]
- Castellani, R.; Hirai, K.; Aliev, G.; Drew, K.L.; Nunomura, A.; Takeda, A.; Cash, A.D.; Obrenovich, M.E.; Perry, G.; Smith, M.A. Role of mitochondrial dysfunction in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Marotta, F.; Dominguez, L.J. Oxidative Stress in Patients with Alzheimer’s Disease: Effect of Extracts of Fermented Papaya Powder. Mediators Inflamm. 2015, 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo-Mora, P.; Luna, R.; Colín-Barenque, L. Amyloid Beta: Multiple Mechanisms of Toxicity and Only Some Protective Effects? Oxid. Med. Cell. Longev. 2014, 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collina, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Tönniesa, E.; Trushinaa, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Atlante, A.; Amadoro, G.; Bobba, A.; de Bari, L.; Corsetti, V.; Pappalardo, G.; Marra, E.; Calissano, P.; Passarella, S. A peptide containing residues 26-44 of tau protein impairs mitochondrial oxidative phosphorylation acting at the level of the adenine nucleotide translocator. Biochim Biophys Acta. 2008, 1777, 1289–1300. [Google Scholar] [CrossRef] [Green Version]
- Florenzano, F.; Veronica, C.; Ciasca, G.; Ciotti, M.T.; Pittaluga, A.; Olivero, G.; Feligioni, M.; Iannuzzi, F.; Latina, V.; Sciacca, M.F.; et al. Extracellular truncated tau causes early presynaptic dysfunction associated with Alzheimer’s disease and other tauopathies. Oncotarget 2017, 8, 64745–64778. [Google Scholar] [CrossRef] [Green Version]
- Bobba, A.; Amadoro, G.; Petragallo, V.A.; Calissano, P.; Atlante, A. Dissecting the molecular mechanism by which NH2htau and Aβ1-42 peptides impair mitochondrial ANT-1 in Alzheimer disease. Biochim Biophys Acta. 2013, 1827, 848–860. [Google Scholar] [CrossRef] [Green Version]
- Bobba, A.; Amadoro, G.; Valenti, D.; Corsetti, V.; Lassandro, R.; Atlante, A. Mitochondrial respiratory chain Complexes I and IV are impaired by β-amyloid via direct interaction and through Complex I-dependent ROS production, respectively. Mitochondrion 2013, 13, 298–311. [Google Scholar] [CrossRef]
- Cenini, G.; Voos, W. Mitochondria as Potential Targets in Alzheimer Disease Therapy: An Update. Front. Pharmacol. 2019, 10, 902. [Google Scholar] [CrossRef]
- Evin, G.; Barakat, A. Critical analysis of the use of β-site amyloid precursor protein-cleaving enzyme 1 inhibitors in the treatment of Alzheimer’s disease. Degener Neurol. Neuromusc Dis. 2014, 4, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Zuo, L.; Hemmelgarn, B.T.; Chuang, C.C.; Best, T.M. The Role of Oxidative Stress-Induced Epigenetic Alterations in Amyloid-𝛽 Production in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinagra, E.; Utzeri, E.; Morreale, G.C.; Fabbri, C.; Pace, F.; Anderloni, A. Microbiota-gut-brain axis and its affect inflammatory bowel disease: Pathophysiological concepts and insights for clinicians. World J. Clin. Cases. 2020, 8, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severia, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. (Encinitas) 2018, 17, 28–32. [Google Scholar]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome–microglia connections via the gut–brain axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef] [Green Version]
- Giau, V.V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.Y.; Hulme, J. Gut Microbiota and Their Neuroinflammatory Implications in Alzheimer’s Disease. Nutrients 2018, 10, 1765. [Google Scholar] [CrossRef] [Green Version]
- Askarova, S.; Umbayev, B.; Masoud, A.R.; Kaiyrlykyzy, A.; Safarova, Y.; Tsoy, A.; Olzhayev, F.; Kushugulova, A. The Links Between the Gut Microbiome, Aging, Modern Lifestyle and Alzheimer’s Disease. Front. Cell. Infect. Microbiol. 2020, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human gut microbiota: The links with dementia development. Protein Cell 2017, 8, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-O.; Holtzman, D.M. Gut Microbiota: From the Forgotten Organ to a Potential Key Player in the Pathology of Alzheimer’s Disease. J. Gerontol A Biol. Sci. Med. Sci. 2020, 75, 1232–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den, H.; Dong, X.; Chen, M.; Zou, Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment-a meta-analysis of randomized controlled trials. Aging 2020, 12, 4010–4039. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, S.; Gillette-Guyonnett, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.N.; Dantoine, T.; Dartigues, J.F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid Beta in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Singh, R. Current Alzheimer’s management with berries fruits therapy. J. Public Health Nutr. 2018, 1, 17–24. [Google Scholar] [CrossRef]
- Rathod, R.; Kale, A.; Joshi, S. Novel insights into the effect of vitamin B12 and omega-3 fatty acids on brain function. J. Biomed. Sci. 2016, 23, 17. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy-A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Mushtaq, M.; Wani, S.M. Poliphenols and human health—A review. Int. J. Pharm. Bio Sci. 2013, 4, 338–360. [Google Scholar]
- Mecocci, P.; Tinarelli, C.; Schulz, R.J.; Polidori, M.C. Nutraceuticals in cognitive impairment and Alzheimer’s disease. Front. Pharmacol. 2014, 5, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl.2), S215–S220. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.; Zhua, X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease. Biochim Biophys Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener 2020, 15, 1–22. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 15. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Jacob, K.D.; Hooten, N.N.; Trzeciak, A.R.; Evans, M.K. Markers of Oxidant Stress that are Clinically Relevant in Aging and Age-related Disease. Mech. Ageing Dev. 2013, 134, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Saharan, S.; Mandal, P.K. The emerging role of glutathione in Alzheimer’s disease. J. Alzheimers Dis. 2014, 40, 519–529. [Google Scholar] [CrossRef]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Saha, S.; Dutta, S.; Mahalanobish, S.; Sil, P.C. Nutraceuticals: An emerging therapeutic approach against the pathogenesis of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 100–114. [Google Scholar] [CrossRef]
- Park, Y.-J.; Ko, J.W.; Jeon, S.; Kwon, Y.H. Protective Effect of Genistein against Neuronal Degeneration in ApoE−/− Mice Fed a High-Fat Diet. Nutrients 2016, 8, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, K.P.; Shanmuganathan, B.; Manayi, A.; Nabavi, S.F.; Nabavi, S.M. Molecular and Therapeutic Targets of Genistein in Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 7028–7041. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Li, L.; Zhang, X.H.; Xiang, L.; Zhang, J.; Feng, J.F.; Xiao, R. Neuroprotective effects of genistein and folic acid on apoptosis of rat cultured cortical neurons induced by β-amyloid 31–35. Br J. Nutr. 2009, 102, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; Wu, M.N.; Qi, J.S.; Qiao, J.T. Amyloid beta-protein fragment 31–35 suppresses long-term potentiation in hippocampal CA1 region of rats in vivo. Synapse 2006, 60, 307–313. [Google Scholar] [CrossRef]
- Clementi, M.E.; Marini, S.; Coletta, M.; Orsini, F.; Giardina, B.; Misiti, F. Abeta(31–35) and Abeta(25–35) fragments of amyloid beta-protein induce cellular death through apoptotic signals: Role of the redox state of methionine-35. FEBS Lett. 2005, 579, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Yuan, L.; Yu, H.; Li, L.; Ma, W.; Bi, X.; Feng, J.; Xiao, R. Genistein and Folic Acid Prevent Oxidative Injury Induced by β-Amyloid Peptide. Basic Clin. Pharmacol. Toxicol 2011, 108, 333–340. [Google Scholar] [CrossRef]
- Atlante, A.; Bobba, A.; Paventi, G.; Pizzuto, R.; Passarella, S. Genistein and daidzein prevent low potassium-dependent apoptosis of cerebellar granule cells. Biochem. Pharmacol. 2010, 79, 758–767. [Google Scholar] [CrossRef] [Green Version]
- Wijeratne, S.S.; Cuppett, S.L. Soy isoflavones protect the intestine from lipid hydroperoxide mediated oxidative damage. J. Agric. Food Chem 2007, 55, 9811–9816. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chemistry 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Kelly, E.; Vyas, P.; Weber, J.T. Biochemical Properties and Neuroprotective Effects of Compounds in Various Species of Berries. Molecules 2017, 23, 26. [Google Scholar] [CrossRef] [Green Version]
- Lyons, M.M.; Yu, C.; Toma, R.; Cho, S.Y.; Reiboldt, W.; Lee, J.; van Breemen, R.B. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 2003, 51, 5867–5870. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Huang, X.; Mazza, G. Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.M.; Rentero, M.P.Z. Bioactive Compounds Contained in Mediterranean Diet and Their Effects on Neurodegenerative Diseases. In Current Topics on Superfoods; InTech Open: London, UK, 2018; Volume 2, pp. 13–31. [Google Scholar] [CrossRef] [Green Version]
- Drygalski, K.; Fereniec, E.; Koryciński, K.; Chomentowski, A.; Kiełczewska, A.; Odrzygóźdź, C.; Modzelewska, B. Resveratrol and Alzheimer’s disease. From molecular pathophysiology to clinical trials. Exp. Gerontol. 2018, 113, 36–47. [Google Scholar] [CrossRef]
- Higashida, K.; Kim, S.H.; Jung, S.R.; Asaka, M.; Holloszy, J.O.; Han, D.H. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013, 11, e1001603. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, G.; Song, J. The association between PGC-1α and Alzheimer’s disease. Anat. Cell Biol. 2016, 49, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Karthick, C.; Periyasamy, S.; Jayachandran, K.S.; Anusuyadevi, M. Intrahippocampal administration of ibotenic acid induced cholinergic dysfunction via NR2A/NR2B expression: Implications of resveratrol against Alzheimer disease pathophysiology. Front. Mol. Neurosci. 2016, 9, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Jiang, T.; Li, W.; Gao, N.; Zhang, T. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol. Lett. 2018, 282, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Nimmagadda, V.K.; Bever, C.T.; Vattikunta, N.R.; Talat, S.; Ahmad, V.; Nagalla, N.K.; Trisler, D.; Judge, S.I.V.; Royal 3rd, W.; Chandrasekaran, K.; et al. Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 targets. J. Immunol. 2013, 190, 4595–4607. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-C.; Lu, K.-T.; Wo, Y.-Y.P.; Wu, Y.-J.; Yang, Y.-L. Resveratrol protects rats from Aβ-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS ONE 2011, 6, e29102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [Green Version]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2017, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Nelson, L.; Tabet, N. Slowing the progression of Alzheimer’s disease; what works? Ageing Res. Rev. 2015, 23, 193–209. [Google Scholar] [CrossRef]
- Howes, M.-J.R.; Perry, N.S.L.; Vásquez-Londoño, C.; Perry, E.K. Role of Phytochemicals as Nutraceuticals for Cognitive Functions Affected in Ageing. Br. J. Pharmacol. 2020, 177, 1294–1315. [Google Scholar] [CrossRef] [Green Version]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 2011, 5, 7119–7131. [Google Scholar] [CrossRef]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef]
- Imamura, T.; Bando, N.; Yamanishi, R. β-carotene Modulates the Immunological Function of RAW264, a Murine Macrophage Cell Line, by Enhancing the level of Intracellular Glutathione. Biosci. Biotechnol. Biochem. 2006, 70, 2112–2120. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Carotenoids and Flavonoids Contribute to Nutritional Protection against Skin Damage from Sunlight. Mol. Biotechnol. 2007, 37, 26–30. [Google Scholar] [CrossRef]
- Min, J.Y.; Min, K.B. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer’s disease mortality in older adults. Dement. Geriatr. Cogn. Disord. 2014, 37, 246–256. [Google Scholar] [CrossRef]
- Grodzicki, W.; Dziendzikowska, K. The Role of Selected Bioactive Compounds in the Prevention of Alzheimer’s Disease. Antioxidants 2020, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wang, W.; Pang, W.; Xiao, Z.; Jiang, Y.; Hong, Y. Dietary Lycopene Supplementation Improves Cognitive Performances in Tau Transgenic Mice Expressing P301L Mutation via Inhibiting Oxidative Stress and Tau Hyperphosphorylation. Alzheimers Dis. 2017, 57, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Yonar, M.E. Protective Effect of Lycopene on Oxidative Stress and Antioxidant Status in Cyprinus Carpio During Cypermethrin Exposure. Environ. Toxicol. 2013, 28, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Caramia, G. Virgin olive oil. From legend to scientific knowledge of the nutraceutical aspects. Pediatr. Med. Chir. 2006, 28, 9–23. [Google Scholar]
- Aiello, A.; Guccione, G.D.; Accardi, G.; Caruso, C. What olive oil for healthy ageing? Maturitas 2015, 80, 117–118. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Gambino, C.M.; Accardi, G.; Aiello, A.; Candore, G.; Dara-Guccione, G.; Mirisola, M.; Procopio, A.; Taormina, G.; Caruso, C. Effect of Extra Virgin Olive Oil and Table Olives on the ImmuneInflammatory Responses: Potential Clinical Applications. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 14–22. [Google Scholar] [CrossRef]
- Rigacci, S. Olive oil phenols as promising multi-targeting agents against Alzheimer’s disease. Adv. Exp. Med. Biol. 2015, 863, 1–20. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.I.; Sola, R.; Fito, M. Up-to date knowledge on the in vivo transcriptomic effect of the Mediterranean diet in humans. Mol. Nutr. Food Res. 2013, 57, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 2003, 2599–2608. [Google Scholar] [CrossRef] [Green Version]
- Kálai, T.; Petrlova, J.; Balog, M.; Aung, H.H.; Voss, J.C.; Hideg, K. Synthesis and study of 2-amino-7-bromofluorenes modified with nitroxides and their precursors as dual anti-amyloid and antioxidant active compounds. Eur. J. Med. Chem. 2011, 46, 1348–1355. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.G.; Shukitt-Hale, B. Berry Fruit Enhances Beneficial Signaling in the Brain. J. Agric. Food Chem. 2012, 60, 5709–5715. [Google Scholar] [CrossRef]
- Shukitt-Hale, B. Blueberries and neuronal aging. Gerontology 2012, 58, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Luo, Y.; Smith, J.V.; Paramasivam, V.; Burdick, A.; Curry, K.J.; Buford, J.P.; Khan, I.; Netzer, W.J.; Xu, H.; Butko, P. Inhibition of amyloid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA 2002, 99, 12197–12202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.; Jin, G.; Zhao, M.; Yang, H. The effect of genistein on the content andactivity of α-and β-Secretase and protein kinase C in Aβ-Injured hippocampal neurons. Basic Clin. Pharmacol. Toxicol. 2013, 112, 182–185. [Google Scholar] [CrossRef]
- Ye, S.; Wang, T.; Cai, B.; Wang, Y.; Li, J.; Zhan, J.; Shen, G. Genistein protects hippocampal neurons against injury by regulating calcium/calmodulin dependent protein kinase IV protein levels in Alzheimer’s disease model rats. Neural Regen. Res. 2017, 12, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M. Nutritional Prevention of Cognitive Decline and Dementia. Acta Biomed. 2018, 89, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Aucoin, D.; Ahmed, M.; Ziliox, M.; Van Nostrand, W.E.; Smith, S.O. Capping of abeta42 oligomers by small molecule inhibitors. Biochemistry 2014, 53, 7893–7903. [Google Scholar] [CrossRef] [Green Version]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Karuppagounder, S.S.; Pinto, J.T.; Xu, H.; Chen, H.L.; Beal, M.F.; Gibson, G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Int. 2009, 54, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Chaterjee, P.; Sharma, P.K.; Singh, A.K.; Gupta, A.; Gill, K.; Tripathi, M.; Dey, A.B.; Dey, S. Sirtuin1: A promising serum protein marker for early detection of Alzheimer’s disease. PLoS ONE 2013, 8, e61560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barger, J.L.; Kayo, T.; Vann, J.M.; Arias, E.B.; Wang, J.; Hacker, T.A.; Wang, Y.; Raederstorff, D.; Morrow, J.D.; Leeuwenburgh, C.; et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE 2008, 3, e2264. [Google Scholar] [CrossRef]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef]
- Sheng, K.; Shui, S.; Yan, L.; Yu, J.; Hao, G.; Qu, H.; Liu, J.; Zhang, Y.; Liu, C.; Zheng, L. The beneficial effects of dietary grape supplementation on improving cognitive deficits in APP/PS1 double transgenic mice. J. Funct. Foods 2018, 49, 224–234. [Google Scholar] [CrossRef]
- Patil, S.P.; Tran, N.; Geekiyanage, H.; Liu, L.; Chan, C. Curcumin-inducedupregulation of the anti-tau cochaperone BAG2 in primary rat corticalneurons. Neurosci. Lett. 2013, 554, 121–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharman, M.J.; Gyengesi, E.; Liang, H.; Chatterjee, P.; Karl, T.; Li, Q.X.; Wenk, M.R.; Halliwell, B.; Martins, R.N.; Münch, G. Assessment of diets containing curcumin, epigallocatechin-3-gallate, docosahexaenoic acid and α-lipoic acid on amyloid load and inflammation in a male transgenic mouse model of Alzheimer’s disease: Are combinations more effective? Neurobiol. Dis. 2019, 124, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology. In Vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem 2007, 102, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin inhibits tau aggregation and disintegrates preformed tau filaments In Vitro. J. Alzheimer’s Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Teng, E.; Hu, S.; Chen, P.P.; Maiti, P.; et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013, 288, 4056–4065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.; Sorribas, A.; Howes, M.-J.R. Natural products as a source of Alzheimer’s drug leads. Nat. Prod. Rep. 2011, 28, 48–77. [Google Scholar] [CrossRef]
- Lin, L.C.; Wang, M.N.; Tseng, T.Y.; Sung, J.S.; Tsai, T.H. Pharmacokinetics of (-)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; do Carmo Pereira, M. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, L.; Wang, Z.; Cui, Y.; Tan, X.; Yuan, T.; Liu, Q.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. 2018, 56, 16–25. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; de la Torre, R. Potential Role of Olive Oil Phenolic Compounds in the Prevention of Neurodegenerative Diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daccache, A.; Lion, C.; Sibille, N.; Gerard, M.; Slomianny, C.; Lippens, G.; Cotelle, P. Oleuropein and dervatives from olive as Tau aggregation inhibitors. Neurochem. Int. 2011, 58, 700–707. [Google Scholar] [CrossRef]
- St-Laurent-Thibault, C.; Arseneault, M.; Longpré, F.; Ramassamy, C. Tyrosol and Hydroxytyrosol Two Main Components of Olive Oil, Protect N2a Cells against Amyloid-β-Induced Toxicity. Involvement of the NF-kB Signaling. Curr. Alzheimer Res. 2011, 8, 543–551. [Google Scholar] [CrossRef]
- Li, W.; Sperry, J.B.; Crowe, A.; Trojanowski, J.Q.; Smith, A.B., III.; Lee, V.M.Y. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J. Neurochem. 2009, 110, 1339–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, W.M.A.D.B.; Martins, I.J.; Goozee, K.G.; Brennan, C.S.; Jayasena, V.; Martins, R.N. The role of dietary coconut for the prevention and treatment of Alzheimer’s disease: Potential mechanisms of action. Br. J. Nutr. 2015, 114, 1–14. [Google Scholar] [CrossRef]
- Marina, A.M.; Man, Y.B.; Nazimah, S.A.; Amin, I. Antioxidant capacity and phenolic acids of virgin coconut oil. Int. J. Food Sci. Nutr. 2009, 60, 114–123. [Google Scholar] [CrossRef]
- Ono, K.; Condron, M.M.; Ho, L.; Wang, J.; Zhao, W.; Pasinetti, G.M.; Teplow, D.B. Effects of grape seed-derived polyphenols on amyloid β-protein self-assembly and cytotoxicity. J. Biol. Chem 2008, 283, 32176–32187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Cai, B.; Shao, J.; Wang, T.T.; Cai, R.Z.; Ma, C.J.; Han, T.; Du, J. Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer’s disease. Neural. Regen. Res. 2016, 11, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-Y.; Ko, H.-A.; Chu, K.-H.; Shieh, T.-M.; Chi, T.-C.; Chen, H.-I.; Chang, W.-C.; Chang, S.-S. The possible mechanism of advanced glycation end products (AGEs) for Alzheimer’s disease. PLoS ONE 2015, 10, e0143345. [Google Scholar] [CrossRef]
- Parker, J.A.; Arango, M.; Abderrahmane, S.; Lambert, E.; Tourette, C.; Catoire, H.; Néri, C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nature Genetics. 2005, 37, 349–350. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, A.; Kumar, A. Implicating the role of lycopene in restoration of mitochondrial enzymes and BDNF levels in β-amyloid induced Alzheimer’s disease. Eur. J. Pharmacol. 2014, 741, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Lim, J.W.; Kim, H. Inhibitory effect of lycopene on amyloid-β-induced apoptosis in neuronal cells. Nutrients 2017, 9, 883. [Google Scholar] [CrossRef] [Green Version]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary intake of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in Aged Blueberry-Fed Rats Are Found Centrally and May Enhance Memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef]
- Veszelka, S.; Tóth, A.; Walter, F.R.; Tóth, A.E.; Gróf, I.; Mészáros, M.; Bocsik, A.; Hellinger, E.; Vastag, M.; Rákhely, G.; et al. Comparison of a Rat Primary Cell-Based Blood-Brain Barrier Model With Epithelial and Brain Endothelial Cell Lines: Gene Expression and Drug Transport. Front. Mol. Neurosci. 2018, 11, 166. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Farzaei, M.H.; Rahimi, R.; Nikfar, S.; Abdollahi, M. Effect of resveratrol on cognitive and memory performance and mood: A meta-analysis of 225 patients. Pharmacol. Res. 2018, 128, 338–344. [Google Scholar] [CrossRef]
- Lalla, R.; Donmez, G. The role of sirtuins in Alzheimer’s disease. Front. Aging Neurosci. 2013, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasinetti, G.M.; Wang, J.; Marambaud, P.; Ferruzzi, M.; Gregor, P.; Knable, L.A.; Ho, L. Neuroprotective and metabolic effects of resveratrol: Therapeutic implications for Huntington’s disease and other neurodegenerative disorders. Exp. Neurol. 2011, 232, 1–6. [Google Scholar] [CrossRef]
- Hishikawa, N.; Takahashi, Y.; Amakusa, Y.; Tanno, Y.; Tuji, Y.; Niwa, H.; Murakami, N.; Krishna, U.K. Effects of turmeric on Alzheimer’s disease with behavioral and psychological symptoms of dementia. Ayu. 2012, 33, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.P.; Chiam, P.C.; Lee, T.; Chua, H.C.; Lim, L.; Kua, E.H. Curry Consumption and Cognitive Function in the Elderly. Am. J. Epidemiol. 2006, 164, 898–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, P.; Dunbar, G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: A randomised, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Christensen, K.; Gleason, C.E.; Mares, J.A. Dietary carotenoids and cognitive function among US adults, NHANES 2011–2014. Nutr. Neurosci. 2020, 23, 554–562. [Google Scholar] [CrossRef]
- Farías, G.A.; Guzmán-Martínez, L.; Delgado, C.; Maccioni, R.B. Nutraceuticals: A Novel Concept in Prevention and Treatment of Alzheimer’s Disease and Related Disorders. J. Alzheimers Dis. 2014, 42, 357–367. [Google Scholar] [CrossRef]
- Kandiah, N.; Ong, P.A.; Yuda, T.; Ng, L.L.; Mamun, K.; Merchant, R.A.; Chen, C.; Dominguez, J.; Marasigan, S.; Ampil, E.; et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci. Ther. 2019, 25, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean Diet, Stroke, Cognitive Impairment, and Depression: A Meta-Analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Aridi, Y.S.; Walker, J.L.; Wright, O.R.L. The Association between the Mediterranean Dietary Pattern and Cognitive Health: A Systematic Review. Nutrients 2017, 9, 674. [Google Scholar] [CrossRef] [Green Version]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Aβ amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef] [PubMed]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30028. [Google Scholar] [CrossRef] [PubMed]
- Minter, M.R.; Hinterleitner, R.; Meisel, M.; Zhang, C.; Leone, V.; Zhang, X.; Oyler-Castrillo, P.; Zhang, X.; Musch, M.W.; Shen, X.; et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1∆E9 murine model of Alzheimer’s disease. Sci. Rep. 2017, 7, 10411. [Google Scholar] [CrossRef]
- Hu, X.; Wang, T.; Jin, F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 2016, 59, 1006–1023. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Raval, U.; Harary, J.M.; Zeng, E.; Pasinetti, G.M. The Dichotomous Role of the Gut Microbiome in Exacerbating and Ameliorating Neurodegenerative Disorders. Expert Rev. Neurother. 2020, 27, 1–14. [Google Scholar] [CrossRef]
- Friedland, R.P. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimers Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef] [Green Version]
- Köhler, C.A.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctôt, K.L.; Carvalho, A.F. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 6152–6166. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saji, N.; Niida, S.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Toba, K.; Sakurai, T. Analysis of the relationship between the gut microbiome and dementia: A cross-sectional study conducted in Japan. Sci. Rep. 2019, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Fujimura, Y.; Mimura, I.; Fujii, Y.; Nguyen, N.L.; Arakawa, K.; Morita, H. Cultivable butyrate-producing bacteria of elderly Japanese diagnosed with Alzheimer’s disease. J. Microbiol. 2018, 56, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [Green Version]
- Bruce-Keller, A.J.; Salbaum, J.M.; Berthoud, H.R. Harnessing Gut Microbes for Mental Health: Getting From Here to There. Biol. Psychiatry 2018, 83, 214–223. [Google Scholar] [CrossRef]
- Szczechowiak, K.; Diniz, B.S.; Leszek, J. Diet and Alzheimer’s dementia-Nutritional approach to modulate inflammation. Pharmacol. Biochem. Behav. 2019, 184, 172743. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, L.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell. 2016, 64, 982–992. [Google Scholar] [CrossRef] [Green Version]
- Bostanciklioğlu, M. The role of gut microbiota in pathogenesis of Alzheimer’s disease. J. Appl. Microbiol. 2019, 127, 954–967. [Google Scholar] [CrossRef]
- Szablewski, L. Human Gut Microbiota in Health and Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics inan adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Rainey-Smith, S.R.; Gu, Y.; Gardener, S.L.; Doecke, J.D.; Villemagne, V.L.; Brown, B.M.; Taddei, K.; Laws, S.M.; Sohrabi, H.R.; Weinborn, M.; et al. Mediterranean Diet Adherence and Rate of Cerebral Aβ-amyloid Accumulation: Data From the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Transl. Psychiatry 2018, 8, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisardi, V.; Panza, F.; Seripa, D.; Imbimbo, B.P.; Vendemiale, G.; Pilotto, A.; Solfrizzi, V. Nutraceutical Properties of Mediterranean Diet and Cognitive Decline: Possible Underlying Mechanisms. J. Alzheimers Dis. 2010, 22, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Suchodolski, J.S.; Nasuti, C.; Fiorini, D.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Berardi, S.; Scarpona, S.; Rossi, G.; Eleuteri, A.M. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol. Neurobiol. 2018, 55, 7987–8000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.Z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease Induced Albino Rats. J. Clin. Diagn. Res. 2017, 11, KC01–KC05. [Google Scholar] [CrossRef]
- Chen, D.; Yang, X.; Yang, J.; Lai, G.; Yong, T.; Tang, X.; Shuai, O.; Zhou, G.; Xie, Y.; Wu, Q. Prebiotic Effect of Fructooligosaccharides From Morinda officinalis on Alzheimer’s Disease in Rodent Models by Targeting the Microbiota-Gut-Brain Axis. Front. Aging Neurosci. 2017, 9, 403. [Google Scholar] [CrossRef] [Green Version]
- Athari Nik Azm, S.; Djazayeri, A.; Safa, M.; Azami, K.; Ahmadvand, B.; Sabbaghziarani, F.; Sharifzadeh, M.; Vafa, M. Lactobacilli and Bifidobacteria Ameliorate Memory and Learning Deficits and Oxidative Stress in β-amyloid (1-42) Injected Rats. Appl Physiol. Nutr. Metab. 2018, 43, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Prakash, S. A novel synbiotic delays Alzheimer’s disease onset via combinatorial gut-brain-axis signaling in Drosophila melanogaster. PLoS ONE 2019, 14, e0214985. [Google Scholar] [CrossRef]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, C.; Santangelo, R. Alzheimer’s disease and gut microbiota modifications: The long way between preclinical studies and clinical evidence. Pharmacol. Res. 2018, 29, 329–336. [Google Scholar] [CrossRef]
- Leblhuber, F.; Steiner, K.; Schuetz, B.; Fuchs, D.; Gostner, J.M. Probiotic Supplementation in Patients With Alzheimer’s Dementia-An Explorative Intervention Study. Curr. Alzheimer Res. 2018, 15, 1106–1113. [Google Scholar] [CrossRef]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef]
- Carlsson, M.; Gustafson, Y.; Haglin, L.; Eriksson, S. The feasibility of serving liquid yoghurt supplemented with probiotic bacteria, Lactobacillus rhamnosus LB 21, and Lactococcus lactis L1A – a pilot study among old people with dementia in a residential care facility. J. Nutr. Health Aging 2009, 13, 813–819. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Esmaeili Taba, S.M.; Salami, M. Does severity of Alzheimer’sdisease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front.Neurol. 2018, 9, 662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamtaji, O.R.; Heidari-soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef]
- Ticinesi, A.; Tana, C.; Nouvenne, A.; Prati, B.; Lauretani, F.; Meschi, T. Gut microbiota, cognitive frailty and dementia in older individuals: A systematic review. Clin. Interv. Aging 2018, 13, 1497–1511. [Google Scholar] [CrossRef] [Green Version]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Gut microbiota and pro/prebiotics in Alzheimer’s disease. Aging 2020, 12, 5539–5550. [Google Scholar] [CrossRef]
- Liu, T.Y.; Hougen, H.; Vollmer, A.C.; Hiebert, S.M. Gut bacteria profiles of Mus musculus at the phylum and family levels are influenced by saturation of dietary fatty acids. Anaerobe 2012, 18, 331–337. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N., Jr.; Frautschy, S.A.; Cole, G.M. A Diet Enriched with the Omega-3 Fatty Acid Docosahexaenoic Acid Reduces Amyloid Burden in an Aged Alzheimer Mouse Model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef] [Green Version]
- Grimm, M.O.; Kuchenbecker, J.; Grosgen, S.; Burg, V.K.; Hundsdorfer, B.; Rothhaar, T.L.; Friess, P.; de Wilde, M.C.; Broersen, L.M.; Penke, N.; et al. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J. Biol. Chem. 2011, 286, 14028–14039. [Google Scholar] [CrossRef] [Green Version]
- Jović, M.; Lončarević-Vasiljković, N.; Ivković, S.; Dinić, J.; Milanović, D.; Zlokovic, B.; Kanazir, S. Short-term Fish Oil Supplementation Applied in Presymptomatic Stage of Alzheimer’s Disease Enhances microglial/macrophage Barrier and Prevents Neuritic Dystrophy in Parietal Cortex of 5xFAD Mouse Model. PLoS ONE 2019, 14, e0216726. [Google Scholar] [CrossRef] [PubMed]
- Calon, F.; Lim, G.P.; Morihara, T.; Yang, F.; Ubeda, O.; Salem, N.; Frautschy, S., Jr.; Cole, G.M. Dietary n-3 Polyunsaturated Fatty Acid Depletion Activates Caspases and Decreases NMDA Receptors in the Brain of a Transgenic Mouse Model of Alzheimer’s Disease. Eur J. Neurosci. 2005, 22, 617–626. [Google Scholar] [CrossRef]
- Wu, K.; Gao, X.; Shi, B.; Chen, S.; Zhou, X.; Li, Z.; Gan, Y.; Cui, L.; Kang, J.X.; Li, W.; et al. Enriched Endogenous n-3 Polyunsaturated Fatty Acids Alleviate Cognitive and Behavioral Deficits in a Mice Model of Alzheimer’s Disease. Neuroscience 2016, 333, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2017, 67, 1974–1983. [Google Scholar] [CrossRef]
- Pu, S.; Khazanehei, H.; Jones, P.J.; Khafipour, E. Interactions between Obesity Status and Dietary Intake of Monounsaturated and Polyunsaturated Oils on Human Gut Microbiome Profiles in the Canola Oil Multicenter Intervention Trial (COMIT). Front. Microbiol. 2016, 7, 1612. [Google Scholar] [CrossRef] [Green Version]
- Noriega, B.S.; Sanchez-Gonzalez, M.A.; Salyakina, D.; Coffman, J.H. Understanding the Impact of Omega-3 Rich Diet on the Gut Microbiota. Case Rep. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef] [Green Version]
- Balfego, M.; Canivell, S.; Hanzu, F.A.; Sala-Vila, A.; Martinez-Medina, M.; Murillo, S.; Mur, T.; Ruano, E.G.; Linares, F.; Porras, N.; et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naive patients with type 2 diabetes: A pilot randomized trial. Lipids Health Dis. 2016, 15, 78. [Google Scholar] [CrossRef] [Green Version]
- Freund-Levi, Y.; Basun, H.; Cederholm, T.; Faxén-Irving, G.; Garlind, A.; Grut, M.; Vedin, I.; Palmblad, J.; Wahlund, L.-O.; Eriksdotter-Jönhagen, M. Omega-3 supplementation in mild to moderate Alzheimer’s disease: Effects on neuropsychiatric symptoms. Int. J. Geriatr. Psychiatry 2008, 23, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R., Jr.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef]
- Shinto, L.; Quinn, J.; Montine, T.; Dodge, H.H.; Woodward, W.; Baldauf-Wagner, S.; Waichunas, D.; Bumgarner, L.; Bourdette, D.; Silbert, L. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J. Alzheimers Dis. 2014, 38, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.A.; Childs, C.E.; Calder, P.C.; Rogers, P.J. No Effect of Omega-3 Fatty Acid Supplementation on Cognition and Mood in Individuals with Cognitive Impairment and Probable Alzheimer’s Disease: A Randomised Controlled Trial. Int. J. Mol. Sci. 2015, 16, 24600–24613. [Google Scholar] [CrossRef] [PubMed]
- Eriksdotter, M.; Vedin, I.; Falahati, F.; Freund-Levi, Y.; Hjorth, E.; Faxen-Irving, G.; Wahlund, L.O.; Schultzberg, M.; Basun, H.; Cederholm, T. Plasma Fatty Acid Profiles in Relation to Cognition and Gender in Alzheimer’s Disease Patients During Oral Omega-3 Fatty Acid Supplementation: The OmegAD Study. J. Alzheimers Dis. 2015, 48, 805–812. [Google Scholar] [CrossRef]
- Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Cederholm, T.; Basun, H.; Faxén-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.O.; Palmblad, J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: A randomized double-blind trial. Arch. Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef] [Green Version]
- Fiala, M.; Restrepo, L.; Pellegrini, M. Immunotherapy of Mild Cognitive Impairment by ω-3 Supplementation: Why Are Amyloid-β Antibodies and ω-3 Not Working in Clinical Trials? J. Alzheimers Dis. 2018, 62, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Famenini, S.; Rigali, E.A.; Olivera-Perez, H.M.; Dang, J.; Chang, M.T.; Halder, R.; Rao, R.V.; Pellegrini, M.; Porter, V.; Bredesen, D. Increased intermediate M1-M2 macrophage polarization and improved cognition in mild cognitive impairment patients on ω-3 supplementation. FASEB J. 2017, 31, 148–160. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Pasker-de Jong, P.C.M.; de Vries, R.B.M.; Ritskes-Hoitinga, M. The Effects of Long-Term omega-3 Fatty Acid Supplementation on Cognition and Alzheimer’s Pathology in Animal Models of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2012, 28, 191–209. [Google Scholar] [CrossRef] [Green Version]
- La Rosa, F.; Clerici, M.; Ratto, D.; Occhinegro, A.; Licito, A.; Romeo, M.; Di Iorio, C.; Rossi, P. The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials. Nutrients 2018, 10, 1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadecka, A.; Bielak-Zmijewska, A. Slowing Down Ageing: The Role of Nutrients and Microbiota in Modulation of the Epigenome. Nutrients 2019, 11, 1251. [Google Scholar] [CrossRef] [Green Version]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural. Regen. Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef]
- Bosscher, D.; Breynaert, A.; Pieters, L.; Hermans, N. Food-based Strategies to Modulate the Composition of the Intestinal Microbiota and Their Associated Health Effects. J. Physiol. Pharmacol. 2009, 60, 5–11. [Google Scholar]
- Di Meo, F.; Donato, S.; Di Pardo, A.; Maglione, V.; Filosa, S.; Crispi, S. New Therapeutic Drugs from Bioactive Natural Molecules: The Role of Gut Microbiota Metabolism in Neurodegenerative Diseases. Curr. Drug Metab. 2018, 19, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s Neuroprotective Effects Against Alzheimer’s Disease Are Mediated by Urolithins, Its Ellagitannin-Gut Microbial Derived Metabolites. ACS Chem Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Shytle, D.; Sun, N.; Mori, T.; Hou, H.; Jeanniton, D.; Ehrhart, J.; Townsend, K.; Zeng, J.; Morgan, D.; et al. Green Tea epigallocatechin-3-gallate (EGCG) Modulates Amyloid Precursor Protein Cleavage and Reduces Cerebral Amyloidosis in Alzheimer Transgenic Mice. J. Neurosci. 2005, 25, 8807–8814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, R.; Januszewski, S.; Ułamek-Kozioł, M. Mutual Two-Way Interactions of Curcumin and Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 1055. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.R.; Mayer, E.A. Gut-Brain Axis and Behavior. Nestle Nutr. Inst Workshop Ser. 2017, 88, 45–53. [Google Scholar] [CrossRef]
- Pasinetti, G.M. Novel Role of Red Wine-Derived Polyphenols in the Prevention of Alzheimer’s Disease Dementia and Brain Pathology: Experimental Approaches and Clinical Implications. Planta. Med. 2012, 78, 1614–1619. [Google Scholar] [CrossRef] [Green Version]
- Lange, K.W.; Guo, J.; Kanaya, S.; Lange, K.M.; Nakamura, Y.; Li, S. Medical foods in Alzheimer’s disease. Food Science and Human Wellness 2019, 8, 1–7. [Google Scholar] [CrossRef]
- Cloud, J. Why Your DNA Isn’t Your Destiny. Time Magazine, 2010; 6. [Google Scholar]
- Robertson, K.D. DNA methylation and human disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.D.; Wolffe, A.P. DNA methylation in health and disease. Nat. Rev. Genet. 2000, 1, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, H.Y.; Beaudet, A.L. Epigenetics and Human Disease. Cold Spring Harb Perspect Biol. 2016, 8, a019497. [Google Scholar] [CrossRef] [PubMed]
- Korzus, E. Manipulating the brain with epigenetics. Nat. Neurosci. 2010, 13, 405–406. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, A.; Wang, Z.J.; Cao, Q.; Wang, W.; Lin, L.; Ma, K.; Zhang, F.; Wei, J.; Matas, E.; et al. Inhibition of EHMT1/2 Rescues Synaptic and Cognitive Functions for Alzheimer’s Disease. Brain 2019, 142, 787–807. [Google Scholar] [CrossRef]
- Monti, N.; Cavallaro, R.A.; Stoccoro, A.; Nicolia, V.; Scarpa, S.; Kovacs, G.G.; Fiorenza, M.T.; Lucarelli, M.; Aronica, E.; Isidre Ferrer, I.; et al. CpG and non-CpG Presenilin1 methylation pattern in course of neurodevelopment and neurodegeneration is associated with gene expression in human and murine brain. Epigenetics 2020, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [Green Version]
- Ayissi, V.B.O.; Ebrahimi, A.; Schluesenner, H. Epigenetic effects of natural polyphenols: A focus on SIRT1-mediated mechanisms. Mol. Nutr. Food Res. 2014, 58, 22–32. [Google Scholar] [CrossRef]
- Kouzarides, T.; Berger, S.L. Chromatin modifications and their mechanism of action. In Epigenetics; Allis, C.D., Jenuwein, T., Reinberg, D., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2007; Volume 191, p. 209. [Google Scholar]
- Li, E.; Bird, A. DNA methylation in mammals. Cold Spring Harbor Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef]
- Kemme, C.A.; Marquez, R.; Luu, R.H.; Iwahara, J. Potential role of DNA methylation as a facilitator of target search processes for transcription factors through interplay with methyl-CpG-binding proteins. Nucleic Acids Res. 2017, 45, 7751–7759. [Google Scholar] [CrossRef] [PubMed]
- Stoccoro, A.; Coppedè, F. Role of epigenetics in Alzheimer’s disease Pathogenesis. Neurodegener Dis. Manag. 2018, 8, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Torrellas, C. Epigenetics of Aging and Alzheimer’s Disease: Implications for Pharmacogenomics and Drug Response. Int. J. Mol. Sci. 2015, 16, 30483–30543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, R.L.; Lee, J.M.; Maroun, L.E. Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. J. Mol. Neurosci. 1995; 6, 141–146. [Google Scholar] [CrossRef]
- Wang, S.C.; Oelze, B.; Schumacher, A. Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS ONE 2008, 3, e2698. [Google Scholar] [CrossRef] [Green Version]
- Di Francesco, A.; Arosio, B.; Falconi, A.; Di Bonaventura, M.V.M.; Karimi, M.; Mari, D.; Casati, M.; Maccarrone, M.; D’addario, C. Global changes in DNA methylation in Alzheimer’s disease peripheral blood mononuclear cells. Brain Behav. Immun. 2015, 45, 139–144. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, B.; Shen, L. The Epigenetics of Alzheimer’s Disease: Factors and Therapeutic Implications. Front. Genetics 2018, 9, 579. [Google Scholar] [CrossRef] [Green Version]
- Wen, K.X.; Milic¸, J.; El-Khodor, B.; Dhana, K.; Nano, J.; Pulido, T.; Kraja, B.; Zaciragic, A.; Bramer, W.M.; Troup, J.; et al. The role of DNA methylation and histone modifications in neurodegenerative diseases: A systematic review. PLoS ONE 2016, 11, e0167201. [Google Scholar] [CrossRef]
- Fransquet, P.D.; Lacaze, P.; Saffery, R.; McNeil, J.; Woods, R.; Ryan, J. Blood DNA methylation as a potential biomarker of dementia: A systematic review. Alzheimers Dement. 2017, 14, 81–103. [Google Scholar] [CrossRef]
- Chen, Y.; Damayanti, N.P.; Irudayaraj, J.; Dunn, K.; Zhou, F.C. Diversity of two forms of DNA methylation in the brain. Front. Genet. 2014, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Bernstein, A.; Chen, D.; Jin, P. 5-Hydroxymethylcytosine: A new player in brain disorders? Exp. Neurol. 2015, 268, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Morales, R.; Esteller, M. Opening up the DNA methylome of dementia. Mol. Psychiatry 2017, 22, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Jiang, T.; Yang, A.F.; Du, Y.J.; Wu, M.; Kong, L.H. Epigenetic Modulation on Tau Phosphorylation in Alzheimer’s Disease. Neural Plasticity 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanasopoulos, D.; Karagiannis, G.; Tsolaki, M. Recent Findings in Alzheimer Disease and Nutrition Focusing on Epigenetics. Adv. Nutr. 2016, 7, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Lardenoije, R.; van den Hove, D.L.A.; Havermans, M.; van Casteren, A.; Le, K.X.; Palmour, R.; Lemere, C.A.; Ruttenb, B.P.F. Age-related epigenetic changes in hippocampal subregions of four animal models of Alzheimer’s disease. Mol. Cell Neurosci. 2018, 86, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tohgi, H.; Utsugisawa, K.; Nagane, Y.; Yoshimura, M.; Genda, Y.; Ukitsu, M. Reduction with age in methylcytosine in the promoter region -224 approximately -101 of the amyloid precursor protein gene in autopsy human cortex. Brain Res. Mol. Brain Res. 1999, 70, 288–292. [Google Scholar] [CrossRef]
- Tohgi, H.; Utsugisawa, K.; Nagane, Y.; Yoshimura, M.; Ukitsu, M.; Genda, Y. The methylation status of cytosines in a tau gene promoter region alters with age to downregulate transcriptional. Neurosci. Lett. 1999, 275, 89–92. [Google Scholar] [CrossRef]
- Li, X.; Bao, X.; Wang, R. Neurogenesis-based epigenetic therapeutics for Alzheimer’s disease. Molec. Med. Rep. 2016, 14, 1043–1053. [Google Scholar] [CrossRef]
- Nicolia, V.; Ciiraci, V.; Cavallaro, R.A.; Ferrer, I.; Scarpa, S.; Fuso, A. GSK3β 5′-flanking DNA methylation and expression in Alzheimer’s disease patients. Curr. Alzheimer Res. 2017, 14, 753–759. [Google Scholar] [CrossRef]
- Martínez-Iglesias, O.; Carrera, I.; Carril, J.C.; Fernández-Novo, L.; Cacabelos, N.; Cacabelos, R. DNA Methylation in Neurodegenerative and Cerebrovascular Disorders. Int. J. Mol. Sci. 2020, 21, 2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, D.; Xu, X. DNA Methyltransferases, DNA Methylation, and Age-Associated Cognitive Function. Int. J. Mol. Sci. 2018, 19, 1315. [Google Scholar] [CrossRef] [Green Version]
- Chouliaras, L.; Mastroeni, D.; Delvaux, E.; Grover, A.; Kenis, G.; Hof, P.R.; Steinbusch, H.W.M.; Coleman, P.D.; Rutten, B.P.F.; van den Hove, D.L.A. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients. Neurobiol. Aging 2013, 34, 2091–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeongsil Kim-Ha, J.; Kim, Y.J. Age-related epigenetic regulation in the brain and its role in neuronal diseases. BMB Rep. 2016, 49, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezgin, Z.; Dincer, Y. Alzheimer’s disease and epigenetic diet. Neurochem. Int. 2014, 78, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, S.; Fuso, A.; D’anselmi, F.; Cavallaro, R.A. Presenilin 1 gene silencing by S-adenosylmethionine: A treatment for Alzheimer disease? FEBS Lett. 2003, 541, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Fuso, A.; Cavallaro, R.A.; Zampelli, A.; D’Anselmi, F.; Piscopo, P.; Confaloni, A.; Scarpa, S. γ-Secretase is differentially modulated by alterations of Homocysteine cycle in neuroblastoma and glioblastoma cells. J. Alzheimer Dis. 2007, 11, 275–290. [Google Scholar] [CrossRef]
- Fuso, A.; Nicolia, V.; Ricceri, L.; Cavallaro, R.A.; Isopi, E.; Mangia, F.; Fiorenza, M.T.; Scarpa, S. S-adenosylmethionine reduces the progress of the Alzheimer-like features induced by B-vitamin deficiency in mice. Neurobiol. Aging 2012, 33, 1482.e1–1482.e16. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Magalhães, L.; Ribeiro-dos-Santos, A.; Vidal, A.F. Mitochondrial Epigenetics: Non-Coding RNAs as a Novel Layer of Complexity. Int. J. Mol. Sci. 2020, 21, 1838. [Google Scholar] [CrossRef] [Green Version]
- Blanch, M.; Mosquera, J.L.; Ansoleaga, B.; Ferrer, I.; Barrachina, M. Altered mitochondrial DNA methylation pattern in Alzheimer disease-related pathology and in Parkinson disease. Am. J. Phys. Anthropol. 2016, 186, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Dzitoyeva, S.; Chen, H.; Manev, H. Effect of aging on 5-hydroxymethylcytosine in brain mitochondria. Neurobiol. Aging 2012, 33, 2881–2891. [Google Scholar] [CrossRef] [Green Version]
- Fuso, A.; Lucarelli, M. CpG and Non-CpG Methylation in the Diet–Epigenetics–Neurodegeneration Connection. Curr. Nutr. Rep. 2019, 8, 74–82. [Google Scholar] [CrossRef]
- Hassan, H.E.; Carlson, S.; Abdallah, I.; Buttolph, T.; Glass, K.C.; Fandy, T.E. Curcumin and dimethoxycurcumin induced epigenetic changes in leukemia cells. Pharm. Res. 2015, 32, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Zhu, B.T. Inhibition of DNAmethylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis 2006, 27, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davinelli, S.; Calabrese, V.; Zella, D.; Scapagnini, G. Epigenetic nutraceutical diets in Alzheimer’s disease. J. Nutr. Health Aging 2014, 18, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Blei, T.; Soukup, S.T.; Schmalbach, K.; Pudenz, M.; Möller, F.J.; Egert, B.; Wörtz, N.; Kurrat, A.; Müller, D.; Vollmer, G.; et al. Dose-dependent effects of isoflavone exposure during early lifetime on the rat mammary gland: Studies on estrogen sensitivity, isoflavone metabolism, and DNA methylation. Mol. Nutr. Food Res. 2015, 59, 270–283. [Google Scholar] [CrossRef]
- Narayan, P.J.; Lill, C.; Faull, R.; Curtis, M.A.; Dragunow, M. Increased acetyl and total histone levels in post-mortem Alzheimer’s disease brain. Neurobiol. Dis. 2015, 74, 281–294. [Google Scholar] [CrossRef]
- Chouliaras, L.; van den Hove, D.L.; Kenis, G.; van Draanen, M.; Hof, P.R.; van Os, J.; Steinbusch, H.W.M.; Schmitz, C.; Rutten, B.P.F. Histone deacetylase 2 in the mouse hippocampus: Attenuation of age-related increase by caloric restriction. Curr. Alzheimer Res. 2013, 10, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Datta, M.; Staszewski, O.; Raschi, E.; Frosch, M.; Hagemeyer, N.; Tay, T.L.; Blank, T.; Kreutzfeldt, M.; Merkler, D.; Ziegler-Waldkirch, S.; et al. Histone deacetylases 1 and 2 regulate microglia function during development, homeostasis, and neurodegeneration in a contextdependent manner. Immunity 2018, 48, 514–529. [Google Scholar] [CrossRef]
- Gonzalez-Zuñiga, M.; Contreras, P.S.; Estrada, L.D.; Chamorro, D.; Villagra, A.; Zanlungo, S.; Seto, E.; Alvarez, A.R. c-Abl stabilizes HDAC2 levels by tyrosine phosphorylation repressing neuronal gene expression in Alzheimer’s disease. Molecular Cell 2014, 56, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Graff, J.; Woldemichael, B.T.; Berchtold, D.; Dewarrat, G.; Mansuy, I.M. Dynamic histone marks in the hippocampus and cortex facilitate memory consolidation. Nat. Commun. 2012, 3, 991. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.; Sananbenesi, F.; Wang, X.Y.; Dobbin, M.; Tsai, L.H. Recovery of learning and memory is associated with chromatin remodelling. Nature 2007, 447, 178–182. [Google Scholar] [CrossRef]
- Mahady, L.; Nadeem, M.; Malek-Ahmadi, M.; Chen, K.; Perez, S.E.; Mufson, E.J. HDAC2 dysregulation in the nucleus basalis of Meynert during the progression of Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2019, 45, 380–397. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.; Rei, D.; Guan, J.S.; Wang, W.Y.; Seo, J.; Hennig, K.M.; Nieland, T.J.F.; Fass, D.M.; Kao, P.F.; Kahn, M.; et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 2012, 483, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, N.; Rao, P.; Burkhardt, S.; Sananbenesi, F.; Schlüter, O.M.; Bradke, F.; Lu, J.; Fischer, A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 52–63. [Google Scholar] [CrossRef]
- Ma, J.; Huo, X.; Jarpe, M.B.; Kavelaars, A.; Heijnen, C.J. Pharmacological inhibition of HDAC6 reverses cognitive impairment and tau pathology as a result of cisplatin treatment. Acta Neuropathol. Commun. 2018, 6, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-Y.; Fan, S.-J.; Huang, F.-I.; Chao, H.-Y.; Hsu, K.-C.; Lin, T.E.; Yeh, T.-K.; Lai, M.-J.; Li, Y.-H.; Huang, H.L.; et al. 5-Aroylindoles act as selective histone deacetylase 6 inhibitors ameliorating Alzheimer’s disease phenotypes. J. Med. Chem. 2018, 61, 7087–7102. [Google Scholar] [CrossRef]
- Kilic, U.; Elibol, B.; Uysal, O.; Kilic, E.; Yulug, B.; Sakul, A.S.; Yildiz, G.B. Specific alterations in the circulating levels of the SIRT1, TLR4, and IL7 proteins in patients with dementia. Exp. Gerontol. 2018, 111, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.L.; Albeck, J.G.; Taha, A.Y.; Ori-McKenney, K.M.; Recanzone, G.H.; Stradleigh, T.W.; Hernandez, B.C.; Tang, F.-Y.V.; Chiang, E.-P.S.; Cruz-Orengo, L. Impact of diet-derived signaling molecules on human cognition: Exploring the food-brain axis. NPJ. Sci. Food. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [Green Version]
- Luccarini, I.; Grossi, C.; Rigacci, S.; Coppi, E.; Pugliese, A.M.; Pantano, D.; la Marca, G.; ed Dami, T.; Berti, A.; Stefani, M.; et al. Oleuropein aglycone protects against pyroglutamylated-3 amyloid- toxicity: Biochemical, epigenetic and functional correlates. Neurobiol. Aging 2015, 36, 648–663. [Google Scholar] [CrossRef]
- Juli, G.; Oliverio, M.; Bellizzi, D.; Gallo Cantafio, M.E.; Grillone, K.; Passarino, G.; Colica, C.; Nardi, M.; Rossi, M.; Procopio, A.; et al. Anti-tumor Activity and Epigenetic Impact of the Polyphenol Oleacein in Multiple Myeloma. Cancers 2019, 11, 990. [Google Scholar] [CrossRef] [Green Version]
- Momtaz, S.; Hassani, S.; Khan, F.; Ziaee, M.; Abdollahi, M. Cinnamon, a promising prospect towards Alzheimer’s disease. Pharmacol. Res. 2018, 130, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Sunagawa, Y.; Kawamura, T.; Takaya, T.; Wada, H.; Nagasawa, A.; Komeda, M.; Fujita, M.; Shimatsu, A.; Kita, T.; et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J. Clin. Invest. 2008, 118, 868–878. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. BioMed Research International-Special Issue: ω-3 PUFAs in the Prevention and Cure of Inflammatory, Degenerative, and Neoplastic Diseases. Biomed. Res. Int 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Dezso, Z.; MacKenzie, C.; Oestreicher, J.; Agoulnik, S.; Byrne, M.; Bernier, F.; Yanagimachi, M.; Aoshima, K.; Oda, Y. Circulating miRNA biomarkers for Alzheimer’s disease. PLoS ONE 2013, 8, e69807. [Google Scholar] [CrossRef]
- Di Meco, A.; Pratico, D. MicroRNAs as therapeutic targets for Alzheimer’s disease. J. Alzheimers Dis. 2016, 53, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Dang, W.; Donahue, G.; Dai, J.; Dorsey, J.; Cao, X.; Liu, W.; Cao, K.; Perry, R.; Lee, J.Y.; et al. H3K36 methylation promotes longevity by enhancing transcriptional fidelity. Genes Dev. 2015, 29, 1362–1376. [Google Scholar] [CrossRef] [Green Version]
- Santa-Maria, I.; Alaniz, M.A.; Renwick, N.; Cela, C.; Fulga, T.A.; Van Vactor, D.; Tuschl, T.; Clark, L.N.; Shelanski, M.L.; McCabe, B.D.; et al. Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. J. Clin. Invest. 2015, 125, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.K.; Narasingappa, R.B.; Vincent, B. Neuro-nutrients as anti-alzheimer’s disease agents: A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2999–3018. [Google Scholar] [CrossRef]
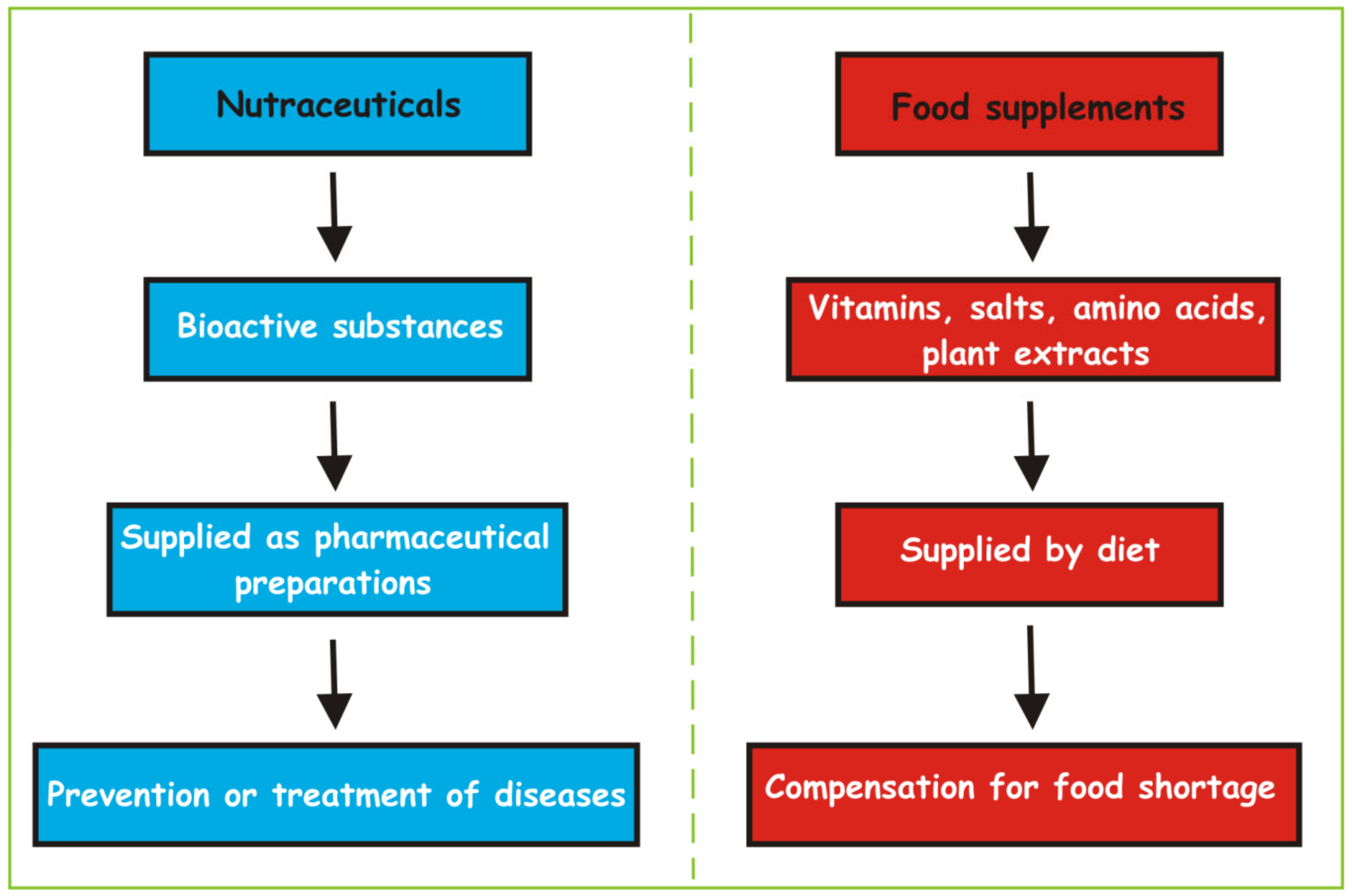
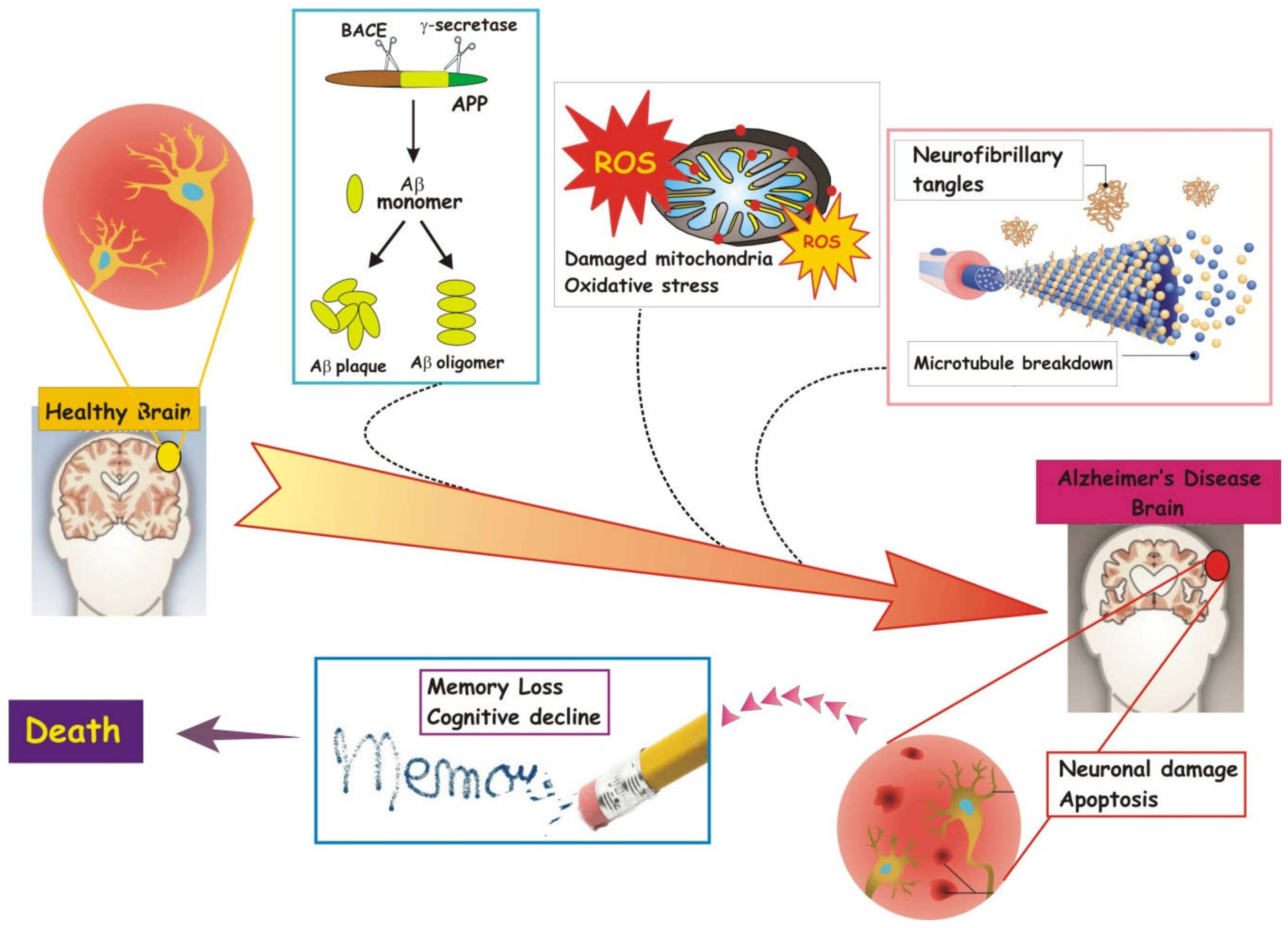


| Advantages of Nutraceuticals |
|
| Disadvantages of Nutraceuticals |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atlante, A.; Amadoro, G.; Bobba, A.; Latina, V. Functional Foods: An Approach to Modulate Molecular Mechanisms of Alzheimer’s Disease. Cells 2020, 9, 2347. https://doi.org/10.3390/cells9112347
Atlante A, Amadoro G, Bobba A, Latina V. Functional Foods: An Approach to Modulate Molecular Mechanisms of Alzheimer’s Disease. Cells. 2020; 9(11):2347. https://doi.org/10.3390/cells9112347
Chicago/Turabian StyleAtlante, Anna, Giuseppina Amadoro, Antonella Bobba, and Valentina Latina. 2020. "Functional Foods: An Approach to Modulate Molecular Mechanisms of Alzheimer’s Disease" Cells 9, no. 11: 2347. https://doi.org/10.3390/cells9112347
APA StyleAtlante, A., Amadoro, G., Bobba, A., & Latina, V. (2020). Functional Foods: An Approach to Modulate Molecular Mechanisms of Alzheimer’s Disease. Cells, 9(11), 2347. https://doi.org/10.3390/cells9112347








