Formation of an RNA Quadruplex-Duplex Hybrid in Living Cells between mRNA of the Epidermal Growth Factor Receptor (EGFR) and a G-Rich Antisense Oligoribonucleotide
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Purification of Unmodified Short DNA and RNA Oligonucleotides
2.2. Synthesis and Purification of Modified Q-ASO and EGFR mRNA Fragment
2.3. Q-ASO-NH2 Labeling with oBMVC-C3
2.4. Denaturing Electrophoresis of RNA Oligonucleotides
2.5. Non-Denaturing Electrophoresis of RNA Oligonucleotides
2.6. NMR Experiments
2.7. UV Thermal Denaturation Curves
2.8. CD Measurements
2.9. Cell Lines and Culture Conditions, Dual Fluorescence Assay
2.10. Cytotoxicity of Antisense Oligomers dASO-C, dASO, rASO, Q-RNA, QF-ASO, QL-ASO in HeLa Cells
2.11. Microscopic Analysis of Fluorescent Cells Expressing EGFP-EGFR Protein from the pEGFP-EGFR Fusion Plasmid
2.12. Microscopic Analysis of Localization of Quadruplexes Formed by Fl-Q-ASO (100 nM) and EGFR mRNA in MCF-7, HeLa and A431 Cells
2.13. Microscopic Analysis of Hybridization of Fl-Q-ASO with Endogenous EGFR mRNA in A431 Cells in the Presence of Various Inhibitory ASOs
3. Results
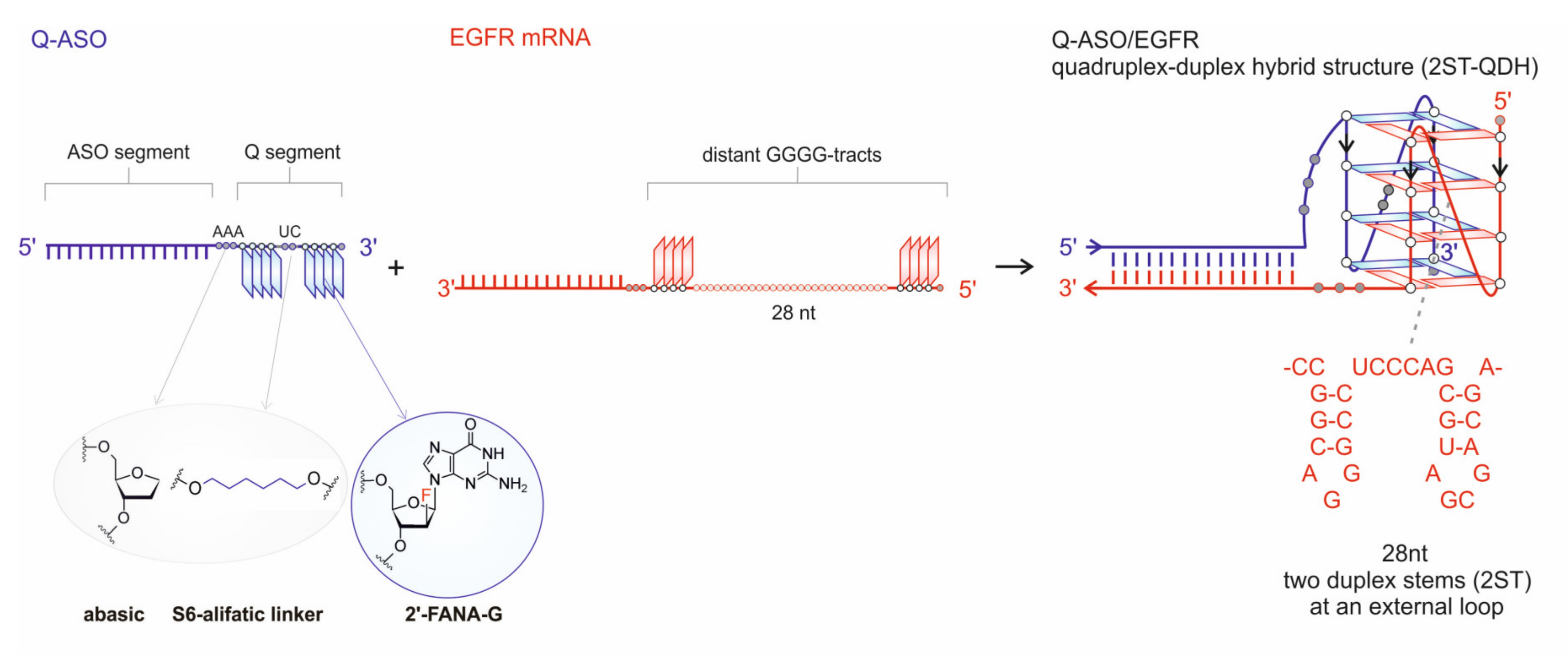
3.1. Hybridization of Q-ASO and EGFR mRNA Induces Quadruplex-Duplex Hybrid Formation
3.2. Native Polyacrylamide Gel Electrophoresis Validation of Secondary Structures of Q-ASO and EGFR and Their Complexes
3.3. NMR Validation of QF-ASO/EGFR and QL-ASO/EGFR Folding Topology
3.4. Thermal Stability of 2ST-QDHs
3.5. Topologies of G-Quadruplex Cores of 2ST-QDHs
3.6. Silencing Activity of Antisense Oligonucleotides in HeLa Cells Assessed by a Dual Fluorescence Assay
3.7. Mitochondrial Activity in HeLa Cells in Response to Transfection with dASO, rASO, Q-RNA, QF-ASO, and QL-ASO Antisense Oligonucleotides
3.8. Analysis of Silencing Activity of QL-ASO Oligonucleotides by Microscopic Fluorescence Imaging
3.9. Visualization of Density of the Endogenous EGFR mRNA Monitored by the G4-Detection in MCF-7, HeLa and A431 Cancer Cells
3.10. Analysis of Hybridization of Fl-Q-ASO with Endogenous EGFR mRNA in A431 Cells in the Presence of Various Inhibitory ASOs
4. Discussion
4.1. Formation of Quadruplex-Duplex Hybrid Structures
4.2. The RNA Q-ASO Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huppert, J.L. Prevalence of quadruplexes in the human genome. Nucleic Acids Res. 2005, 33, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.W.; Nguyen, T.Q.N.; Phan, A.T. Joining of Multiple Duplex Stems at a Single Quadruplex Loop. J. Am. Chem. Soc. 2014, 136, 17969–17973. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.W.; Phan, A.T. Structural Basis of DNA Quadruplex-Duplex Junction Formation. Angew. Chem. Int. Ed. 2013, 52, 8566–8569. [Google Scholar] [CrossRef]
- Lim, K.W.; Khong, Z.J.; Phan, A.T. Thermal Stability of DNA Quadruplex–Duplex Hybrids. Biochemistry 2013, 53, 247–257. [Google Scholar] [CrossRef]
- Lim, K.W.; Jenjaroenpun, P.; Low, Z.J.; Khong, Z.J.; Ng, Y.S.; Kuznetsov, V.A.; Phan, A.T. Duplex stem-loop-containing quadruplex motifs in the human genome: A combined genomic and structural study. Nucleic Acids Res. 2015, 43, 5630–5646. [Google Scholar] [CrossRef]
- Chambers, V.S.; Marsico, G.; Boutell, J.M.; Di Antonio, M.; Smith, G.P.; Balasubramanian, S. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat. Biotechnol. 2015, 33, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.K.; Marsico, G.; Sahakyan, A.B.; Chambers, V.S.; Balasubramanian, S. rG4-seq reveals widespread formation of G-quadruplex structures in the human transcriptome. Nat. Methods 2016, 13, 841–844. [Google Scholar] [CrossRef]
- Chilka, P.; Desai, N.; Datta, B. Small Molecule Fluorescent Probes for G- Quadruplex Visualization as Potential Cancer Theranostic Agents. Molecules 2019, 24, 752. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Chen, Y.-Q.; Wang, S.-R.; Zhou, X. G-Quadruplex: A Regulator of Gene Expression and Its Chemical Targeting. Chem 2018, 4, 1314–1344. [Google Scholar] [CrossRef]
- Biffi, G.; Di Antonio, M.; Tannahill, D.; Balasubramanian, S. Visualization and selective chemical targeting of RNA G-quadruplex structures in the cytoplasm of human cells. Nat. Chem. 2013, 6, 75–80. [Google Scholar] [CrossRef]
- Spiegel, J.; Adhikari, S.; Balasubramanian, S. The Structure and Function of DNA G-Quadruplexes. Trends Chem. 2020, 2, 123–136. [Google Scholar] [CrossRef]
- Rhodes, D.; Lipps, H.J. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015, 43, 8627–8637. [Google Scholar] [CrossRef]
- Fay, M.M.; Lyons, S.M.; Ivanov, P. RNA G-Quadruplexes in Biology: Principles and Molecular Mechanisms. J. Mol. Biol. 2017, 429, 2127–2147. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.L.; Singh, K.; Zhong, Y.; Drewe, P.; Rajasekhar, V.K.; Sanghvi, V.R.; Mavrakis, K.J.; Jiang, M.; Roderick, J.E.; Van der Meulen, J.; et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature 2014, 513, 65–70. [Google Scholar] [CrossRef]
- Maizels, N. G4-associated human diseases. EMBO Rep. 2015, 16, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, A.R.; Donnelly, C.J.; Periz, G.; Simko, E.A.J.; Shaw, P.G.; Kim, M.; Maragakis, N.J.; Troncoso, J.C.; Pandey, A.; Sattler, R.; et al. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature 2014, 507, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ribeyre, C.; Lopes, J.; Boulé, J.-B.; Piazza, A.; Guédin, A.; Zakian, V.A.; Mergny, J.-L.; Nicolas, A. The Yeast Pif1 Helicase Prevents Genomic Instability Caused by G-Quadruplex-Forming CEB1 Sequences In Vivo. PLoS Genet. 2009, 5, e1000475. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-Y.; Wang, X.-N.; Cheng, S.-Q.; Su, X.-X.; Ou, T.-M. Developing Novel G-Quadruplex Ligands: From Interaction with Nucleic Acids to Interfering with Nucleic Acid–Protein Interaction. Molecules 2019, 24, 396. [Google Scholar] [CrossRef]
- Katsuda, Y.; Sato, S.I.; Asano, L.; Morimura, Y.; Furuta, T.; Sugiyama, H.; Hagihara, M.; Uesugi, M. A Small Molecule That Represses Translation of G-Quadruplex-Containing mRNA. J. Am. Chem. Soc. 2016, 138, 9037–9040. [Google Scholar] [CrossRef] [PubMed]
- Asamitsu, S.; Obata, S.; Yu, Z.; Bando, T.; Sugiyama, H. Recent Progress of Targeted G-Quadruplex-Preferred Ligands Toward Cancer Therapy. Molecules 2019, 24, 429. [Google Scholar] [CrossRef]
- Chen, M.C.; Murat, P.; Abecassis, K.; Amar, A.R.F. Insights into the mechanism of a G-quadruplex-unwinding DEAH-box helicase. Nucleic Acids Res. 2015, 43, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Go, S.; Komiyama, M.; Xu, Y. Inhibition of translation by small RNA-stabilized mRNA structures in human cells. J. Am. Chem. Soc. 2011, 133, 19153–19159. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Nguyen, K.; Basu, S. Rationally Induced RNA:DNA G-Quadruplex Structures Elicit an Anticancer Effect by Inhibiting Endogenous eIF-4E Expression. Biochemistry 2014, 53, 5461–5470. [Google Scholar] [CrossRef]
- Hagihara, M.; Yamauchi, L.; Seo, A.; Yoneda, K.; Senda, M.; Nakatani, K. Antisense-induced guanine quadruplexes inhibit reverse transcription by HIV-1 reverse transcriptase. J. Am. Chem. Soc. 2010, 132, 11171–11178. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Kujtan, L.; Subramanian, J. Epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer. Expert Rev. Anticancer Ther. 2019, 19, 547–559. [Google Scholar] [CrossRef]
- El Guerrab, A.; Bamdad, M.; Kwiatkowski, F.; Bignon, Y.J.; Penault-Llorca, F.; Aubel, C. Anti-EGFR monoclonal antibodies and EGFR tyrosine kinase inhibitors as combination therapy for triple-negative breast cancer. Oncotarget 2016, 7, 73618–73637. [Google Scholar] [CrossRef]
- Kaniowski, D.; Ebenryter-Olbińska, K.; Sobczak, M.; Wojtczak, B.; Janczak, S.; Lésnikowski, Z.J.; Nawrot, B. High boron-loaded DNA-oligomers as potential boron neutron capture therapy and antisense oligonucleotide dual-action anticancer agents. Molecules 2017, 22, 1393. [Google Scholar] [CrossRef]
- Cheung, A.H.-K.; Chow, C.; Zhang, J.; Zhou, Y.; Huang, T.; Ng, K.C.-K.; Or, T.C.-T.; Yao, Y.Y.; Dong, Y.; Fung, J.M.-W.; et al. Specific targeting of point mutations in EGFR L858R-positive lung cancer by CRISPR/Cas9. Lab. Investig. 2018, 98, 968–976. [Google Scholar] [CrossRef]
- Ebenryter-Olbińska, K.; Kaniowski, D.; Sobczak, M.; Wojtczak, B.A.; Janczak, S.; Wielgus, E.; Nawrot, B.; Leśnikowski, Z.J. Versatile Method for the Site-Specific Modification of DNA with Boron Clusters: Anti-Epidermal Growth Factor Receptor (EGFR) Antisense Oligonucleotide Case. Chem. Eur. J. 2017, 23, 16535–16546. [Google Scholar] [CrossRef]
- Kaniowski, D.; Ebenryter-Olbinska, K.; Kulik, K.; Janczak, S.; Maciaszek, A.; Bednarska-Szczepaniak, K.; Nawrot, B.; Lesnikowski, Z. Boron clusters as a platform for new materials: Composites of nucleic acids and oligofunctionalized carboranes (C2B10H12) and their assembly into functional nanoparticles. Nanoscale 2020, 12, 103–114. [Google Scholar] [CrossRef]
- Kolesnikova, S.; Hubálek, M.; Bednárová, L.; Cvačka, J.; Curtis, E.A. Multimerization rules for G-quadruplexes. Nucleic Acids Res. 2017, 45, 8684–8696. [Google Scholar] [CrossRef] [PubMed]
- Jackowiak, P.; Hojka-Osinska, A.; Gasiorek, K.; Stelmaszczuk, M.; Gudanis, D.; Gdaniec, Z.; Figlerowicz, M. Effects of G-quadruplex topology on translational inhibition by tRNA fragments in mammalian and plant systems in vitro. Int. J. Biochem. Cell Biol. 2017, 92, 148–154. [Google Scholar] [CrossRef]
- Risitano, A.; Fox, K.R. Influence of loop size on the stability of intramolecular DNA quadruplexes. Nucleic Acids Res. 2004, 32, 2598–2606. [Google Scholar] [CrossRef]
- Rachwal, P.A.; Brown, T.; Fox, K.R. Sequence effects of single base loops in intramolecular quadruplex DNA. FEBS Lett. 2007, 581, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Liy, Z.; Lechy, C.J.; Phan, A.T. Sugar-modified G-quadruplexes: Effects of LNA-, 2′F-RNA-and 2′F-ANA-guanosine chemistries on G-quadruplex structure and stability. Nucleic Acids Res. 2014, 42, 4068–4079. [Google Scholar] [CrossRef] [PubMed]
- Varani, G.; Tinoco, I. RNA structure and NMR spectroscopy. Q. Rev. Biophys. 1991, 24, 479–532. [Google Scholar] [CrossRef]
- Nguyen, T.Q.N.; Lim, K.W.; Phan, A.T. Folding Kinetics of G-Quadruplexes: Duplex Stem Loops Drive and Accelerate G-Quadruplex Folding. J. Phys. Chem. B 2020, 124, 5122–5130. [Google Scholar] [CrossRef]
- Rachwal, P.A.; Fox, K.R. Quadruplex melting. Methods 2007, 43, 291–301. [Google Scholar] [CrossRef]
- Małgowska, M.; Gudanis, D.; Teubert, A.; Dominiak, G.; Gdaniec, Z. How to study G-quadruplex structures. Biotechnologia 2013, 93, 381–390. [Google Scholar] [CrossRef]
- Vorlíčková, M.; Kejnovská, I.; Bednářová, K.; Renčiuk, D.; Kypr, J. Circular Dichroism Spectroscopy of DNA: From Duplexes to Quadruplexes. Chirality 2012, 24, 691–698. [Google Scholar] [CrossRef]
- Xiao, C.-D.; Shibata, T.; Yamamoto, Y.; Xu, Y. An intramolecular antiparallel G-quadruplex formed by human telomere RNA. Chem. Commun. 2018, 54, 3944–3946. [Google Scholar] [CrossRef] [PubMed]
- Warner, K.D.; Chen, M.C.; Song, W.; Strack, R.L.; Thorn, A.; Jaffrey, S.R.; Ferré-D’Amaré, A.R. Structural basis for activity of highly efficient RNA mimics of green fluorescent protein. Nat. Struct. Mol. Biol. 2014, 21, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Sipa, K.; Sochacka, E.; Kazmierczak-Baranska, J.; Maszewska, M.; Janicka, M.; Nowak, G.; Nawrot, B. Effect of base modifications on structure, thermodynamic stability, and gene silencing activity of short interfering RNA. RNA 2007, 13, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, S.; Yin, L.; Yang, Y.; Guan, Y.; Wang, W.; Xu, H.; Tao, N. Quantification of Epidermal Growth Factor Receptor Expression Level and Binding Kinetics on Cell Surfaces by Surface Plasmon Resonance Imaging. Anal. Chem. 2015, 87, 9960–9965. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chou, C.-K.; Kim, M.; Vasisht, R.; Kuo, Y.-A.; Ang, P.; Liu, C.; Perillo, E.P.; Chen, Y.-A.; Blocher, K.; et al. Assessing metastatic potential of breast cancer cells based on EGFR dynamics. Sci. Rep. 2019, 9, 3395. [Google Scholar] [CrossRef] [PubMed]
- Palizban, A.A.; Sadeghi-Aliabadi, H.; Abdollahpour, F. Effect of cerium lanthanide on Hela and MCF-7 cancer cell growth in the presence of transferring. Res. Pharm. Sci. 2010, 5, 119–125. [Google Scholar]
- Grenman, S.; Shapira, A.; Carey, T.E. In vitro response of cervical cancer cell lines CaSki, HeLa, and ME-180 to the antiestrogen tamoxifen. Gynecol. Oncol. 1988, 30, 228–238. [Google Scholar] [CrossRef]
- Tien, Y.; Tsai, C.-L.; Hou, W.-H.; Chiang, Y.; Hsu, F.-M.; Tsai, Y.-C.; Cheng, J.C.-H. Targeting human epidermal growth factor receptor 2 enhances radiosensitivity and reduces the metastatic potential of Lewis lung carcinoma cells. Radiat. Oncol. 2020, 15, 58. [Google Scholar] [CrossRef]
- Stratigos, A.; Garbe, C.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Becker, J.C.; Zalaudek, I.; Saiag, P.; et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 1989–2007. [Google Scholar] [CrossRef]
- Fotticchia, I.; Amato, J.; Pagano, B.; Novellino, E.; Petraccone, L.; Giancola, C. How are thermodynamically stable G-quadruplex-duplex hybrids? J. Therm. Anal. Calorim. 2015, 121, 1121–1127. [Google Scholar] [CrossRef]
- Phan, A.T.; Kuryavyi, V.; Darnell, J.C.; Serganov, A.; Majumdar, A.; Ilin, S.; Raslin, T.; Polonskaia, A.; Chen, C.; Clain, D.; et al. Structure-function studies of FMRP RGG peptide recognition of an RNA duplex-quadruplex junction. Nat. Struct. Mol. Biol. 2011, 18, 796–804. [Google Scholar] [CrossRef]
- Ouellet, J. RNA Fluorescence with Light-Up Aptamers. Front. Chem. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Fernandez-Millan, P.; Autour, A.; Ennifar, E.; Westhof, E.; Ryckelynck, M. Crystal structure and fluorescence properties of the iSpinach aptamer in complex with DFHBI. RNA 2017, 23, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, B.H.; Giles, R.V.; Spiller, D.G.; Grzybowski, J.; Tidd, D.M.; Sibson, D.R. Clatterbridge Determination of optimal sites of antisense oligonucleotide cleavage within TNFalpha mRNA. Nucleic Acids Res. 2001, 29, 3664–3673. [Google Scholar] [CrossRef]
- Bennett, C.F. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu. Rev. Med. 2019, 70, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Gurav, B.; Srinivasan, G. Antisense oligonucleotides as therapeutics and their delivery. Curr. Sci. 2017, 112, 490–498. [Google Scholar] [CrossRef]
- Shen, X.; Corey, D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2017, 1–17. [Google Scholar] [CrossRef]
- Seth, P.P.; Vasquez, G.; Allerson, C.A.; Berdeja, A.; Gaus, H.; Kinberger, G.A.; Prakash, T.P.; Migawa, M.T.; Bhat, B.; Swayze, E.E. Synthesis and Biophysical Evaluation of 2′,4′-Constrained 2′ O -Methoxyethyl and 2′,4′-Constrained 2′ O -Ethyl Nucleic Acid Analogues. J. Org. Chem. 2010, 75, 1569–1581. [Google Scholar] [CrossRef]
- Kalota, A.; Karabon, L.; Swider, C.R.; Viazovkina, E.; Elzagheid, M.; Damha, M.J.; Gewirtz, A.M. 2′-Deoxy-2′-fluoro-β-D-arabinonucleic acid (2′F-ANA) modified oligonucleotides (ON) effect highly efficient, and persistent, gene silencing. Nucleic Acids Res. 2006, 34, 451–461. [Google Scholar] [CrossRef]
- Braasch, D.A.; Corey, D.R. Locked nucleic acid (LNA): Fine-tuning the recognition of DNA and RNA. Chem. Biol. 2001, 8, 1–7. [Google Scholar] [CrossRef]
- Smith, C.I.E.; Zain, R. Therapeutic Oligonucleotides: State of the Art. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 605–630. [Google Scholar] [CrossRef]
- Deleavey, G.F.; Damha, M.J. Designing Chemically Modified Oligonucleotides for Targeted Gene Silencing. Chem. Biol. 2012, 19, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.U.; Bartel, D.P. RNA G-quadruplexes are globally unfolded in eukaryotic cells and depleted in bacteria. Science 2016, 353, aaf5371. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.K.; Marsico, G.; Balasubramanian, S. Detecting RNA G-quadruplexes (rG4s) in the transcriptome. Cold Spring Harb. Perspect. Biol. 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Chen, X.-C.; Chen, S.-B.; Dai, J.; Yuan, J.-H.; Ou, T.-M.; Huang, Z.-S.; Tan, J.-H. Tracking the Dynamic Folding and Unfolding of RNA G-Quadruplexes in Live Cells. Angew. Chem. Int. Ed. 2018, 57, 4702–4706. [Google Scholar] [CrossRef]
- Chen, S.-B.; Hu, M.-H.; Liu, G.-C.; Wang, J.; Ou, T.-M.; Gu, L.-Q.; Huang, Z.-S.; Tan, J.-H. Visualization of NRAS RNA G-Quadruplex Structures in Cells with an Engineered Fluorogenic Hybridization Probe. J. Am. Chem. Soc. 2016, 138, 10382–10385. [Google Scholar] [CrossRef]
- Lyu, K.; Chen, S.B.; Chan, C.Y.; Tan, J.H.; Kwok, C.K. Structural analysis and cellular visualization of APP RNA G-quadruplex. Chem. Sci. 2019, 10, 11095–11102. [Google Scholar] [CrossRef]
- Tseng, T.-Y.; Chu, I.-T.; Lin, S.-J.; Li, J.; Chang, T.-C. Binding of Small Molecules to G-quadruplex DNA in Cells Revealed by Fluorescence Lifetime Imaging Microscopy of o-BMVC Foci. Molecules 2018, 24, 35. [Google Scholar] [CrossRef]









| Reference Antisense Oligonucleotides | Name |
|---|---|
| d(AGCAGCGCCAGGAGCG) | dASO |
| r(AGCAGCGCCAGGAGCG) | rASO |
| r(AGGGGUCGGGGA) | Q-RNA |
| d(TTTCTTTTCCTCCAGAGCCCGA) | dASO-R |
| d(ATGAAGGTTCAATCTGATTTT) | dASO-C |
| Q-ASO: G-rich antisense oligoribonucleotides folded into the quadruplex-duplex hybrid | |
| AGCAGCGCCAGGAGCG-AAA-GFFG-UC-GFFGA | QF-ASO |
| AGCAGCGCCAGGAGCG-aaa-GGGG-S6-GGGGA | QL-ASO |
| AGCAGCGCCAGGAGCG-AAA-GGGG-UC-GGGGA-linker-NH2 | Q-ASO-NH2 |
| AGCAGCGCCAGGAGCG-AAA-GGGG-UC-GGGGA-linker-oBMVCC3 | Fl-Q-ASO |
| EGFR: EGFR-235-290 mRNA target sequence | |
| CGGGG-AGCAGCGAUGCGACCCUCCGGGACGGCC-GGGG-CAG- CGCUCCUGGCGCUGCU | EGFR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudanis, D.; Kaniowski, D.; Kulik, K.; Baranowski, D.; Gdaniec, Z.; Nawrot, B. Formation of an RNA Quadruplex-Duplex Hybrid in Living Cells between mRNA of the Epidermal Growth Factor Receptor (EGFR) and a G-Rich Antisense Oligoribonucleotide. Cells 2020, 9, 2375. https://doi.org/10.3390/cells9112375
Gudanis D, Kaniowski D, Kulik K, Baranowski D, Gdaniec Z, Nawrot B. Formation of an RNA Quadruplex-Duplex Hybrid in Living Cells between mRNA of the Epidermal Growth Factor Receptor (EGFR) and a G-Rich Antisense Oligoribonucleotide. Cells. 2020; 9(11):2375. https://doi.org/10.3390/cells9112375
Chicago/Turabian StyleGudanis, Dorota, Damian Kaniowski, Katarzyna Kulik, Daniel Baranowski, Zofia Gdaniec, and Barbara Nawrot. 2020. "Formation of an RNA Quadruplex-Duplex Hybrid in Living Cells between mRNA of the Epidermal Growth Factor Receptor (EGFR) and a G-Rich Antisense Oligoribonucleotide" Cells 9, no. 11: 2375. https://doi.org/10.3390/cells9112375
APA StyleGudanis, D., Kaniowski, D., Kulik, K., Baranowski, D., Gdaniec, Z., & Nawrot, B. (2020). Formation of an RNA Quadruplex-Duplex Hybrid in Living Cells between mRNA of the Epidermal Growth Factor Receptor (EGFR) and a G-Rich Antisense Oligoribonucleotide. Cells, 9(11), 2375. https://doi.org/10.3390/cells9112375





