A Novel FRET Approach Quantifies the Interaction Strength of Peroxisomal Targeting Signals and Their Receptor in Living Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. A Novel System to Investigate the Interaction between PEX5 and PTS1 by FRET
3.2. Quantitative Interaction Studies by Flow Cytometry-Based FRET Measurements (FlowFRET)
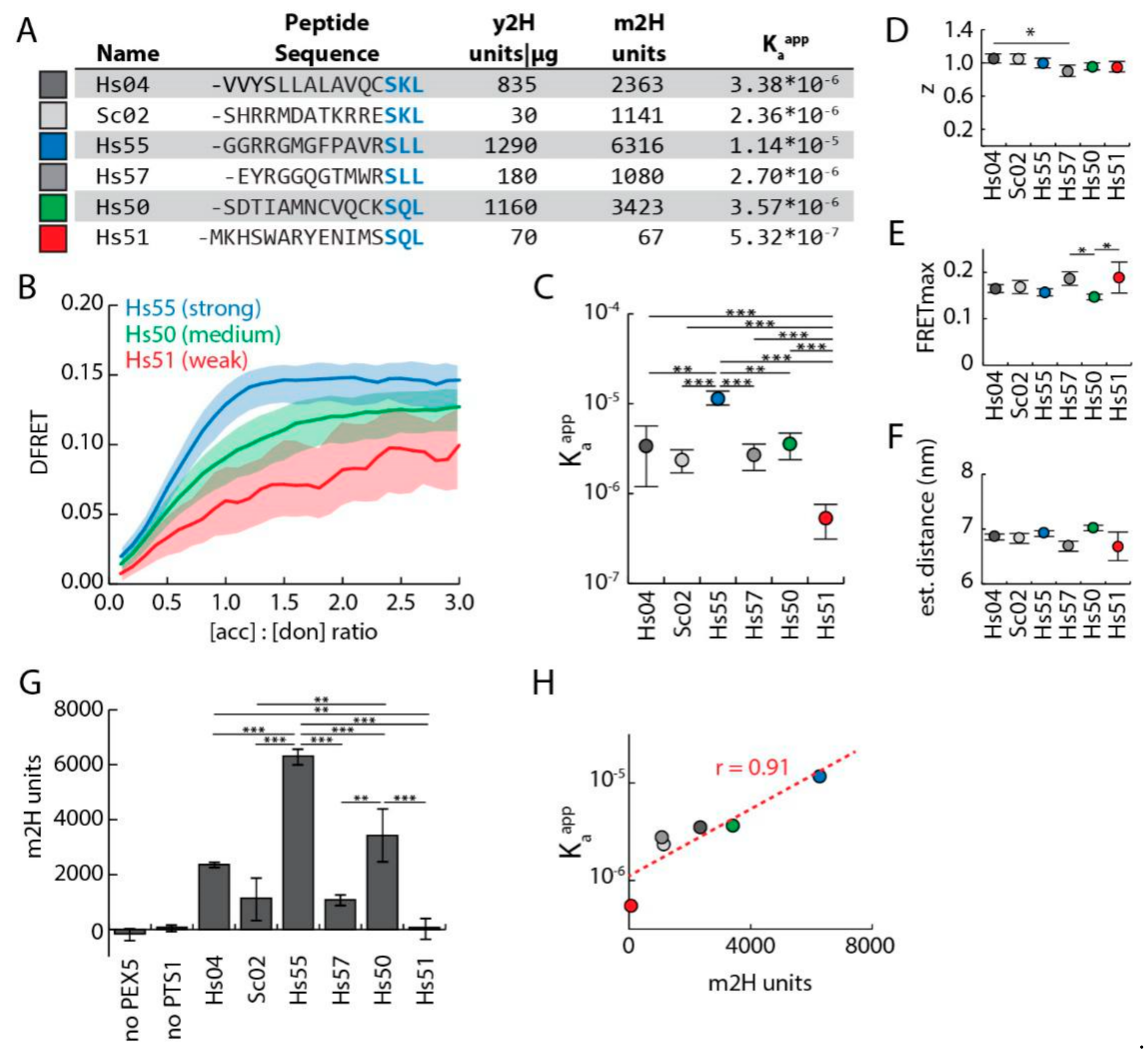
3.3. Discrimination of Affinities by FlowFRET
3.4. Live-Cell Competition Experiments by FlowFRET
3.5. The Upstream Sequence of PTS1 Motifs Determines Their Binding Strength to PEX5
3.6. Computational Verification
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DFRET | donor-normalized FRET |
| FRET | Förster-resonance-energy-transfer |
| FRETmax | plateau level of DFRET |
| Kaapp | apparent affinity |
| PPI | protein–protein interaction |
| PEX | Peroxin |
| PTS | Peroxisomal targeting signal |
| TPR | tetratricopeptide |
| z | stoichiometry factor of the complex |
References
- Wanders, R.J.A.; Waterham, H.R. Biochemistry of Mammalian Peroxisomes Revisited. Annu. Rev. Biochem. 2006, 75, 295–332. [Google Scholar] [CrossRef] [PubMed]
- Reumann, S.; Bartel, B. Plant peroxisomes: Recent discoveries in functional complexity, organelle homeostasis, and morphological dynamics. Curr. Opin. Plant Biol. 2016, 34, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Waterham, H.R.; Ferdinandusse, S.; Wanders, R.J.A. Human disorders of peroxisome metabolism and biogenesis. Biochim. Biophys. Acta 2016, 1863, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Reumann, S.; Chowdhary, G.; Lingner, T. Characterization, prediction and evolution of plant peroxisomal targeting signals type 1 (PTS1s). Biochim. Biophys. Bioenerg. 2016, 1863, 790–803. [Google Scholar] [CrossRef]
- Brocard, C.; Hartig, A. Peroxisome targeting signal 1: Is it really a simple tripeptide? Biochim. Biophys. Acta 2006, 1763, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Kunze, M.; Berger, J. The similarity between N-terminal targeting signals for protein import into different organelles and its evolutionary relevance. Front. Physiol. 2015, 6, 259. [Google Scholar] [CrossRef] [PubMed]
- Lanyon-Hogg, T.; Warriner, S.; Baker, A. Getting a camel through the eye of a needle: The import of folded proteins by peroxisomes. Biol. Cell 2010, 102, 245–263. [Google Scholar] [CrossRef]
- Waterham, H.R.; Ebberink, M.S. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim. Biophys. Acta 2012, 1822, 1430–1441. [Google Scholar] [CrossRef]
- Francisco, T.; Rodrigues, T.A.; Dias, A.F.; Barros-Barbosa, A.; Bicho, D.; Azevedo, J.E. Protein transport into peroxisomes: Knowns and unknowns. BioEssays 2017, 39, 39. [Google Scholar] [CrossRef]
- Gould, S.G.; Keller, G.A.; Subramani, S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J. Cell Biol. 1987, 105, 2923–2931. [Google Scholar] [CrossRef]
- Gould, S.J.; Keller, G.-A.; Hosken, N.; Wilkinson, J.; Subramani, S. A conserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 1989, 108, 1657–1664. [Google Scholar] [CrossRef]
- Lametschwandtner, G.; Brocard, C.; Fransen, M.; Van Veldhoven, P.; Berger, J.; Hartig, A. The Difference in Recognition of Terminal Tripeptides as Peroxisomal Targeting Signal 1 between Yeast and Human Is Due to Different Affinities of Their Receptor Pex5p to the Cognate Signal and to Residues Adjacent to It. J. Biol. Chem. 1998, 273, 33635–33643. [Google Scholar] [CrossRef]
- Neuberger, G.; Maurer-Stroh, S.; Eisenhaber, B.; Hartig, A.; Eisenhaber, F. Motif Refinement of the Peroxisomal Targeting Signal 1 and Evaluation of Taxon-specific Differences. J. Mol. Biol. 2003, 328, 567–579. [Google Scholar] [CrossRef]
- Van Der Leij, I.; Franse, M.M.; Elgersma, Y.; Distel, B.; Tabak, H.F. PAS10 is a tetratricopeptide-repeat protein that is essential for the import of most matrix proteins into peroxisomes of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1993, 90, 11782–11786. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, E.A.; Nuttley, W.M.; Bertolaet, B.L.; Li, X.; Francke, U.; Wheelock, M.J.; Anné, U.K.; Johnson, K.R.; Subramani, S. Human peroxisomal targeting signal-1 receptor restores peroxisomal protein import in cells from patients with fatal peroxisomal disorders. J. Cell Biol. 1995, 130, 51–65. [Google Scholar] [CrossRef]
- Ghosh, D.; Berg, J. A Proteome-Wide Perspective on Peroxisome Targeting Signal 1(PTS1)-Pex5p Affinities. J. Am. Chem. Soc. 2010, 132, 3973–3979. [Google Scholar] [CrossRef]
- Gatto, G.J.; Maynard, E.L.; Guerrerio, A.L.; Geisbrecht, B.V.; Gould, S.J.; Berg, J. Correlating Structure and Affinity for PEX5:PTS1 Complexes. Biochem. 2003, 42, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Maynard, E.L.; Berg, J. Quantitative Analysis of Peroxisomal Targeting Signal Type-1 Binding to Wild-type and Pathogenic Mutants of Pex5p Supports an Affinity Threshold for Peroxisomal Protein Targeting. J. Mol. Biol. 2007, 368, 1259–1266. [Google Scholar] [CrossRef]
- Skoulding, N.S.; Chowdhary, G.; Deus, M.J.; Baker, A.; Reumann, S.; Warriner, S. Experimental Validation of Plant Peroxisomal Targeting Prediction Algorithms by Systematic Comparison of In Vivo Import Efficiency and In Vitro PTS1 Binding Affinity. J. Mol. Biol. 2015, 427, 1085–1101. [Google Scholar] [CrossRef]
- Cross, L.L.; Paudyal, R.; Kamisugi, Y.; Berry, A.; Cuming, A.C.; Baker, A.; Warriner, S. Towards designer organelles by subverting the peroxisomal import pathway. Nat. Commun. 2017, 8, 454. [Google Scholar] [CrossRef]
- Zimmerman, S.B.; Minton, A.P. Macromolecular Crowding: Biochemical, Biophysical, and Physiological Consequences. Annu. Rev. Biophys. Biomol. Struct. 1993, 22, 27–65. [Google Scholar] [CrossRef] [PubMed]
- Musiani, F.; Giorgetti, A. Protein Aggregation and Molecular Crowding: Perspectives from Multiscale Simulations. Int. Rev. Cell Mol. Bio. 2017, 329, 49–77. [Google Scholar] [CrossRef]
- Elcock, A.H. Models of macromolecular crowding effects and the need for quantitative comparisons with experiment. Curr. Opin. Struct. Biol. 2010, 20, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Fields, S. Interactive learning: Lessons from two hybrids over two decades. Proteomics 2009, 9, 5209–5213. [Google Scholar] [CrossRef] [PubMed]
- Lievens, S.; Lemmens, I.; Tavernier, J. Mammalian two-hybrids come of age. Trends Biochem. Sci. 2009, 34, 579–588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okamoto, K.; Sako, Y. Recent advances in FRET for the study of protein interactions and dynamics. Curr. Opin. Struct. Biol. 2017, 46, 16–23. [Google Scholar] [CrossRef]
- Förster, T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. der Phys. 1948, 437, 55–75. [Google Scholar] [CrossRef]
- Shrestha, D.; Jenei, A.; Nagy, P.; Vereb, G.; Szollosi, J. Understanding FRET as a Research Tool for Cellular Studies. Int. J. Mol. Sci. 2015, 16, 6718–6756. [Google Scholar] [CrossRef]
- Zeug, A.; Woehler, A.; Neher, E.; Ponimaskin, E. Quantitative Intensity-Based FRET Approaches—A Comparative Snapshot. Biophys. J. 2012, 103, 1821–1827. [Google Scholar] [CrossRef]
- Hochreiter, B.; Kunze, M.; Moser, B.; Schmid, J.A. Advanced FRET normalization allows quantitative analysis of protein interactions including stoichiometries and relative affinities in living cells. Sci. Rep. 2019, 9, 8233. [Google Scholar] [CrossRef]
- He, L.; Olson, D.P.; Wu, X.; Karpova, T.S.; McNally, J.G.; Lipsky, P.E. A flow cytometric method to detect protein-protein interaction in living cells by directly visualizing donor fluorophore quenching during CFP→YFP fluorescence resonance energy transfer (FRET). Cytometry 2003, 55, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Banning, C.; Votteler, J.; Hoffmann, D.; Koppensteiner, H.; Warmer, M.; Reimer, R.; Kirchhoff, F.; Schubert, U.; Hauber, J.; Schindler, M. A Flow Cytometry-Based FRET Assay to Identify and Analyse Protein-Protein Interactions in Living Cells. PLoS ONE 2010, 5, e9344. [Google Scholar] [CrossRef] [PubMed]
- Szalóki, N.; Doan-Xuan, Q.M.; Szöllősi, J.; Tóth, K.; Vámosi, G.; Bacsó, Z. High throughput FRET analysis of protein-protein interactions by slide-based imaging laser scanning cytometry. Cytometry 2013, 83, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Baes, M.; Gressens, P.; Baumgart, E.; Carmeliet, P.; Casteels, M.; Fransen, M.; Evrard, P.; Fahimi, D.; Declercq, P.E.; Collen, D.; et al. A mouse model for Zellweger syndrome. Nat. Genet. 1997, 17, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kunze, M.; Malkani, N.; Maurer-Stroh, S.; Wiesinger, C.; Schmid, J.A.; Berger, J. Mechanistic insights into PTS2-mediated peroxisomal protein import: The co-receptor PEX5L drastically increases the interaction strength between the cargo protein and the receptor PEX7. J. Biol. Chem. 2015, 290, 4928–4940. [Google Scholar] [CrossRef]
- Gatto, G.J.; Geisbrecht, B.V.; Gould, S.J.; Berg, J. Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nat. Genet. 2000, 7, 1091–1095. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Grou, C.P.; Pinto, M.P.; Alencastre, I.S.; Costa-Rodrigues, J.; Fransen, M.; Miranda, M.C.S.; Azevedo, J.E. Functional characterization of two missense mutations in Pex5p—C11S and N526K. Biochim. Biophys. Acta 2007, 1773, 1141–1148. [Google Scholar] [CrossRef]
- Akrap, N.; Seidel, T.; Barisas, B. Förster distances for fluorescence resonant energy transfer between mCherry and other visible fluorescent proteins. Anal. Biochem. 2010, 402, 105–106. [Google Scholar] [CrossRef]
- Evers, T.H.; Van Dongen, E.M.W.M.; Faesen, A.C.; Meijer, E.B.; Merkx, M. Quantitative Understanding of the Energy Transfer between Fluorescent Proteins Connected via Flexible Peptide Linkers. Biochemistry 2006, 45, 13183–13192. [Google Scholar] [CrossRef]
- A Rizzo, M.; Springer, G.H.; Granada, B.; Piston, D.W. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 2004, 22, 445–449. [Google Scholar] [CrossRef]
- Neuberger, G.; Maurer-Stroh, S.; Eisenhaber, B.; Hartig, A.; Eisenhaber, F. Prediction of Peroxisomal Targeting Signal 1 Containing Proteins from Amino Acid Sequence. J. Mol. Biol. 2003, 328, 581–592. [Google Scholar] [CrossRef]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX web server: An online force field. Nucleic Acids Res. 2005, 33, W382–W388. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.A.; Filipp, F.V.; Kursula, P.; Schüller, N.; Erdmann, R.; Schliebs, W.; Sattler, M.; Wilmanns, M. Recognition of a Functional Peroxisome Type 1 Target by the Dynamic Import Receptor Pex5p. Mol. Cell 2006, 24, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Christensen, K.; Swanson, J.A. Fluorescence Resonance Energy Transfer-Based Stoichiometry in Living Cells. Biophys. J. 2002, 83, 3652–3664. [Google Scholar] [CrossRef]
- Kofoed, E.M.; Guerbadot, M.; Schaufele, F. Structure, Affinity, and Availability of Estrogen Receptor Complexes in the Cellular Environment. J. Biol. Chem. 2009, 285, 2428–2437. [Google Scholar] [CrossRef]
- Chen, H.; Puhl, H.L.; Koushik, S.V.; Vogel, S.S.; Ikeda, S.R. Measurement of FRET Efficiency and Ratio of Donor to Acceptor Concentration in Living Cells. Biophys. J. 2006, 91, L39–L41. [Google Scholar] [CrossRef]
- Butz, E.S.; Ben-Johny, M.; Shen, M.; Yang, P.S.; Sang, L.; Biel, M.; Yue, D.T.; Wahl-Schott, C. Quantifying macromolecular interactions in living cells using FRET two-hybrid assays. Nat. Protoc. 2016, 11, 2470–2498. [Google Scholar] [CrossRef]
- Lingner, T.; Kataya, A.R.A.; Antonicelli, G.E.; Benichou, A.; Nilssen, K.; Chen, X.-Y.; Siemsen, T.; Morgenstern, B.; Meinicke, P.; Reumann, S. Identification of Novel Plant Peroxisomal Targeting Signals by a Combination of Machine Learning Methods and in Vivo Subcellular Targeting Analyses. Plant Cell 2011, 23, 1556–1572. [Google Scholar] [CrossRef]
- Fodor, K.; Wolf, J.; Reglinski, K.; Passon, D.M.; Lou, Y.; Schliebs, W.; Erdmann, R.; Wilmanns, M. Ligand-Induced Compaction of the PEX5 Receptor-Binding Cavity Impacts Protein Import Efficiency into Peroxisomes. Traffic 2014, 16, 85–98. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochreiter, B.; Chong, C.-S.; Hartig, A.; Maurer-Stroh, S.; Berger, J.; Schmid, J.A.; Kunze, M. A Novel FRET Approach Quantifies the Interaction Strength of Peroxisomal Targeting Signals and Their Receptor in Living Cells. Cells 2020, 9, 2381. https://doi.org/10.3390/cells9112381
Hochreiter B, Chong C-S, Hartig A, Maurer-Stroh S, Berger J, Schmid JA, Kunze M. A Novel FRET Approach Quantifies the Interaction Strength of Peroxisomal Targeting Signals and Their Receptor in Living Cells. Cells. 2020; 9(11):2381. https://doi.org/10.3390/cells9112381
Chicago/Turabian StyleHochreiter, Bernhard, Cheng-Shoong Chong, Andreas Hartig, Sebastian Maurer-Stroh, Johannes Berger, Johannes A. Schmid, and Markus Kunze. 2020. "A Novel FRET Approach Quantifies the Interaction Strength of Peroxisomal Targeting Signals and Their Receptor in Living Cells" Cells 9, no. 11: 2381. https://doi.org/10.3390/cells9112381
APA StyleHochreiter, B., Chong, C.-S., Hartig, A., Maurer-Stroh, S., Berger, J., Schmid, J. A., & Kunze, M. (2020). A Novel FRET Approach Quantifies the Interaction Strength of Peroxisomal Targeting Signals and Their Receptor in Living Cells. Cells, 9(11), 2381. https://doi.org/10.3390/cells9112381







