An Index Combining Lost and Remaining Nerve Fibers Correlates with Pain Hypersensitivity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Chronic Constriction Injury
2.4. Nociceptive Tests for Pain Threshold Measurement
2.4.1. Assessment of Mechanical Hypersensitivity
2.4.2. Assessment of Thermal Hyperalgesia
2.5. Immunohistochemistry
2.6. Intravital Microscopy
2.6.1. Global Imaging Using Fluorescence Microscopy
2.6.2. Local Imaging Using Two-Photon Fluorescence Microscopy
2.6.3. D Intravital Image Processing and Analysis
2.7. Statistical Analysis
3. Results
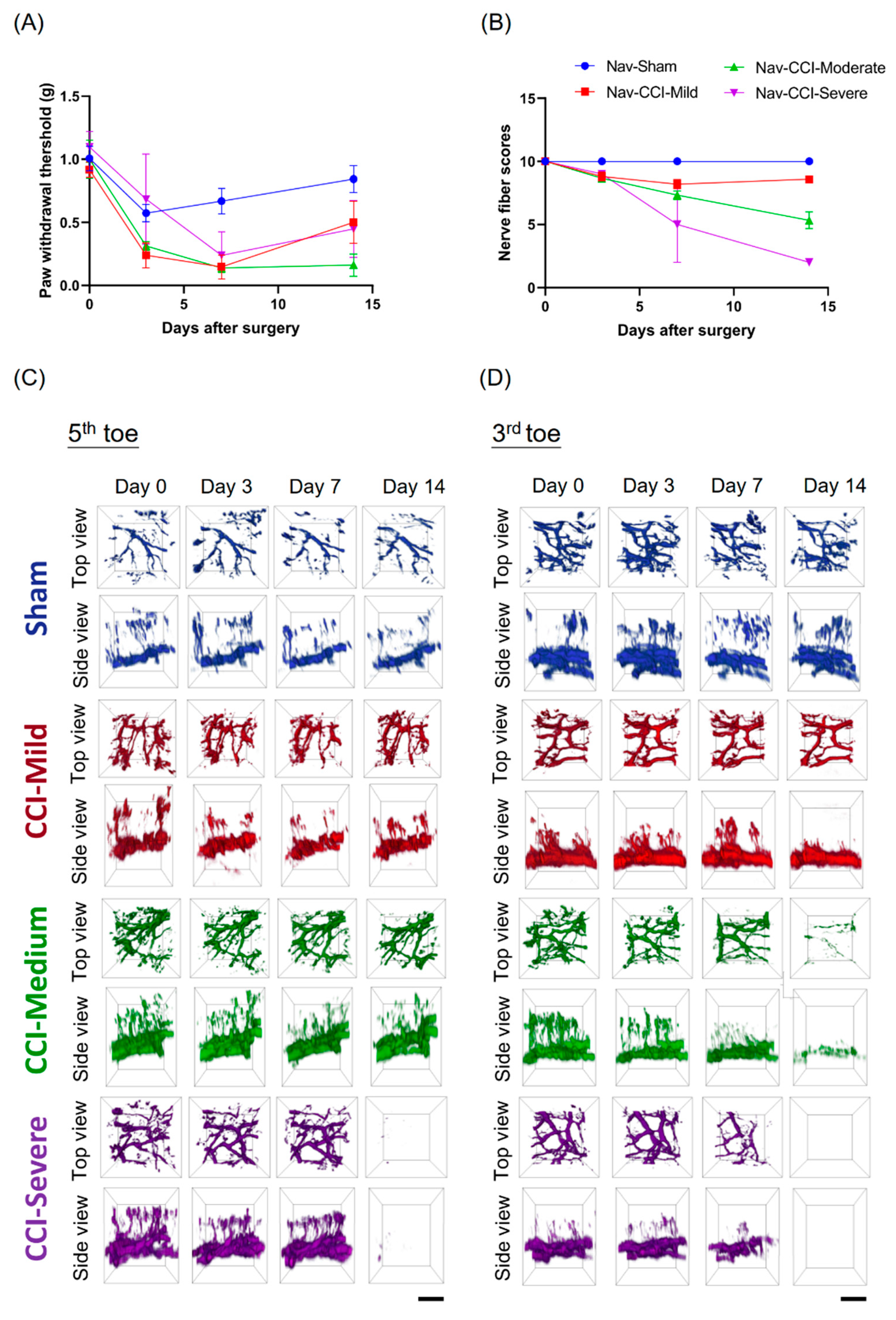
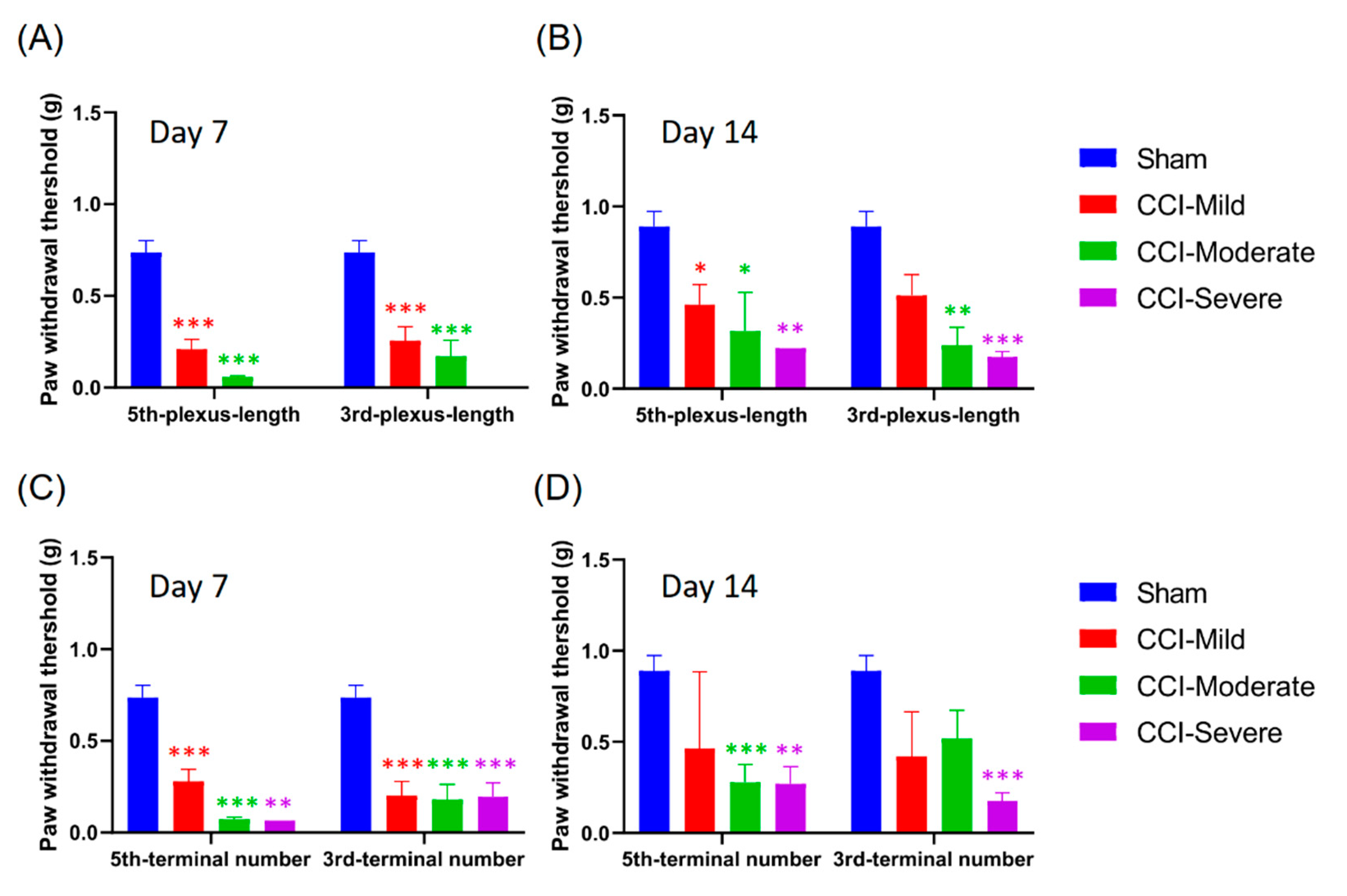
3.1. CCI-Induced Mechanical Hypersensitivity
3.2. CCI-Induced Thermal Hyperalgesia
3.3. Mechanical Hypersensitivity Did Not Associate with Nerve-Injury Severity
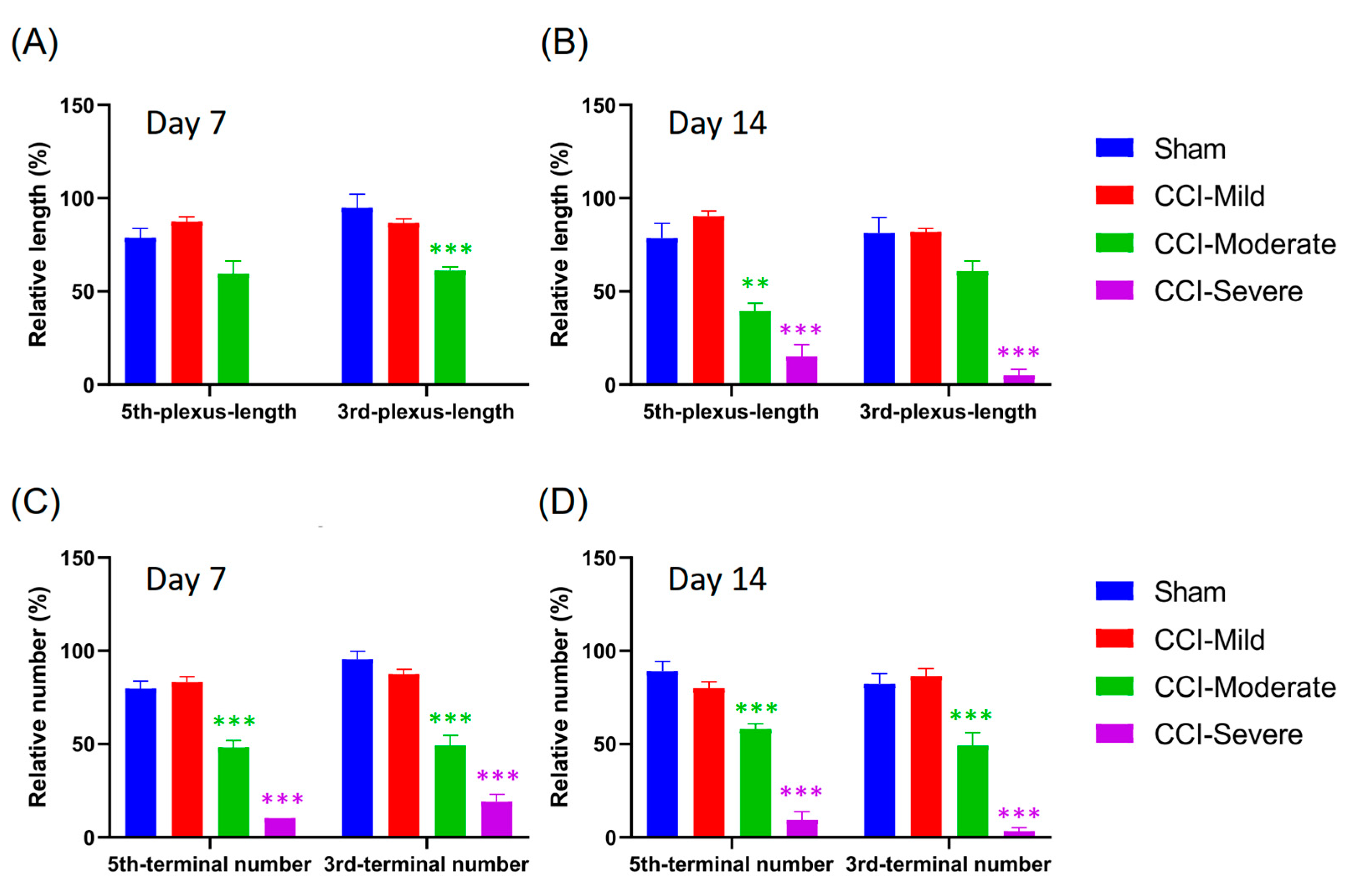
3.4. Degeneration of the Nerve Plexus and Terminals Was Associated with Nerve-Injury Severity
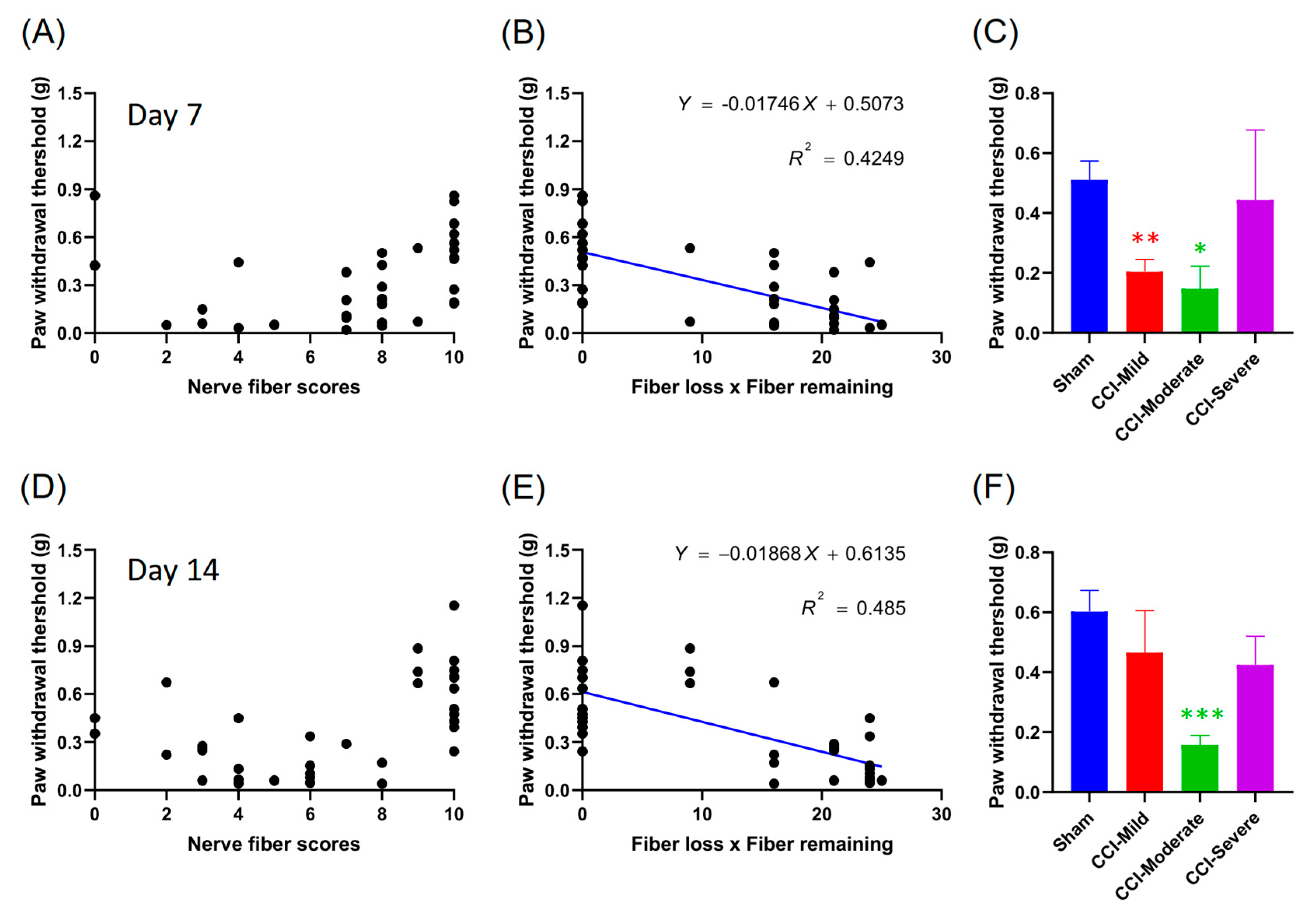
3.5. Mechanical Hypersensitivity Was Correlated with the Product of the Nerve Score and Number of Fibers Lost
3.6. Nerve Score at 7 d Post CCI Was Correlated with the Von Frey Test Threshold at 14 d
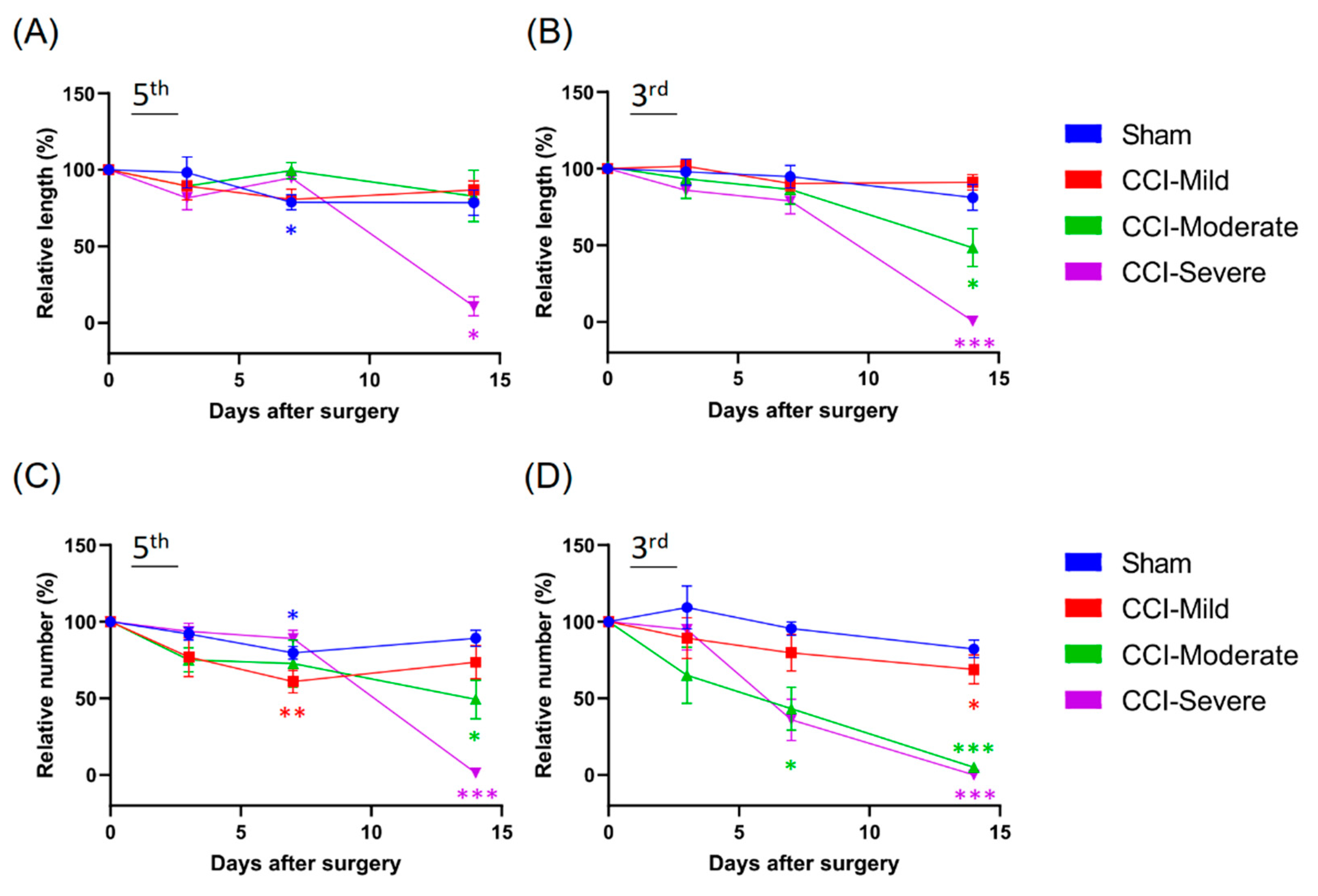
3.7. Quantification of Nerve Plexus and Terminals with Different Indices in Recategorized CCI Subgroups
3.8. Mechanical Hypersensitivity Was Associated with Indexing by Both the Relative Length and Number of Nerve Terminals Remaining After CCI
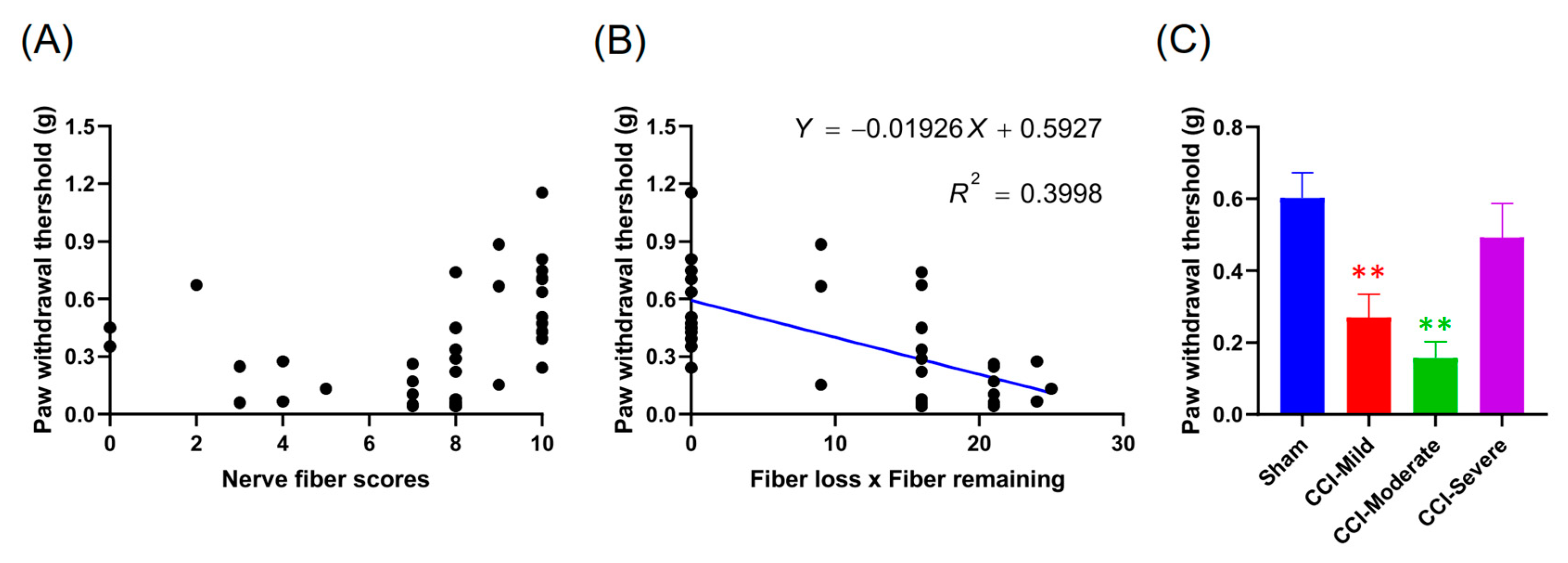
3.9. Indexing by the Relative Length and Number of Nerves Remaining at 7 D Post CCI Was Associated with the Von Frey Test Threshold at 14 d
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiang, H.; Chang, K.C.; Kan, H.W.; Wu, S.W.; Tseng, M.T.; Hsueh, H.W.; Lin, Y.H.; Chao, C.C.; Hsieh, S.T. Physiological and pathological characterization of capsaicin-induced reversible nerve degeneration and hyperalgesia. Eur. J. Pain 2018, 22, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Devigili, G.; Tugnoli, V.; Penza, P.; Camozzi, F.; Lombardi, R.; Melli, G.; Broglio, L.; Granieri, E.; Lauria, G. The diagnostic criteria for small fibre neuropathy: From symptoms to neuropathology. Brain 2008, 131, 1912–1925. [Google Scholar] [CrossRef]
- Mosconi, T.; Kruger, L. Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: Ultrastructural morphometric analysis of axonal alterations. Pain 1996, 64, 37–57. [Google Scholar] [CrossRef]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932. [Google Scholar] [CrossRef]
- Guo, S.-H.; Lin, J.-P.; Huang, L.-E.; Yang, Y.; Chen, C.-Q.; Li, N.-N.; Su, M.-Y.; Zhao, X.; Zhu, S.-M.; Yao, Y.-X. Silencing of spinal Trpv1 attenuates neuropathic pain in rats by inhibiting CAMKII expression and ERK2 phosphorylation. Sci. Rep. 2019, 9, 2769. [Google Scholar] [CrossRef]
- Rivat, C.; Sar, C.; Mechaly, I.; Leyris, J.-P.; Diouloufet, L.; Sonrier, C.; Philipson, Y.; Lucas, O.; Mallié, S.; Jouvenel, A. Inhibition of neuronal FLT3 receptor tyrosine kinase alleviates peripheral neuropathic pain in mice. Nat. Commun. 2018, 9, 1042. [Google Scholar] [CrossRef]
- Tseng, T.-J.; Hsieh, Y.-L.; Ko, M.-H.; Hsieh, S.-T. Redistribution of voltage-gated sodium channels after nerve decompression contributes to relieve neuropathic pain in chronic constriction injury. Brain Res. 2014, 1589, 15–25. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, H.; Singh, A. Neuropathic pain models caused by damage to central or peripheral nervous system. Pharmacol. Rep. 2018, 70, 206–216. [Google Scholar] [CrossRef]
- Shields, S.D.; Ahn, H.-S.; Yang, Y.; Han, C.; Seal, R.P.; Wood, J.N.; Waxman, S.G.; Dib-Hajj, S.D. Nav1. 8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain 2012, 153, 2017–2030. [Google Scholar] [CrossRef]
- Lakomá, J.; Rimondini, R.; Donadio, V.; Liguori, R.; Caprini, M. Pain related channels are differentially expressed in neuronal and non-neuronal cells of glabrous skin of fabry knockout male mice. PLoS ONE 2014, 9, e108641. [Google Scholar] [CrossRef]
- Persson, A.-K.; Black, J.A.; Gasser, A.; Cheng, X.; Fischer, T.Z.; Waxman, S.G. Sodium-calcium exchanger and multiple sodium channel isoforms in intra-epidermal nerve terminals. Mol. Pain 2010, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.-K.; Kim, I.; Zhao, P.; Estacion, M.; Black, J.A.; Waxman, S.G. Sodium channels contribute to degeneration of dorsal root ganglion neurites induced by mitochondrial dysfunction in an in vitro model of axonal injury. J. Neurosci. 2013, 33, 19250–19261. [Google Scholar] [CrossRef]
- Daou, I.; Beaudry, H.; Ase, A.R.; Wieskopf, J.S.; Ribeiro-da-Silva, A.; Mogil, J.S.; Séguéla, P. Optogenetic silencing of Nav1. 8-positive afferents alleviates inflammatory and neuropathic pain. eNeuro 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-W.; Goregoaker, S.; Engler, H.; Zhou, X.; Mark, L.; Crona, J.; Terry, R.; Hunter, J.; Priestley, T. Small interfering RNA-mediated selective knockdown of NaV1. 8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience 2007, 146, 812–821. [Google Scholar] [CrossRef]
- Ito, A.; Takeda, M.; Yoshimura, T.; Komatsu, T.; Ohno, T.; Kuriyama, H.; Matsuda, A.; Yoshimura, M. Anti-hyperalgesic effects of calcitonin on neuropathic pain interacting with its peripheral receptors. Mol. Pain 2012, 8, 42. [Google Scholar] [CrossRef]
- Wilson-Gerwing, T.D.; Stucky, C.L.; McComb, G.W.; Verge, V.M. Neurotrophin-3 significantly reduces sodium channel expression linked to neuropathic pain states. Exp. Neurol. 2008, 213, 303–314. [Google Scholar] [CrossRef]
- Hai, T.; Wolfgang, C.D.; Marsee, D.K.; Allen, A.E.; Sivaprasad, U. ATF3 and stress responses. Gene Expr. J. Liver Res. 1999, 7, 321–335. [Google Scholar]
- Obata, K.; Yamanaka, H.; Fukuoka, T.; Yi, D.; Tokunaga, A.; Hashimoto, N.; Yoshikawa, H.; Noguchi, K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain 2003, 101, 65–77. [Google Scholar] [CrossRef]
- Grace, P.M.; Fabisiak, T.J.; Green-Fulgham, S.M.; Anderson, N.D.; Strand, K.A.; Kwilasz, A.J.; Galer, E.L.; Walker, F.R.; Greenwood, B.N.; Maier, S.F. Prior voluntary wheel running attenuates neuropathic pain. Pain 2016, 157, 2012. [Google Scholar] [CrossRef]
- Stirling, L.C.; Forlani, G.; Baker, M.D.; Wood, J.N.; Matthews, E.A.; Dickenson, A.H.; Nassar, M.A. Nociceptor-specific gene deletion using heterozygous NaV1. 8-Cre recombinase mice. Pain 2005, 113, 27–36. [Google Scholar] [CrossRef]
- Tseng, T.-J.; Chen, C.-C.; Hsieh, Y.-L.; Hsieh, S.-T. Effects of decompression on neuropathic pain behaviors and skin reinnervation in chronic constriction injury. Exp. Neurol. 2007, 204, 574–582. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lee, C.-H.; Sun, W.-H.; Chen, C.-C. Involvement of advillin in somatosensory neuron subtype-specific axon regeneration and neuropathic pain. Proc. Natl. Acad. Sci. USA 2018, 115, E8557–E8566. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Hsieh, S.-T.; Chiang, H.-Y.; Lin, W.-M. Pathology of nerve terminal degeneration in the skin. J. Neuropathol. Exp. Neurol. 2000, 59, 297–307. [Google Scholar] [CrossRef]
- Kennedy, W.R. Opportunities afforded by the study of unmyelinated nerves in skin and other organs. Muscle Nerve: Off. J. Am. Assoc. Electrodiagn. Med. 2004, 29, 756–767. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.; Hsieh, S.-T.; Stocks, A.; Hauer, P.; Macko, C.; Cornblath, D.; Griffin, J.; McArthur, J.C. Cutaneous innervation in sensory neuropathies: Evaluation by skin biopsy. Neurology 1995, 45, 1848–1855. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Tseng, T.-J.; Lin, W.-M.; Hsieh, S.-T. Cutaneous nerve terminal degeneration in painful mononeuropathy. Exp. Neurol. 2001, 170, 290–296. [Google Scholar] [CrossRef]
- Peleshok, J.C.; Ribeiro-da-Silva, A. Delayed reinnervation by nonpeptidergic nociceptive afferents of the glabrous skin of the rat hindpaw in a neuropathic pain model. J. Comp. Neurol. 2011, 519, 49–63. [Google Scholar] [CrossRef]
- Yen, L.D.; Bennett, G.J.; Ribeiro-da-Silva, A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J. Comp. Neurol. 2006, 495, 679–690. [Google Scholar] [CrossRef]
- Casals-Díaz, L.; Vivó, M.; Navarro, X. Nociceptive responses and spinal plastic changes of afferent C-fibers in three neuropathic pain models induced by sciatic nerve injury in the rat. Exp. Neurol. 2009, 217, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Braz, J.M.; Nassar, M.A.; Wood, J.N.; Basbaum, A.I. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron 2005, 47, 787–793. [Google Scholar] [CrossRef]
- Lawson, S.; Waddell, P. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J. Physiol. 1991, 435, 41–63. [Google Scholar] [CrossRef]
- Scherrer, G.; Imamachi, N.; Cao, Y.-Q.; Contet, C.; Mennicken, F.; O’Donnell, D.; Kieffer, B.L.; Basbaum, A.I. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 2009, 137, 1148–1159. [Google Scholar] [CrossRef]
- Zylka, M.J.; Rice, F.L.; Anderson, D.J. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 2005, 45, 17–25. [Google Scholar] [CrossRef]
- Akopian, A.N.; Sivilotti, L.; Wood, J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996, 379, 257. [Google Scholar] [CrossRef]
- Rush, A.; Bräu, M.; Elliott, A.; Elliott, J. Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia. J. Physiol. 1998, 511, 771–789. [Google Scholar] [CrossRef]
- Blair, N.T.; Bean, B.P. Role of tetrodotoxin-resistant Na+ current slow inactivation in adaptation of action potential firing in small-diameter dorsal root ganglion neurons. J. Neurosci. 2003, 23, 10338–10350. [Google Scholar] [CrossRef]
- Joshi, S.; Honore, P.; Hernandez, G.; Schmidt, R.; Gomtsyan, A.; Scanio, M.; Kort, M.; Jarvis, M.F. Additive antinociceptive effects of the selective Nav1. 8 blocker A-803467 and selective TRPV1 antagonists in rat inflammatory and neuropathic pain models. J. Pain 2009, 10, 306–315. [Google Scholar] [CrossRef]
- Joshi, S.; Mikusa, J.P.; Hernandez, G.; Baker, S.; Shieh, C.-C.; Neelands, T.; Zhang, X.-F.; Niforatos, W.; Kage, K.; Han, P. Involvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic but not post-operative, pain states. Pain 2006, 123, 75–82. [Google Scholar] [CrossRef]
- Porreca, F.; Lai, J.; Bian, D.; Wegert, S.; Ossipov, M.H.; Eglen, R.M.; Kassotakis, L.; Novakovic, S.; Rabert, D.K.; Sangameswaran, L. A comparison of the potential role of the tetrodotoxin-insensitive sodium channels, PN3/SNS and NaN/SNS2, in rat models of chronic pain. Proc. Natl. Acad. Sci. USA 1999, 96, 7640–7644. [Google Scholar] [CrossRef]
- Ko, M.-H.; Hsieh, Y.-L.; Hsieh, S.-T.; Tseng, T.-J. Nerve demyelination increases metabotropic glutamate receptor subtype 5 expression in peripheral painful mononeuropathy. Int. J. Mol. Sci. 2015, 16, 4642–4665. [Google Scholar] [CrossRef] [PubMed]
- Yonehara, N.; Yoshimura, M. Influence of painful chronic neuropathy on neurogenic inflammation. Pain 2001, 92, 259–265. [Google Scholar] [CrossRef]
- La Rana, G.; Russo, R.; D’Agostino, G.; Sasso, O.; Raso, G.M.; Iacono, A.; Meli, R.; Piomelli, D.; Calignano, A. AM404, an anandamide transport inhibitor, reduces plasma extravasation in a model of neuropathic pain in rat: Role for cannabinoid receptors. Neuropharmacology 2008, 54, 521–529. [Google Scholar] [CrossRef]
- Shimada, N.; Sakata, A.; Igarashi, T.; Takeuchi, M.; Nishimura, S. M1 macrophage infiltration exacerbate muscle/bone atrophy after peripheral nerve injury. BMC Musculoskelet. Disord. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Clatworthy, A.; Illich, P.; Castro, G.; Walters, E. Role of peri-axonal inflammation in the development of thermal hyperalgesia and guarding behavior in a rat model of neuropathic pain. Neurosci. Lett. 1995, 184, 5–8. [Google Scholar] [CrossRef]
- Okamoto, K.; Martin, D.P.; Schmelzer, J.D.; Mitsui, Y.; Low, P.A. Pro-and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp. Neurol. 2001, 169, 386–391. [Google Scholar] [CrossRef]
- Malek, N.; Pajak, A.; Kolosowska, N.; Kucharczyk, M.; Starowicz, K. The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol. Cell. Neurosci. 2015, 65, 1–10. [Google Scholar] [CrossRef]
- Kolter, J.; Feuerstein, R.; Zeis, P.; Hagemeyer, N.; Paterson, N.; d’Errico, P.; Baasch, S.; Amann, L.; Masuda, T.; Lösslein, A. A Subset of Skin Macrophages Contributes to the Surveillance and Regeneration of Local Nerves. Immunity 2019. [Google Scholar] [CrossRef] [PubMed]
- Yuryev, M.; Molotkov, D. In vivo two-photon microscopy of single nerve endings in skin. Jove (J. Vis. Exp.) 2014, e51045. [Google Scholar] [CrossRef] [PubMed]
- Yuryev, M.; Khiroug, L. Dynamic longitudinal investigation of individual nerve endings in the skin of anesthetized mice using in vivo two-photon microscopy. J. Biomed. Opt. 2012, 17, 046007. [Google Scholar] [CrossRef]
- Chiang, M.-C.; Lin, Y.-H.; Pan, C.-L.; Tseng, T.-J.; Lin, W.-M.; Hsieh, S.-T. Cutaneous innervation in chronic inflammatory demyelinating polyneuropathy. Neurology 2002, 59, 1094–1098. [Google Scholar] [CrossRef]
- Tsujino, H.; Kondo, E.; Fukuoka, T.; Dai, Y.; Tokunaga, A.; Miki, K.; Yonenobu, K.; Ochi, T.; Noguchi, K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol. Cell. Neurosci. 2000, 15, 170–182. [Google Scholar] [CrossRef]
- Tsuzuki, K.; Kondo, E.; Fukuoka, T.; Yi, D.; Tsujino, H.; Sakagami, M.; Noguchi, K. Differential regulation of P2X3 mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain 2001, 91, 351–360. [Google Scholar] [CrossRef]
- Shortland, P.J.; Baytug, B.; Krzyzanowska, A.; McMahon, S.B.; Priestley, J.V.; Averill, S. ATF3 expression in L4 dorsal root ganglion neurons after L5 spinal nerve transection. Eur. J. Neurosci. 2006, 23, 365–373. [Google Scholar] [CrossRef]
- Ivanavicius, S.P.; Ball, A.D.; Heapy, C.G.; Westwood, F.R.; Murray, F.; Read, S.J. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: Increased expression of ATF-3 and pharmacological characterisation. Pain 2007, 128, 272–282. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, H.-H.; Lee, J.-C.; Chen, C.-C.; Chen, S.-K.; Yen, C.-T. An Index Combining Lost and Remaining Nerve Fibers Correlates with Pain Hypersensitivity in Mice. Cells 2020, 9, 2414. https://doi.org/10.3390/cells9112414
Chi H-H, Lee J-C, Chen C-C, Chen S-K, Yen C-T. An Index Combining Lost and Remaining Nerve Fibers Correlates with Pain Hypersensitivity in Mice. Cells. 2020; 9(11):2414. https://doi.org/10.3390/cells9112414
Chicago/Turabian StyleChi, Han-Hsiung, Jye-Chang Lee, Chih-Cheng Chen, Shih-Kuo Chen, and Chen-Tung Yen. 2020. "An Index Combining Lost and Remaining Nerve Fibers Correlates with Pain Hypersensitivity in Mice" Cells 9, no. 11: 2414. https://doi.org/10.3390/cells9112414
APA StyleChi, H.-H., Lee, J.-C., Chen, C.-C., Chen, S.-K., & Yen, C.-T. (2020). An Index Combining Lost and Remaining Nerve Fibers Correlates with Pain Hypersensitivity in Mice. Cells, 9(11), 2414. https://doi.org/10.3390/cells9112414






