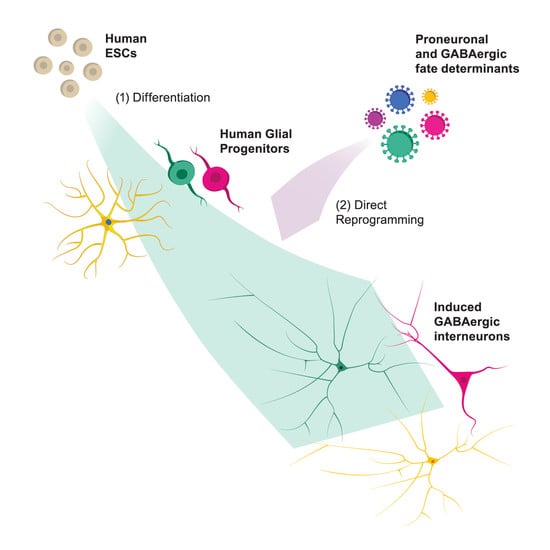
Direct Conversion of Human Stem Cell-Derived Glial Progenitor Cells into GABAergic Interneurons
Abstract
:1. Introduction
2. Materials and Methods
2.1. hESC-Derived GPC Culturing
2.2. Flow Cytometry Analysis
2.3. Viral Vectors
2.4. Neural Direct Conversion
2.5. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Immunocytochemistry and Microscopy
2.7. High-Content Screening
2.8. Electrophysiology
2.9. Statistical Analysis
3. Results
3.1. Five Factor Combination Converts hESC-Derived GPCs into Induced Neurons
3.2. The Induced Cultures Consist of GABAergic Interneurons and Show PV Expression
3.3. Functional Characterization of Induced GABAergic Neurons Obtained in 26 Days
3.4. A Heterogenous Population of GABAergic Induced Neurons
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pang, Z.P.; Yang, N.; Vierbuchen, T.; Ostermeier, A.; Fuentes, D.R.; Yang, T.Q.; Citri, A.; Sebastiano, V.; Marro, S.; Sudhof, T.C.; et al. Induction of human neuronal cells by defined transcription factors. Nature 2011, 476, 220–223. [Google Scholar] [CrossRef]
- Pfisterer, U.; Wood, J.; Nihlberg, K.; Hallgren, O.; Bjermer, L.; Westergren-Thorsson, G.; Lindvall, O.; Parmar, M. Efficient induction of functional neurons from adult human fibroblasts. Cell Cycle 2011, 10, 3311–3316. [Google Scholar] [CrossRef] [Green Version]
- Drouin-Ouellet, J.; Lau, S.; Brattas, P.L.; Rylander Ottosson, D.; Pircs, K.; Grassi, D.A.; Collins, L.M.; Vuono, R.; Andersson Sjoland, A.; Westergren-Thorsson, G.; et al. REST suppression mediates neural conversion of adult human fibroblasts via microRNA-dependent and -independent pathways. EMBO Mol. Med. 2017, 9, 1117–1131. [Google Scholar] [CrossRef]
- Mertens, J.; Reid, D.; Lau, S.; Kim, Y.; Gage, F.H. Aging in a Dish: iPSC-Derived and Directly Induced Neurons for Studying Brain Aging and Age-Related Neurodegenerative Diseases. Annu. Rev. Genet. 2018, 52, 271–293. [Google Scholar] [CrossRef]
- Fang, L.; El Wazan, L.; Tan, C.; Nguyen, T.; Hung, S.S.C.; Hewitt, A.W.; Wong, R.C.B. Potentials of Cellular Reprogramming as a Novel Strategy for Neuroregeneration. Front. Cell. Neurosci. 2018, 12, 460. [Google Scholar] [CrossRef] [Green Version]
- Grealish, S.; Drouin-Ouellet, J.; Parmar, M. Brain repair and reprogramming: The route to clinical translation. J. Intern. Med. 2016, 280, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Vignoles, R.; Lentini, C.; d’Orange, M.; Heinrich, C. Direct Lineage Reprogramming for Brain Repair: Breakthroughs and Challenges. Trends Mol. Med. 2019, 25, 897–914. [Google Scholar] [CrossRef]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Gotz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef]
- Windrem, M.S.; Schanz, S.J.; Morrow, C.; Munir, J.; Chandler-Militello, D.; Wang, S.; Goldman, S.A. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J. Neurosci. 2014, 34, 16153–16161. [Google Scholar] [CrossRef]
- Lin, S.C.; Bergles, D.E. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 2004, 7, 24–32. [Google Scholar] [CrossRef]
- Grande, A.; Sumiyoshi, K.; Lopez-Juarez, A.; Howard, J.; Sakthivel, B.; Aronow, B.; Campbell, K.; Nakafuku, M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat. Commun. 2013, 4, 2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 2014, 14, 188–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, C.; Bergami, M.; Gascon, S.; Lepier, A.; Vigano, F.; Dimou, L.; Sutor, B.; Berninger, B.; Gotz, M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 2014, 3, 1000–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, W.; Zang, T.; Smith, D.K.; Vue, T.Y.; Zou, Y.; Bachoo, R.; Johnson, J.E.; Zhang, C.L. SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Rep. 2015, 4, 780–794. [Google Scholar] [CrossRef] [Green Version]
- Torper, O.; Ottosson, D.R.; Pereira, M.; Lau, S.; Cardoso, T.; Grealish, S.; Parmar, M. In Vivo Reprogramming of Striatal NG2 Glia into Functional Neurons that Integrate into Local Host Circuitry. Cell Rep. 2015, 12, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.; Birtele, M.; Rylander Ottosson, D. In Vivo Direct Reprogramming of Resident Glial Cells into Interneurons by Intracerebral Injection of Viral Vectors. J. Vis. Exp. 2019, 148, e59465. [Google Scholar] [CrossRef]
- Pereira, M.; Birtele, M.; Shrigley, S.; Benitez, J.A.; Hedlund, E.; Parmar, M.; Ottosson, D.R. Direct Reprogramming of Resident NG2 Glia into Neurons with Properties of Fast-Spiking Parvalbumin-Containing Interneurons. Stem Cell Rep. 2017, 9, 742–751. [Google Scholar] [CrossRef]
- Kreitzer, A.C. Physiology and pharmacology of striatal neurons. Annu. Rev. Neurosci. 2009, 32, 127–147. [Google Scholar] [CrossRef]
- Lim, L.; Mi, D.; Llorca, A.; Marin, O. Development and Functional Diversification of Cortical Interneurons. Neuron 2018, 100, 294–313. [Google Scholar] [CrossRef] [Green Version]
- Petilla Interneuron Nomenclature Group; Ascoli, G.A.; Alonso-Nanclares, L.; Anderson, S.A.; Barrionuevo, G.; Benavides-Piccione, R.; Burkhalter, A.; Buzsaki, G.; Cauli, B.; Defelipe, J.; et al. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008, 9, 557–568. [Google Scholar] [CrossRef] [Green Version]
- Southwell, D.G.; Nicholas, C.R.; Basbaum, A.I.; Stryker, M.P.; Kriegstein, A.R.; Rubenstein, J.L.; Alvarez-Buylla, A. Interneurons from embryonic development to cell-based therapy. Science 2014, 344, 1240622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Spatazza, J.; Mancia Leon, W.R.; Alvarez-Buylla, A. Transplantation of GABAergic interneurons for cell-based therapy. Prog. Brain Res. 2017, 231, 57–85. [Google Scholar] [PubMed]
- Sim, F.J.; McClain, C.R.; Schanz, S.J.; Protack, T.L.; Windrem, M.S.; Goldman, S.A. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nat. Biotechnol. 2011, 29, 934–941. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Bates, J.; Li, X.; Schanz, S.; Chandler-Militello, D.; Levine, C.; Maherali, N.; Studer, L.; Hochedlinger, K.; Windrem, M.; et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 2013, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Colasante, G.; Lignani, G.; Rubio, A.; Medrihan, L.; Yekhlef, L.; Sessa, A.; Massimino, L.; Giannelli, S.G.; Sacchetti, S.; Caiazzo, M.; et al. Rapid Conversion of Fibroblasts into Functional Forebrain GABAergic Interneurons by Direct Genetic Reprogramming. Cell Stem Cell 2015, 17, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Birtele, M.; Sharma, Y.; Kidnapillai, S.; Lau, S.; Stoker, T.; Barker, R.A.; Rylander Ottosson, D.; Drouin-Ouellet, J.; Parmar, M. Dual modulation of neuron-specific microRNAs and the REST complex promotes functional maturation of human adult induced neurons. FEBS Lett. 2019, 593, 3370–3380. [Google Scholar] [CrossRef]
- Nolbrant, S.; Giacomoni, J.; Hoban, D.B.; Bruzelius, A.; Birtele, M.; Chandler-Militello, D.; Pereira, M.; Ottosson, D.R.; Goldman, S.A.; Parmar, M. Direct Reprogramming of Human Fetal- and Stem Cell-Derived Glial Progenitor Cells into Midbrain Dopaminergic Neurons. Stem Cell Rep. 2020, 15, 869–882. [Google Scholar] [CrossRef]
- Zufferey, R.; Nagy, D.; Mandel, R.J.; Naldini, L.; Trono, D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 1997, 15, 871–875. [Google Scholar] [CrossRef]
- Georgievska, B.; Jakobsson, J.; Persson, E.; Ericson, C.; Kirik, D.; Lundberg, C. Regulated delivery of glial cell line-derived neurotrophic factor into rat striatum, using a tetracycline-dependent lentiviral vector. Hum. Gene Ther. 2004, 15, 934–944. [Google Scholar] [CrossRef]
- Richner, M.; Victor, M.B.; Liu, Y.; Abernathy, D.; Yoo, A.S. MicroRNA-based conversion of human fibroblasts into striatal medium spiny neurons. Nat. Protoc. 2015, 10, 1543–1555. [Google Scholar] [CrossRef]
- Rothman, J.S.; Silver, R.A. NeuroMatic: An Integrated Open-Source Software Toolkit for Acquisition, Analysis and Simulation of Electrophysiological Data. Front. Neuroinform. 2018, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, C.R.; Chen, J.; Tang, Y.; Southwell, D.G.; Chalmers, N.; Vogt, D.; Arnold, C.M.; Chen, Y.J.; Stanley, E.G.; Elefanty, A.G.; et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 2013, 12, 573–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caiazzo, M.; Dell’Anno, M.T.; Dvoretskova, E.; Lazarevic, D.; Taverna, S.; Leo, D.; Sotnikova, T.D.; Menegon, A.; Roncaglia, P.; Colciago, G.; et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011, 476, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, U.; Kirkeby, A.; Torper, O.; Wood, J.; Nelander, J.; Dufour, A.; Bjorklund, A.; Lindvall, O.; Jakobsson, J.; Parmar, M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 10343–10348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hevner, R.F.; Shi, L.; Justice, N.; Hsueh, Y.; Sheng, M.; Smiga, S.; Bulfone, A.; Goffinet, A.M.; Campagnoni, A.T.; Rubenstein, J.L. Tbr1 regulates differentiation of the preplate and layer 6. Neuron 2001, 29, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Niu, W.; Liu, M.L.; Zou, Y.; Zhang, C.L. In Vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014, 5, 3338. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, J.C.; Yeh, H.; Ma, N.X.; Lee, G.; Chen, X.A.; Wang, Y.; Lin, L.; Chen, L.; Jin, P.; et al. Small Molecules Efficiently Reprogram Human Astroglial Cells into Functional Neurons. Cell Stem Cell 2015, 17, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.C.; Zhang, L.; Ma, N.X.; Wang, Y.; Lee, G.; Hou, X.Y.; Lei, Z.F.; Zhang, F.Y.; Dong, F.P.; Wu, G.Y.; et al. Chemical Conversion of Human Fetal Astrocytes into Neurons through Modulation of Multiple Signaling Pathways. Stem Cell Rep. 2019, 12, 488–501. [Google Scholar] [CrossRef] [Green Version]
- Karow, M.; Sanchez, R.; Schichor, C.; Masserdotti, G.; Ortega, F.; Heinrich, C.; Gascon, S.; Khan, M.A.; Lie, D.C.; Dellavalle, A.; et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell 2012, 11, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Dimou, L.; Gotz, M. Glial cells as progenitors and stem cells: New roles in the healthy and diseased brain. Physiol. Rev. 2014, 94, 709–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, M.; Birtele, M.; Rylander Ottosson, D. Direct reprogramming into interneurons: Potential for brain repair. Cell. Mol. Life Sci. 2019, 76, 3953–3967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casarosa, S.; Fode, C.; Guillemot, F. Mash1 regulates neurogenesis in the ventral telencephalon. Development 1999, 126, 525–534. [Google Scholar] [PubMed]
- Fode, C.; Ma, Q.; Casarosa, S.; Ang, S.L.; Anderson, D.J.; Guillemot, F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000, 14, 67–80. [Google Scholar]
- Wang, Y.; Dye, C.A.; Sohal, V.; Long, J.E.; Estrada, R.C.; Roztocil, T.; Lufkin, T.; Deisseroth, K.; Baraban, S.C.; Rubenstein, J.L. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J. Neurosci. 2010, 30, 5334–5345. [Google Scholar] [CrossRef]
- Ferri, A.; Favaro, R.; Beccari, L.; Bertolini, J.; Mercurio, S.; Nieto-Lopez, F.; Verzeroli, C.; La Regina, F.; De Pietri Tonelli, D.; Ottolenghi, S.; et al. Sox2 is required for embryonic development of the ventral telencephalon through the activation of the ventral determinants Nkx2.1 and Shh. Development 2013, 140, 1250–1261. [Google Scholar] [CrossRef] [Green Version]
- Manuel, M.; Martynoga, B.; Yu, T.; West, J.D.; Mason, J.O.; Price, D.J. The transcription factor Foxg1 regulates the competence of telencephalic cells to adopt subpallial fates in mice. Development 2010, 137, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Maroof, A.M.; Keros, S.; Tyson, J.A.; Ying, S.W.; Ganat, Y.M.; Merkle, F.T.; Liu, B.; Goulburn, A.; Stanley, E.G.; Elefanty, A.G.; et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 2013, 12, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Regal, M.P.; Luengas-Escuza, I.; Bayon-Cordero, L.; Ibarra-Aizpurua, N.; Alberdi, E.; Perez-Samartin, A.; Matute, C.; Sanchez-Gomez, M.V. Oligodendrocyte Differentiation and Myelination Is Potentiated via GABAB Receptor Activation. Neuroscience 2020, 439, 163–180. [Google Scholar] [CrossRef]
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006, 9, 173–179. [Google Scholar] [CrossRef]
- Miyoshi, G.; Butt, S.J.; Takebayashi, H.; Fishell, G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J. Neurosci. 2007, 27, 7786–7798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kepecs, A.; Fishell, G. Interneuron cell types are fit to function. Nature 2014, 505, 318–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, C.; Li, M. Understanding neurodevelopmental disorders using human pluripotent stem cell-derived neurons. Brain Pathol. 2017, 27, 508–517. [Google Scholar] [CrossRef] [Green Version]
- Sun, A.X.; Yuan, Q.; Tan, S.; Xiao, Y.; Wang, D.; Khoo, A.T.; Sani, L.; Tran, H.D.; Kim, P.; Chiew, Y.S.; et al. Direct Induction and Functional Maturation of Forebrain GABAergic Neurons from Human Pluripotent Stem Cells. Cell Rep. 2016, 16, 1942–1953. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Chen, X.; Fang, K.H.; Wang, Y.; Lin, M.; Xu, S.B.; Huo, H.Q.; Xu, M.; Ma, L.; Chen, Y.; et al. Induction of human somatostatin and parvalbumin neurons by expressing a single transcription factor LIM homeobox 6. Elife 2018, 7, e37382. [Google Scholar] [CrossRef]
- Ballas, N.; Grunseich, C.; Lu, D.D.; Speh, J.C.; Mandel, G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 2005, 121, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, H.F.; Chen, Z.F.; Merkenschlager, M.; Fisher, A.G. Is REST required for ESC pluripotency? Nature 2009, 457, E4–E5, discussion E7. [Google Scholar] [CrossRef]
- Dewald, L.E.; Rodriguez, J.P.; Levine, J.M. The RE1 binding protein REST regulates oligodendrocyte differentiation. J. Neurosci. 2011, 31, 3470–3483. [Google Scholar] [CrossRef] [Green Version]
- Kohyama, J.; Sanosaka, T.; Tokunaga, A.; Takatsuka, E.; Tsujimura, K.; Okano, H.; Nakashima, K. BMP-induced REST regulates the establishment and maintenance of astrocytic identity. J. Cell Biol. 2010, 189, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Abrajano, J.J.; Qureshi, I.A.; Gokhan, S.; Zheng, D.; Bergman, A.; Mehler, M.F. REST and CoREST modulate neuronal subtype specification, maturation and maintenance. PLoS ONE 2009, 4, e7936. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chanda, S.; Marro, S.; Ng, Y.H.; Janas, J.A.; Haag, D.; Ang, C.E.; Tang, Y.; Flores, Q.; Mall, M.; et al. Generation of pure GABAergic neurons by transcription factor programming. Nat. Methods 2017, 14, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Linaro, D.; Vermaercke, B.; Iwata, R.; Ramaswamy, A.; Libe-Philippot, B.; Boubakar, L.; Davis, B.A.; Wierda, K.; Davie, K.; Poovathingal, S.; et al. Xenotransplanted Human Cortical Neurons Reveal Species-Specific Development and Functional Integration into Mouse Visual Circuits. Neuron 2019, 104, 972–986.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacomoni, J.; Bruzelius, A.; Stamouli, C.-A.; Rylander Ottosson, D. Direct Conversion of Human Stem Cell-Derived Glial Progenitor Cells into GABAergic Interneurons. Cells 2020, 9, 2451. https://doi.org/10.3390/cells9112451
Giacomoni J, Bruzelius A, Stamouli C-A, Rylander Ottosson D. Direct Conversion of Human Stem Cell-Derived Glial Progenitor Cells into GABAergic Interneurons. Cells. 2020; 9(11):2451. https://doi.org/10.3390/cells9112451
Chicago/Turabian StyleGiacomoni, Jessica, Andreas Bruzelius, Christina-Anastasia Stamouli, and Daniella Rylander Ottosson. 2020. "Direct Conversion of Human Stem Cell-Derived Glial Progenitor Cells into GABAergic Interneurons" Cells 9, no. 11: 2451. https://doi.org/10.3390/cells9112451