Scavenger Receptors as Biomarkers and Therapeutic Targets in Cardiovascular Disease
Abstract
1. Introduction
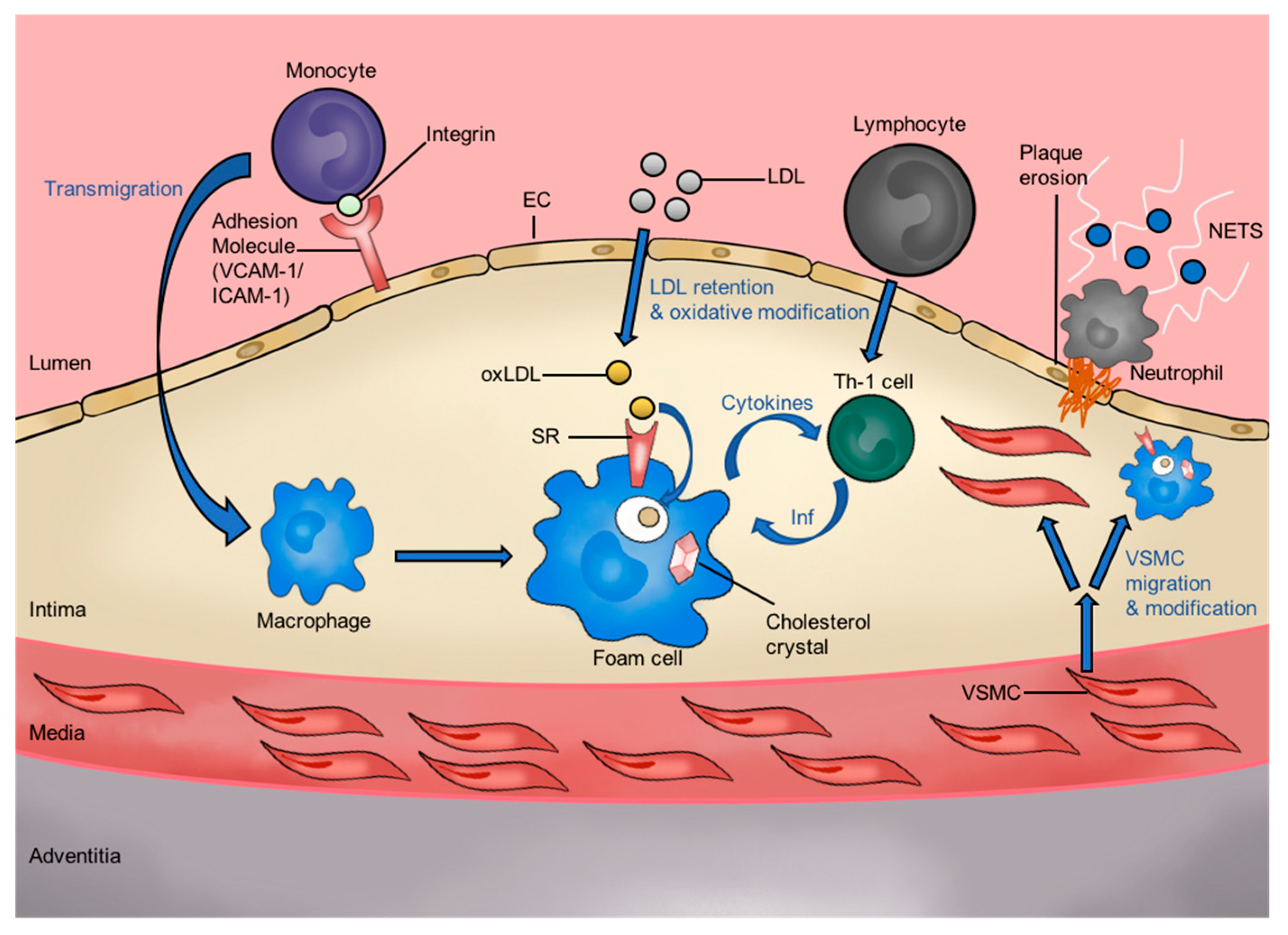
1.1. Oxidized Low-Density Lipoprotein and Atherogenesis
1.2. The Role of Atherosclerotic Plaque in CVD
1.3. A Brief Introduction to SRs
2. SRs as Biomarkers in CVD
3. SRs as Therapeutic Targets in CVD
3.1. Current Therapy
3.2. Novel Therapeutics
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Falk, E. Pathogenesis of Atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Gistera, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, N.; Formoso, G.; Pandolfi, A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc. Pharm. 2016, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Thomas, A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N. Engl. J. Med. 1984, 310, 1137–1140. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie Frank, D.; Burke Allen, P.; Finn Aloke, V.; Gold Herman, K.; Tulenko Thomas, N.; Wrenn Steven, P.; Narula, J. Atherosclerotic Plaque Progression and Vulnerability to Rupture. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef]
- Stary Herbert, C.; Chandler, A.B.; Dinsmore Robert, E.; Fuster, V.; Glagov, S.; Insull, W.; Rosenfeld Michael, E.; Schwartz Colin, J.; Wagner William, D.; Wissler Robert, W. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Kolodgie, F.D.; Farb, A. Vulnerable plaque: The pathology of unstable coronary lesions. J. Interv. Cardiol. 2002, 15, 439–446. [Google Scholar] [CrossRef]
- Burke, A.P.; Virmani, R.; Galis, Z.; Haudenschild, C.C.; Muller, J.E. Task force #2—What is the pathologic basis for new atherosclerosis imaging techniques? J. Am. Coll. Cardiol. 2003, 41, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.K. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Libby, P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatment. Circulation 2017, 136, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Partida, R.A.; Libby, P.; Crea, F.; Jang, I.K. Plaque erosion: A new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur. Heart J. 2018, 39, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Obama, T.; Ohinata, H.; Takaki, T.; Iwamoto, S.; Sawada, N.; Aiuchi, T.; Kato, R.; Itabe, H. Cooperative Action of Oxidized Low-Density Lipoproteins and Neutrophils on Endothelial Inflammatory Responses Through Neutrophil Extracellular Trap Formation. Front. Immunol. 2019, 10, 1899. [Google Scholar] [CrossRef]
- Prabhudas, M.; Bowdish, D.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; Means, T.K.; Moestrup, S.K.; et al. Standardizing scavenger receptor nomenclature. J. Immunol. 2014, 192, 1997–2006. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Ho, Y.K.; Basu, S.K.; Brown, M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 1979, 76, 333–337. [Google Scholar] [CrossRef]
- Brown, M.S.; Basu, S.K.; Falck, J.R.; Ho, Y.K.; Goldstein, J.L. The scavenger cell pathway for lipoprotein degradation: Specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J. Supramol. Struct. 1980, 13, 67–81. [Google Scholar] [CrossRef]
- Zani, I.A.; Stephen, S.L.; Mughal, N.A.; Russell, D.; Homer-Vanniasinkam, S.; Wheatcroft, S.B.; Ponnambalam, S. Scavenger receptor structure and function in health and disease. Cells 2015, 4, 178–201. [Google Scholar] [CrossRef]
- Wilkinson, K.; El Khoury, J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 2012, 489456. [Google Scholar] [CrossRef]
- Singh, T.D.; Park, S.Y.; Bae, J.S.; Yun, Y.; Bae, Y.C.; Park, R.W.; Kim, I.S. MEGF10 functions as a receptor for the uptake of amyloid-beta. FEBS Lett. 2010, 584, 3936–3942. [Google Scholar] [CrossRef] [PubMed]
- Bachli, E.B.; Schaer, D.J.; Walter, R.B.; Fehr, J.; Schoedon, G. Functional expression of the CD163 scavenger receptor on acute myeloid leukemia cells of monocytic lineage. J. Leukoc. Biol. 2006, 79, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Leelahavanichkul, A.; Bocharov, A.V.; Kurlander, R.; Baranova, I.N.; Vishnyakova, T.G.; Souza, A.C.; Hu, X.; Doi, K.; Vaisman, B.; Amar, M.; et al. Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J. Immunol. 2012, 188, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, N.; Kashiwagi, M.; Wait, R.; Nagayoshi, R.; Nakamura, M.; Matsuda, T.; Hogger, P.; Guyre, P.M.; Nagase, H.; Matsuyama, T. Elevated levels of soluble CD163 in sera and fluids from rheumatoid arthritis patients and inhibition of the shedding of CD163 by TIMP-3. Clin. Exp. Immunol. 2002, 130, 156–161. [Google Scholar] [CrossRef]
- Nagai, M.; Hirayama, K.; Ebihara, I.; Higuchi, T.; Shimohata, H.; Kobayashi, M. Serum levels of the soluble haemoglobin scavenger receptor CD163 in MPO-ANCA-associated renal vasculitis. Scand. J. Rheumatol. 2016, 45, 397–403. [Google Scholar] [CrossRef]
- Hughes, D.A.; Fraser, I.P.; Gordon, S. Murine macrophage scavenger receptor: In vivo expression and function as receptor for macrophage adhesion in lymphoid and non-lymphoid organs. Eur. J. Immunol. 1995, 25, 466–473. [Google Scholar] [CrossRef]
- Plüddemann, A.; Neyen, C.; Gordon, S. Macrophage scavenger receptors and host-derived ligands. Methods 2007, 43, 207–217. [Google Scholar] [CrossRef]
- Gough, P.J.; Greaves, D.R.; Suzuki, H.; Hakkinen, T.; Hiltunen, M.O.; Turunen, M.; Herttuala, S.Y.; Kodama, T.; Gordon, S. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 461–471. [Google Scholar] [CrossRef]
- Kelley, J.L.; Ozment, T.R.; Li, C.; Schweitzer, J.B.; Williams, D.L. Scavenger receptor-A (CD204): A two-edged sword in health and disease. Crit. Rev. Immunol. 2014, 34, 241–261. [Google Scholar] [CrossRef]
- Pluddemann, A.; Mukhopadhyay, S.; Gordon, S. Innate immunity to intracellular pathogens: Macrophage receptors and responses to microbial entry. Immunol. Rev. 2011, 240, 11–24. [Google Scholar] [CrossRef]
- Christie, R.H.; Freeman, M.; Hyman, B.T. Expression of the macrophage scavenger receptor, a multifunctional lipoprotein receptor, in microglia associated with senile plaques in Alzheimer’s disease. Am. J. Pathol. 1996, 148, 399–403. [Google Scholar] [PubMed]
- El Khoury, J.; Hickman, S.E.; Thomas, C.A.; Cao, L.; Silverstein, S.C.; Loike, J.D. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature 1996, 382, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Talle, M.A.; Rao, P.E.; Westberg, E.; Allegar, N.; Makowski, M.; Mittler, R.S.; Goldstein, G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983, 78, 83–99. [Google Scholar] [CrossRef]
- Yokoi, H.; Yanagita, M. Targeting the fatty acid transport protein CD36, a class B scavenger receptor, in the treatment of renal disease. Kidney Int. 2016, 89, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.C.; Han, J.; Febbraio, M.; Silversterin, R.L.; Hajjar, D.P. Role of CD36, the macrophage class B scavenger receptor, in atherosclerosis. Ann. N. Y. Acad. Sci. 2001, 947, 224–228. [Google Scholar] [CrossRef]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef]
- Greaves, D.R.; Gordon, S. Macrophage-Specific Gene Expression: Current Paradigms and Future Challenges. Int. J. Hematol. 2002, 76, 6–15. [Google Scholar] [CrossRef]
- Yoshida, H.; Quehenberger, O.; Kondratenko, N.; Green, S.; Steinberg, D. Minimally oxidized low-density lipoprotein increases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 794–802. [Google Scholar] [CrossRef]
- De Beer, M.C.; Zhao, Z.; Webb, N.R.; van der Westhuyzen, D.R.; de Villiers, W.J. Lack of a direct role for macrosialin in oxidized LDL metabolism. J. Lipid Res. 2003, 44, 674–685. [Google Scholar] [CrossRef]
- Song, L.; Lee, C.; Schindler, C. Deletion of the murine scavenger receptor CD68. J. Lipid Res. 2011, 52, 1542–1550. [Google Scholar] [CrossRef]
- Herre, J.; Willment, J.A.; Gordon, S.; Brown, G.D. The role of Dectin-1 in antifungal immunity. Crit. Rev. Immunol. 2004, 24, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Mango, R.; Predazzi, I.M.; Romeo, F.; Novelli, G. LOX-1/LOXIN: The yin/yang of atheroscleorosis. Cardiovasc. Drugs 2011, 25, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Adachi, H.; Aoki, J.; Koizumi, H.; Tomita, S.; Suzuki, T.; Tsujimoto, M.; Inoue, K.; Arai, H. SREC-II, a new member of the scavenger receptor type F family, trans-interacts with SREC-I through its extracellular domain. J. Biol. Chem. 2002, 277, 39696–39702. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Tsujimoto, M. Structure and function of a novel scavenger receptor expressed in human endothelial cells. Tanpakushitsu Kakusan Koso 1999, 44, 1282–1286. [Google Scholar]
- Hofnagel, O.; Luechtenborg, B.; Plenz, G.; Robenek, H. Expression of the novel scavenger receptor SR-PSOX in cultured aortic smooth muscle cells and umbilical endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 710–711. [Google Scholar] [CrossRef]
- Wågsäter, D.; Olofsson, P.S.; Norgren, L.; Stenberg, B.; Sirsjö, A. The chemokine and scavenger receptor CXCL16/SR-PSOX is expressed in human vascular smooth muscle cells and is induced by interferon gamma. Biochem. Biophys. Res. Commun. 2004, 325, 1187–1193. [Google Scholar] [CrossRef]
- Ma, Z.; Jin, X.; He, L.; Wang, Y. CXCL16 regulates renal injury and fibrosis in experimental renal artery stenosis. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H815–H821. [Google Scholar] [CrossRef]
- Hu, Z.B.; Chen, Y.; Gong, Y.X.; Gao, M.; Zhang, Y.; Wang, G.H.; Tang, R.N.; Liu, H.; Liu, B.C.; Ma, K.L. Activation of the CXCL16/CXCR6 Pathway by Inflammation Contributes to Atherosclerosis in Patients with End-stage Renal Disease. Int. J. Med. Sci. 2016, 13, 858–867. [Google Scholar] [CrossRef][Green Version]
- Zhou, F.; Wang, J.; Wang, K.; Zhu, X.; Pang, R.; Li, X.; Zhu, G.; Pan, X. Serum CXCL16 as a Novel Biomarker of Coronary Artery Disease in Type 2 Diabetes Mellitus: A Pilot Study. Ann. Clin. Lab. Sci. 2016, 46, 184–189. [Google Scholar]
- Adachi, H.; Tsujimoto, M. FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating activities. J. Biol. Chem. 2002, 277, 34264–34270. [Google Scholar] [CrossRef]
- Zhou, B.; Weigel, J.A.; Fauss, L.; Weigel, P.H. Identification of the hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 2000, 275, 37733–37741. [Google Scholar] [CrossRef] [PubMed]
- Irjala, H.; Elima, K.; Johansson, E.L.; Merinen, M.; Kontula, K.; Alanen, K.; Grenman, R.; Salmi, M.; Jalkanen, S. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur. J. Immunol. 2003, 33, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Irjala, H.; Alanen, K.; Grénman, R.; Heikkilä, P.; Joensuu, H.; Jalkanen, S. Mannose receptor (MR) and common lymphatic endothelial and vascular endothelial receptor (CLEVER)-1 direct the binding of cancer cells to the lymph vessel endothelium. Cancer Res. 2003, 63, 4671–4676. [Google Scholar] [PubMed]
- Etzerodt, A.; Maniecki, M.B.; Graversen, J.H.; Møller, H.J.; Torchilin, V.P.; Moestrup, S.K. Efficient intracellular drug-targeting of macrophages using stealth liposomes directed to the hemoglobin scavenger receptor CD163. J. Control Release 2012, 160, 72–80. [Google Scholar] [CrossRef]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. RAGE: Therapeutic target and biomarker of the inflammatory response--the evidence mounts. J. Leukoc. Biol. 2009, 86, 505–512. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. The RAGE axis and endothelial dysfunction: Maladaptive roles in the diabetic vasculature and beyond. Trends Cardiovasc. Med. 2005, 15, 237–243. [Google Scholar] [CrossRef]
- Che, J.J.; Shao, Y.X.; Li, G.P. Association between rs1049673 polymorphism in CD36 and premature coronary heart disease. Genet. Mol. Res. 2014, 13, 7708–7717. [Google Scholar] [CrossRef]
- Jayewardene, A.F.; Gwinn, T.; Hancock, D.P.; Mavros, Y.; Rooney, K.B. The associations between polymorphisms in the CD36 gene, fat oxidation and cardiovascular disease risk factors in a young adult Australian population: A pilot study. Obes. Res. Clin. Pract. 2014, 8, e618–e621. [Google Scholar] [CrossRef]
- Morini, E.; Rizzacasa, B.; Pucci, S.; Polidoro, C.; Ferrè, F.; Caporossi, D.; Helmer Citterich, M.; Novelli, G.; Amati, F. The human rs1050286 polymorphism alters LOX-1 expression through modifying miR-24 binding. J. Cell. Mol. Med. 2016, 20, 181–187. [Google Scholar] [CrossRef]
- Mango, R.; Clementi, F.; Borgiani, P.; Forleo, G.B.; Federici, M.; Contino, G.; Giardina, E.; Garza, L.; Fahdi, I.E.; Lauro, R.; et al. Association of single nucleotide polymorphisms in the oxidised LDL receptor 1 (OLR1) gene in patients with acute myocardial infarction. J. Med. Genet. 2003, 40, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, K.; Liu, E.; Wang, X.; Li, F.; Liu, T.; Zheng, X.; Li, G.; Che, J. Gender-specific associations of CD36 polymorphisms with the lipid profile and susceptibility to premature multi-vessel coronary artery heart disease in the Northern Han Chinese. Gene 2020, 753, 144806. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Kudoh, T.; Kaikita, K.; Yoshimura, M.; Oshima, S.; Miyamoto, Y.; Takeya, M.; Ogawa, H. Class A macrophage scavenger receptor gene expression levels in peripheral blood mononuclear cells specifically increase in patients with acute coronary syndrome. Atherosclerosis 2008, 198, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Emura, I.; Usuda, H.; Fujita, T.; Ebe, K.; Nagai, T. Scavenger receptor A index and coronary thrombus in patients with acute ST elevation myocardial infarction. Pathol. Int. 2011, 61, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Emura, I.; Usuda, H.; Fujita, T.; Ebe, K.; Nagai, T. Increase of scavenger receptor A-positive monocytes in patients with acute coronary syndromes. Pathol. Int. 2007, 57, 502–508. [Google Scholar] [CrossRef]
- Teupser, D.; Mueller, M.A.; Koglin, J.; Wilfert, W.; Ernst, J.; von Scheidt, W.; Steinbeck, G.; Seidel, D.; Thiery, J. CD36 mRNA expression is increased in CD14+ monocytes of patients with coronary heart disease. Clin. Exp. Pharmacol. Physiol. 2008, 35, 552–556. [Google Scholar] [CrossRef]
- Ye, M.; Zhou, J.; Zhong, Y.; Xu, J.; Hou, J.; Wang, X.; Wang, Z.; Guo, D. SR-A-Targeted Phase-Transition Nanoparticles for the Detection and Treatment of Atherosclerotic Vulnerable Plaques. ACS Appl. Mater. Interfaces 2019, 11, 9702–9715. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, J.; Martinez-Zaguilan, R.; Sennoune, S.; Hossen, M.N.; Lichtenstein, A.H.; Cao, J.; Meyerrose, G.E.; Paone, R.; Soontrapa, S.; et al. Detection of atherosclerotic lesions and intimal macrophages using CD36-targeted nanovesicles. J. Control Release 2015, 220, 61–70. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Anand, A.; Strachan, F.E.; Ferry, A.V.; Lee, K.K.; Chapman, A.R.; Sandeman, D.; Stables, C.L.; Adamson, P.D.; Andrews, J.P.M.; et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: A stepped-wedge, cluster-randomised controlled trial. Lancet 2018, 392, 919–928. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Handberg, A.; Overvad, K.; Tjønneland, A.; Rimm, E.B.; Jensen, M.K. Association between plasma CD36 levels and incident risk of coronary heart disease among Danish men and women. Atherosclerosis 2018, 277, 163–168. [Google Scholar] [CrossRef]
- Rac, M.; Krzystolik, A.; Safranow, K.; Dziedziejko, V.; Goschorska, M.; Poncyljusz, W.; Chlubek, D. Is plasma-soluble CD36 associated with density of atheromatous plaque and ankle-brachial index in early-onset coronary artery disease patients? Kardiol. Pol. 2016, 74, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Castelblanco, E.; Sanjurjo, L.; Barranco-Altirriba, M.; Falguera, M.; Hernández, M.; Soldevila, B.; Sarrias, M.-R.; Franch-Nadal, J.; Arroyo, J.A.; Fernandez-Real, J.-M.; et al. The Circulating Fatty Acid Transporter Soluble CD36 Is Not Associated with Carotid Atherosclerosis in Subjects with Type 1 and Type 2 Diabetes Mellitus. J. Clin. Med. 2020, 9, 1700. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.A. Role of Plasma Soluble Lectin Like Oxidized Low-Density Lipoprotein Receptor-1 in Severity of CAD Patients and Relationship with Microrna-98. Balk. Med. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Stankova, T.; Delcheva, G.; Maneva, A.; Vladeva, S. Serum Levels of Carbamylated LDL and Soluble Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Are Associated with Coronary Artery Disease in Patients with Metabolic Syndrome. Medicina 2019, 55, 493. [Google Scholar] [CrossRef]
- Misaka, T.; Suzuki, S.; Sakamoto, N.; Yamaki, T.; Sugimoto, K.; Kunii, H.; Nakazato, K.; Saitoh, S.-i.; Sawamura, T.; Ishibashi, T.; et al. Significance of soluble lectin-like oxidized LDL receptor-1 levels in systemic and coronary circulation in acute coronary syndrome. BioMed Res. Int. 2014, 2014, 649185. [Google Scholar] [CrossRef]
- Kobayashi, N.; Hata, N.; Kume, N.; Shinada, T.; Tomita, K.; Shirakabe, A.; Kitamura, M.; Nozaki, A.; Inami, T.; Seino, Y.; et al. Soluble lectin-like oxidized LDL receptor-1 and high-sensitivity troponin T as diagnostic biomarkers for acute coronary syndrome. Improved values with combination usage in emergency rooms. Circ. J. 2011, 75, 2862–2871. [Google Scholar] [CrossRef]
- Kume, N.; Mitsuoka, H.; Hayashida, K.; Tanaka, M.; Kominami, G.; Kita, T. Soluble lectin-like oxidized LDL receptor-1 (sLOX-1) as a sensitive and specific biomarker for acute coronary syndrome—Comparison with other biomarkers. J. Cardiol. 2010, 56, 159–165. [Google Scholar] [CrossRef]
- Lee, A.S.; Wang, Y.C.; Chang, S.S.; Lo, P.H.; Chang, C.M.; Lu, J.; Burns, A.R.; Chen, C.H.; Kakino, A.; Sawamura, T.; et al. Detection of a High Ratio of Soluble to Membrane-Bound LOX-1 in Aspirated Coronary Thrombi From Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014008. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Xu, Y.W.; Li, S.M.; Guo, J.J.; Yi, T.; Chen, L.L. Higher serum lectin-like oxidized low-density lipoprotein receptor-1 in patients with stable coronary artery disease is associated with major adverse cardiovascular events: A multicentre pilot study. Biochem. Med. (Zagreb) 2019, 29, 010705. [Google Scholar] [CrossRef]
- Hu, C.; Chen, J.; Dandapat, A.; Fujita, Y.; Inoue, N.; Kawase, Y.; Jishage, K.; Suzuki, H.; Li, D.; Hermonat, P.L.; et al. LOX-1 abrogation reduces myocardial ischemia-reperfusion injury in mice. J. Mol. Cell. Cardiol. 2008, 44, 76–83. [Google Scholar] [CrossRef]
- Hu, C.; Dandapat, A.; Chen, J.; Fujita, Y.; Inoue, N.; Kawase, Y.; Jishage, K.; Suzuki, H.; Sawamura, T.; Mehta, J.L. LOX-1 deletion alters signals of myocardial remodeling immediately after ischemia-reperfusion. Cardiovasc. Res. 2007, 76, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Iwai-Kanai, E.; Hasegawa, K.; Sawamura, T.; Fujita, M.; Yanazume, T.; Toyokuni, S.; Adachi, S.; Kihara, Y.; Sasayama, S. Activation of Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Induces Apoptosis in Cultured Neonatal Rat Cardiac Myocytes. Circulation 2001, 104, 2948–2954. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Yoshida, K.; Nakano, S.; Ohno, T.; Honda, T.; Tsubokou, Y.; Matsuoka, H. Cardioprotective Mechanisms of Eplerenone on Cardiac Performance and Remodeling in Failing Rat Hearts. Hypertension 2006, 47, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Besli, F.; Gullulu, S.; Sag, S.; Kecebas, M.; Acikgoz, E.; Sarandol, E.; Aydinlar, A. The relationship between serum lectin-like oxidized LDL receptor-1 levels and systolic heart failure. Acta Cardiol. 2016, 71, 185–190. [Google Scholar] [CrossRef]
- Koenig, W. High-sensitivity C-reactive protein and atherosclerotic disease: From improved risk prediction to risk-guided therapy. Int. J. Cardiol. 2013, 168, 5126–5134. [Google Scholar] [CrossRef]
- Li, L.; Roumeliotis, N.; Sawamura, T.; Renier, G. C-reactive protein enhances LOX-1 expression in human aortic endothelial cells: Relevance of LOX-1 to C-reactive protein-induced endothelial dysfunction. Circ. Res. 2004, 95, 877–883. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhang, M.W.; Wang, F.; Zhao, Y.X.; Li, J.J.; Wang, X.P.; Bu, P.L.; Yang, J.M.; Liu, X.L.; Zhang, M.X.; et al. CRP enhances soluble LOX-1 release from macrophages by activating TNF-α converting enzyme. J. Lipid Res. 2011, 52, 923–933. [Google Scholar] [CrossRef]
- Hofmann, A.; Brunssen, C.; Wolk, S.; Reeps, C.; Morawietz, H. Soluble LOX-1: A Novel Biomarker in Patients with Coronary Artery Disease, Stroke, and Acute Aortic Dissection? J. Am. Heart Assoc. 2020, 9, e013803. [Google Scholar] [CrossRef]
- Jin, P.; Cong, S. LOX-1 and atherosclerotic-related diseases. Clin. Chim. Acta 2019, 491, 24–29. [Google Scholar] [CrossRef]
- Huang, W.; Li, Q.; Chen, X.; Lin, Y.; Xue, J.; Cai, Z.; Zhang, W.; Wang, H.; Jin, K.; Shao, B. Soluble lectin-like oxidized low-density lipoprotein receptor-1 as a novel biomarker for large-artery atherosclerotic stroke. Int. J. Neurosci. 2017, 127, 881–886. [Google Scholar] [CrossRef]
- Markstad, H.; Edsfeldt, A.; Yao Mattison, I.; Bengtsson, E.; Singh, P.; Cavalera, M.; Asciutto, G.; Björkbacka, H.; Fredrikson, G.N.; Dias, N.; et al. High Levels of Soluble Lectinlike Oxidized Low-Density Lipoprotein Receptor-1 Are Associated With Carotid Plaque Inflammation and Increased Risk of Ischemic Stroke. J. Am. Heart Assoc. 2019, 8, e009874. [Google Scholar] [CrossRef] [PubMed]
- Skarpengland, T.; Skjelland, M.; Kong Xiang, Y.; Skagen, K.; Holm, S.; Otterdal, K.; Dahl Christen, P.; Krohg-Sørensen, K.; Sagen Ellen, L.; Bjerkeli, V.; et al. Increased Levels of Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 in Ischemic Stroke and Transient Ischemic Attack. J. Am. Heart Assoc. 2018, 7, e006479. [Google Scholar] [CrossRef] [PubMed]
- Aslanian Ara, M.; Charo Israel, F. Targeted Disruption of the Scavenger Receptor and Chemokine CXCL16 Accelerates Atherosclerosis. Circulation 2006, 114, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Jin, G. The relationship between serum CXCL16 level and carotid vulnerable plaque in patients with ischemic stroke. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3911–3915. [Google Scholar]
- Mitsuoka, H.; Toyohara, M.; Kume, N.; Hayashida, K.; Jinnai, T.; Tanaka, M.; Kita, T. Circulating Soluble SR-PSOX/CXCL16 as a Biomarker for Acute Coronary Syndrome -Comparison with High-Sensitivity C-Reactive Protein. J. Atheroscler. Thromb. 2009, 16, 586–593. [Google Scholar] [CrossRef]
- Jansson, A.M.; Aukrust, P.; Ueland, T.; Smith, C.; Omland, T.; Hartford, M.; Caidahl, K. Soluble CXCL16 Predicts Long-Term Mortality in Acute Coronary Syndromes. Circulation 2009, 119, 3181–3188. [Google Scholar] [CrossRef]
- Andersen, T.; Ueland, T.; Ghukasyan Lakic, T.; Åkerblom, A.; Bertilsson, M.; Aukrust, P.; Michelsen, A.E.; James, S.K.; Becker, R.C.; Storey, R.F.; et al. C-X-C Ligand 16 Is an Independent Predictor of Cardiovascular Death and Morbidity in Acute Coronary Syndromes. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2402–2410. [Google Scholar] [CrossRef]
- Laugsand, L.E.; Åsvold, B.O.; Vatten, L.J.; Janszky, I.; Platou, C.; Michelsen, A.E.; Arain, F.; Damås, J.K.; Aukrust, P.; Ueland, T. Soluble CXCL16 and risk of myocardial infarction: The HUNT study in Norway. Atherosclerosis 2016, 244, 188–194. [Google Scholar] [CrossRef]
- Borst, O.; Schaub, M.; Walker, B.; Sauter, M.; Muenzer, P.; Gramlich, M.; Mueller, K.; Geisler, T.; Lang, F.; Klingel, K.; et al. CXCL16 is a novel diagnostic marker and predictor of mortality in inflammatory cardiomyopathy and heart failure. Int. J. Cardiol. 2014, 176, 896–903. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamagishi, S.-i.; Adachi, H.; Kurita-Nakamura, Y.; Matsui, T.; Yoshida, T.; Sato, A.; Imaizumi, T. Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in diabetic subjects with coronary artery disease. Diabetes Metab. Res. Rev. 2007, 23, 368–371. [Google Scholar] [CrossRef]
- Nin, J.W.M.; Jorsal, A.; Ferreira, I.; Schalkwijk, C.G.; Prins, M.H.; Parving, H.-H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D.A. Higher Plasma Soluble Receptor for Advanced Glycation End Products (sRAGE) Levels Are Associated With Incident Cardiovascular Disease and All-Cause Mortality in Type 1 Diabetes. Diabetes 2010, 59, 2027–2032. [Google Scholar] [CrossRef] [PubMed]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.; Hitman, G.; Neil, A.; Livingstone, S.; Charlton-Menys, V.; Bao, W.; DeMicco, D.A.; Preston, G.M.; et al. Total Soluble and Endogenous Secretory Receptor for Advanced Glycation End Products as Predictive Biomarkers of Coronary Heart Disease Risk in Patients With Type 2 Diabetes. Diabetes 2011, 60, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Katakami, N.; Kaneto, H.; Naka, T.; Takahara, M.; Sakamoto, F.; Irie, Y.; Miyashita, K.; Kubo, F.; Yasuda, T.; et al. Circulating soluble RAGE as a predictive biomarker of cardiovascular event risk in patients with type 2 diabetes. Atherosclerosis 2013, 227, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Del Turco, S.; Navarra, T.; Mazzarisi, A.; Cocci, F.; Coceani, M.; Bianchi, M.; Schlueter, M.; Marraccini, P. Inverse Association between Circulating Levels of Soluble Receptor for Advanced Glycation End-Products and Coronary Plaque Burden. J. Atheroscler. Thromb. 2012, 19, 941–948. [Google Scholar] [CrossRef][Green Version]
- Falcone, C.; Emanuele, E.; D’Angelo, A.; Buzzi Maria, P.; Belvito, C.; Cuccia, M.; Geroldi, D. Plasma Levels of Soluble Receptor for Advanced Glycation End Products and Coronary Artery Disease in Nondiabetic Men. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1032–1037. [Google Scholar] [CrossRef]
- Jensen, L.J.N.; Flyvbjerg, A.; Bjerre, M. Soluble Receptor for Advanced Glycation End Product: A Biomarker for Acute Coronary Syndrome. BioMed Res. Int. 2015, 2015, 815942. [Google Scholar] [CrossRef]
- Grauen Larsen, H.; Yndigegn, T.; Marinkovic, G.; Grufman, H.; Mares, R.; Nilsson, J.; Goncalves, I.; Schiopu, A. The soluble receptor for advanced glycation end-products (sRAGE) has a dual phase-dependent association with residual cardiovascular risk after an acute coronary event. Atherosclerosis 2019, 287, 16–23. [Google Scholar] [CrossRef]
- Hawkes, N. NICE guidelines could put 12 million UK adults on statins. BMJ 2017, 358, j3674. [Google Scholar] [CrossRef]
- Liao, J.K. Beyond lipid lowering: The role of statins in vascular protection. Int. J. Cardiol. 2002, 86, 5–18. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Zhu, X.L.; Li, R.T.; Liu, T.B.; Shang, D.Y.; Zhang, Y. Atorvastatin inhibits scavenger receptor A and monocyte chemoattractant protein-1 expressions in foam cell. Zhonghua Xin Xue Guan Bing Za Zhi 2007, 35, 666–669. [Google Scholar]
- Han, J.; Parsons, M.; Zhou, X.; Nicholson Andrew, C.; Gotto Antonio, M.; Hajjar David, P. Functional Interplay Between the Macrophage Scavenger Receptor Class B Type I and Pitavastatin (NK-104). Circulation 2004, 110, 3472–3479. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Wang, N.; Bezouevski, M.; Welch, C.; Tall, A.R. Decreased Atherosclerosis in Heterozygous Low Density Lipoprotein Receptor-deficient Mice Expressing the Scavenger Receptor BI Transgene. J. Biol. Chem. 1999, 274, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Xu, Y.; Sahebkar, A.; Xu, S. CD36 in Atherosclerosis: Pathophysiological Mechanisms and Therapeutic Implications. Curr. Atheroscler. Rep. 2020, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Liu, Q.; Yu, L.; Yang, Y.; Lu, M.; Wang, H.; Luo, D.; Rong, X.; Tang, F.; Guo, J. Downregulations of CD36 and Calpain-1, Inflammation, and Atherosclerosis by Simvastatin in Apolipoprotein E Knockout Mice. J. Vasc. Res. 2017, 54, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Biocca, S.; Iacovelli, F.; Matarazzo, S.; Vindigni, G.; Oteri, F.; Desideri, A.; Falconi, M. Molecular mechanism of statin-mediated LOX-1 inhibition. Cell Cycle 2015, 14, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Uzui, H.; Hayashi, H.; Nakae, I.; Matsumoto, T.; Uenishi, H.; Hayasaki, H.; Asaji, T.; Matsui, S.; Miwa, K.; Lee, J.D.; et al. Pitavastatin decreases serum LOX-1 ligand levels and MT1-MMP expression in CD14-positive mononuclear cells in hypercholesterolemic patients. Int. J. Cardiol. 2014, 176, 1230–1232. [Google Scholar] [CrossRef]
- Mehta, J.L.; Li, D.Y.; Chen, H.J.; Joseph, J.; Romeo, F. Inhibition of LOX-1 by Statins May Relate to Upregulation of eNOS. Biochem. Biophys. Res. Commun. 2001, 289, 857–861. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Romeo, F.; Sawamura, T.; Saldeen, T.; Mehta, J.L. Statins Modulate Oxidized Low-Density Lipoprotein-Mediated Adhesion Molecule Expression in Human Coronary Artery Endothelial Cells: Role of LOX-1. J. Pharmacol. Exp. Ther. 2002, 302, 601. [Google Scholar] [CrossRef]
- Puccetti, L.; Pasqui, A.L.; Bruni, F.; Pastorelli, M.; Ciani, F.; Palazzuoli, A.; Pontani, A.; Ghezzi, A.; Auteri, A. Lectin-like oxidized-LDL receptor-1 (LOX-1) polymorphisms influence cardiovascular events rate during statin treatment. Int. J. Cardiol. 2007, 119, 41–47. [Google Scholar] [CrossRef]
- Cuccurullo, C.; Iezzi, A.; Fazia Maria, L.; De Cesare, D.; Di Francesco, A.; Muraro, R.; Bei, R.; Ucchino, S.; Spigonardo, F.; Chiarelli, F.; et al. Suppression of Rage as a Basis of Simvastatin-Dependent Plaque Stabilization in Type 2 Diabetes. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2716–2723. [Google Scholar] [CrossRef]
- Santilli, F.; Bucciarelli, L.; Noto, D.; Cefalù, A.B.; Davì, V.; Ferrante, E.; Pettinella, C.; Averna, M.R.; Ciabattoni, G.; Davì, G. Decreased plasma soluble RAGE in patients with hypercholesterolemia: Effects of statins. Free Radic. Biol. Med. 2007, 43, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.P. PCSK9 Inhibitors: Novel Therapeutic Strategies for Lowering LDLCholesterol. Mini Rev. Med. Chem. 2019, 19, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Shahanawaz, J.; Shmookler Reis, R.J.; Varughese, K.I.; Sawamura, T.; Mehta, J.L. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc. Res. 2015, 107, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Pothineni, N.V.K.; Goel, A.; Lüscher, T.F.; Mehta, J.L. PCSK9 and inflammation: Role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc. Res. 2020, 116, 908–915. [Google Scholar] [CrossRef]
- Glintborg, D.; Højlund, K.; Andersen, M.; Henriksen, J.E.; Beck-Nielsen, H.; Handberg, A. Soluble CD36 and Risk Markers of Insulin Resistance and Atherosclerosis Are Elevated in Polycystic Ovary Syndrome and Significantly Reduced During Pioglitazone Treatment. Diabetes Care 2008, 31, 328–334. [Google Scholar] [CrossRef][Green Version]
- Gao, H.; Li, H.; Li, W.; Shen, X.; Di, B. Pioglitazone Attenuates Atherosclerosis in Diabetic Mice by Inhibition of Receptor for Advanced Glycation End-Product (RAGE) Signaling. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 6121–6131. [Google Scholar] [CrossRef][Green Version]
- Li, Q.; Zhao, W.; Zeng, X.; Hao, Z. Ursolic Acid Attenuates Atherosclerosis in ApoE(-/-) Mice: Role of LOX-1 Mediated by ROS/NF-κB Pathway. Molecules 2018, 23, 1101. [Google Scholar] [CrossRef]
- Song, G.; Tian, H.; Liu, J.; Zhang, H.; Sun, X.; Qin, S. H2 inhibits TNF-α-induced lectin-like oxidized LDL receptor-1 expression by inhibiting nuclear factor κB activation in endothelial cells. Biotechnol. Lett. 2011, 33, 1715–1722. [Google Scholar] [CrossRef]
- Taye, A.; Sawamura, T.; Morawietz, H. Aldosterone augments LOX-1-mediated low-density lipoprotein uptake in human umbilical artery endothelial cells. Pharmacol. Rep. 2010, 62, 311–318. [Google Scholar] [CrossRef]
- Morawietz, H.; Rueckschloss, U.; Niemann, B.; Duerrschmidt, N.; Galle, J.; Hakim, K.; Zerkowski, H.R.; Sawamura, T.; Holtz, J. Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation 1999, 100, 899–902. [Google Scholar] [CrossRef]
- Babaev, V.R.; Gleaves, L.A.; Carter, K.J.; Suzuki, H.; Kodama, T.; Fazio, S.; Linton, M.F. Reduced Atherosclerotic Lesions in Mice Deficient for Total or Macrophage-Specific Expression of Scavenger Receptor-A. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Y.; Li, F.; Fan, S.; Chen, X.; Lu, Y.; Wei, Y.; Chen, Q.; Xia, L.; Tang, J.; et al. Stimulation of the class-A scavenger receptor induces neutrophil extracellular traps (NETs) by ERK dependent NOX2 and ROMO1 activation. Biochem. Biophys. Res. Commun. 2019, 511, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Hehir, S.; Plourde, N.M.; Gu, L.; Poree, D.E.; Welsh, W.J.; Moghe, P.V.; Uhrich, K.E. Carbohydrate composition of amphiphilic macromolecules influences physicochemical properties and binding to atherogenic scavenger receptor A. Acta Biomater. 2012, 8, 3956–3962. [Google Scholar] [CrossRef] [PubMed]
- Iverson, N.M.; Plourde, N.M.; Sparks, S.M.; Wang, J.; Patel, E.N.; Shah, P.S.; Lewis, D.R.; Zablocki, K.R.; Nackman, G.B.; Uhrich, K.E.; et al. Dual use of amphiphilic macromolecules as cholesterol efflux triggers and inhibitors of macrophage athero-inflammation. Biomaterials 2011, 32, 8319–8327. [Google Scholar] [CrossRef] [PubMed]
- Fichtlscherer, S.; Breuer, S.; Heeschen, C.; Dimmeler, S.; Zeiher, A.M. Interleukin-10 serum levels and systemic endothelial vasoreactivity in patients with coronary artery disease. J. Am. Coll. Cardiol. 2004, 44, 44–49. [Google Scholar] [CrossRef]
- Anguera, I.; Miranda-Guardiola, F.; Bosch, X.; Filella, X.; Sitges, M.; Marin, J.L.; Betriu, A.; Sanz, G. Elevation of serum levels of the anti-inflammatory cytokine interleukin-10 and decreased risk of coronary events in patients with unstable angina. Am. Heart J. 2002, 144, 811–817. [Google Scholar] [CrossRef]
- Yang, H.; Chen, S.; Tang, Y.; Dai, Y. Interleukin-10 down-regulates oxLDL induced expression of scavenger receptor A and Bak-1 in macrophages derived from THP-1 cells. Arch. Biochem. Biophys. 2011, 512, 30–37. [Google Scholar] [CrossRef]
- Dai, X.-Y.; Cai, Y.; Mao, D.-D.; Qi, Y.-F.; Tang, C.; Xu, Q.; Zhu, Y.; Xu, M.-J.; Wang, X. Increased stability of phosphatase and tensin homolog by intermedin leading to scavenger receptor A inhibition of macrophages reduces atherosclerosis in apolipoprotein E-deficient mice. J. Mol. Cell. Cardiol. 2012, 53, 509–520. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, M.-J.; Teng, X.; Zhou, Y.B.; Chen, L.; Zhu, Y.; Wang, X.; Tang, C.S.; Qi, Y.F. Intermedin inhibits vascular calcification by increasing the level of matrix γ-carboxyglutamic acid protein. Cardiovasc. Res. 2009, 85, 864–873. [Google Scholar] [CrossRef][Green Version]
- Usui, H.K.; Shikata, K.; Sasaki, M.; Okada, S.; Matsuda, M.; Shikata, Y.; Ogawa, D.; Kido, Y.; Nagase, R.; Yozai, K.; et al. Macrophage Scavenger Receptor-A–Deficient Mice Are Resistant against Diabetic Nephropathy Through Amelioration of Microinflammation. Diabetes 2007, 56, 363–372. [Google Scholar] [CrossRef]
- Mäkinen, P.I.; Lappalainen, J.P.; Heinonen, S.E.; Leppänen, P.; Lähteenvuo, M.T.; Aarnio, J.V.; Heikkilä, J.; Turunen, M.P.; Ylä-Herttuala, S. Silencing of either SR-A or CD36 reduces atherosclerosis in hyperlipidaemic mice and reveals reciprocal upregulation of these receptors. Cardiovasc. Res. 2010, 88, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Seizer, P.; Schiemann, S.; Merz, T.; Daub, K.; Bigalke, B.; Stellos, K.; Muller, I.; Stockle, C.; Muller, K.; Gawaz, M.; et al. CD36 and macrophage scavenger receptor a modulate foam cell formation via inhibition of lipid-laden platelet phagocytosis. Semin. Thromb. Hemost. 2010, 36, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Manning-Tobin, J.J.; Moore, K.J.; Seimon, T.A.; Bell, S.A.; Sharuk, M.; Alvarez-Leite, J.I.; de Winther, M.P.; Tabas, I.; Freeman, M.W. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhotla, S.; Vanegas, D.; Kennedy, D.J.; Guy, E.; Nimako, G.; Morton, R.E.; Febbraio, M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 2008, 78, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, J.F.; Anhê, F.F.; Foley, K.P.; Denou, E.; Chan, R.W.; Bowdish, D.M.E.; Schertzer, J.D. Targeting macrophage scavenger receptor 1 promotes insulin resistance in obese male mice. Physiol. Rep. 2018, 6, e13930. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.G.; Tran, J.L.; Erion, D.M.; Vera, N.B.; Febbraio, M.; Weiss, E.J. Hepatocyte-Specific Disruption of CD36 Attenuates Fatty Liver and Improves Insulin Sensitivity in HFD-Fed Mice. Endocrinology 2016, 157, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, E. CD36: A multi-modal target for acute stroke therapy. J. Neurochem. 2009, 109 (Suppl. 1), 126–132. [Google Scholar] [CrossRef] [PubMed]
- Cho, S. CD36 as a therapeutic target for endothelial dysfunction in stroke. Curr. Pharm. Des. 2012, 18, 3721–3730. [Google Scholar] [CrossRef][Green Version]
- Mwaikambo, B.R.; Yang, C.; Ong, H.; Chemtob, S.; Hardy, P. Emerging roles for the CD36 scavenger receptor as a potential therapeutic target for corneal neovascularization. Endocr. Metab. Immune Disord Drug Targets 2008, 8, 255–272. [Google Scholar] [CrossRef]
- Zhao, L.; Varghese, Z.; Moorhead, J.F.; Chen, Y.; Ruan, X.Z. CD36 and lipid metabolism in the evolution of atherosclerosis. Br. Med. Bull. 2018, 126, 101–112. [Google Scholar] [CrossRef]
- Qin, L.; Kim, E.; Ratan, R.; Lee, F.S.; Cho, S. Genetic Variant of BDNF (Val66Met) Polymorphism Attenuates Stroke-Induced Angiogenic Responses by Enhancing Anti-Angiogenic Mediator CD36 Expression. J. Neurosci. 2011, 31, 775. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Szeto, H.H.; Kim, E.; Kim, H.; Tolhurst, A.T.; Pinto, J.T. A Novel Cell-permeable Antioxidant Peptide, SS31, Attenuates Ischemic Brain Injury by Down-regulating CD36. J. Biol. Chem. 2007, 282, 4634–4642. [Google Scholar] [CrossRef] [PubMed]
- Chnari, E.; Nikitczuk, J.S.; Wang, J.; Uhrich, K.E.; Moghe, P.V. Engineered Polymeric Nanoparticles for Receptor-Targeted Blockage of Oxidized Low Density Lipoprotein Uptake and Atherogenesis in Macrophages. Biomacromolecules 2006, 7, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Marleau, S.; Harb, D.; Bujold, K.; Avallone, R.; Iken, K.; Wang, Y.; Demers, A.; Sirois, M.G.; Febbraio, M.; Silverstein, R.L.; et al. EP 80317, a ligand of the CD36 scavenger receptor, protects apolipoprotein E-deficient mice from developing atherosclerotic lesions. FASEB J. 2005, 19, 1869–1871. [Google Scholar] [CrossRef] [PubMed]
- Bujold, K.; Mellal, K.; Zoccal, K.F.; Rhainds, D.; Brissette, L.; Febbraio, M.; Marleau, S.; Ong, H. EP 80317, a CD36 selective ligand, promotes reverse cholesterol transport in apolipoprotein E-deficient mice. Atherosclerosis 2013, 229, 408–414. [Google Scholar] [CrossRef]
- Wang, C.; Xu, W.; Liang, M.; Huang, D.; Huang, K. CTRP13 inhibits atherosclerosis via autophagy-lysosome-dependent degradation of CD36. FASEB J. 2019, 33, 2290–2300. [Google Scholar] [CrossRef]
- De Siqueira, J.; Abdul Zani, I.; Russell, D.A.; Wheatcroft, S.B.; Ponnambalam, S.; Homer-Vanniasinkam, S. Clinical and Preclinical Use of LOX-1-Specific Antibodies in Diagnostics and Therapeutics. J. Cardiovasc. Transl. Res. 2015, 8, 458–465. [Google Scholar] [CrossRef]
- Mehta Jawahar, L.; Sanada, N.; Hu Chang, P.; Chen, J.; Dandapat, A.; Sugawara, F.; Satoh, H.; Inoue, K.; Kawase, Y.; Jishage, K.-I.; et al. Deletion of LOX-1 Reduces Atherogenesis in LDLR Knockout Mice Fed High Cholesterol Diet. Circ. Res. 2007, 100, 1634–1642. [Google Scholar] [CrossRef]
- Li, D.; Williams, V.; Liu, L.; Chen, H.; Sawamura, T.; Romeo, F.; Mehta, J.L. Expression of lectin-like oxidized low-density lipoprotein receptors during ischemia-reperfusion and its role in determination of apoptosis and left ventricular dysfunction. J. Am. Coll. Cardiol. 2003, 41, 1048–1055. [Google Scholar] [CrossRef]
- Hinagata, J.; Kakutani, M.; Fujii, T.; Naruko, T.; Inoue, N.; Fujita, Y.; Mehta, J.L.; Ueda, M.; Sawamura, T. Oxidized LDL receptor LOX-1 is involved in neointimal hyperplasia after balloon arterial injury in a rat model. Cardiovasc. Res. 2006, 69, 263–271. [Google Scholar] [CrossRef]
- Li, D.; Williams, V.; Liu, L.; Chen, H.; Sawamura, T.; Antakli, T.; Mehta, J.L. LOX-1 inhibition in myocardial ischemia-reperfusion injury: Modulation of MMP-1 and inflammation. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1795–H1801. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Inoue, N.; Sato, Y.; Nishimichi, N.; Takikawa, K.; Fujita, Y.; Kakino, A.; Otsui, K.; Yamaguchi, S.; Matsuda, H.; et al. LOX-1 mediates vascular lipid retention under hypertensive state. J. Hypertens. 2010, 28, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.H.; Mehta, J.L.; Li, D.; Wu, P.; Kelly, K.J.; Packer, C.S.; Temm, C.; Goss, E.; Cheng, L.; Zhang, S.; et al. Anti-LOX-1 therapy in rats with diabetes and dyslipidemia: Ablation of renal vascular and epithelial manifestations. Am. J. Physiol. Ren. Physiol. 2008, 294, F110–F119. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.A.; Hata, A.; MacLean, M.R.; Morrell, N.W.; Baker, A.H. MicroRNA and vascular remodelling in acute vascular injury and pulmonary vascular remodelling. Cardiovasc. Res. 2012, 93, 594–604. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, X.; Dai, D.; Li, J.; Mehta, J.L. MicroRNA-98 regulates foam cell formation and lipid accumulation through repression of LOX-1. Redox Biol. 2018, 16, 255–262. [Google Scholar] [CrossRef]
- Liu, M.; Tao, G.; Liu, Q.; Liu, K.; Yang, X. MicroRNA let-7g alleviates atherosclerosis via the targeting of LOX-1 in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 57–64. [Google Scholar] [CrossRef]
- Arjuman, A.; Chandra, N.C. LOX-1: A potential target for therapy in atherosclerosis; an in vitro study. Int. J. Biochem. Cell Biol. 2017, 91, 65–80. [Google Scholar] [CrossRef]
- Gavrilov, K.; Saltzman, W.M. Therapeutic siRNA: Principles, challenges, and strategies. Yale J. Biol. Med. 2012, 85, 187–200. [Google Scholar]
- Ishigaki, T.; Ohki, I.; Utsunomiya-Tate, N.; Tate, S.I. Chimeric structural stabilities in the coiled-coil structure of the NECK domain in human lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1). J. Biochem. 2007, 141, 855–866. [Google Scholar] [CrossRef]
- Thakkar, S.; Wang, X.; Khaidakov, M.; Dai, Y.; Gokulan, K.; Mehta, J.L.; Varughese, K.I. Structure-based Design Targeted at LOX-1, a Receptor for Oxidized Low-Density Lipoprotein. Sci. Rep. 2015, 5, 16740. [Google Scholar] [CrossRef]
- Fan, Q.; Cai, H.; Yang, H.; Li, L.; Yuan, C.; Lu, X.; Wan, L. Biological evaluation of 131I- and CF750-labeled Dmab(scFv)-Fc antibodies for xenograft imaging of CD25-positive tumors. BioMed Res. Int. 2014, 2014, 459676. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xie, Q.; Xiang, H. Improved scFv Anti-LOX-1 Binding Activity by Fusion with LOX-1-Binding Peptides. BioMed Res. Int. 2017, 2017, 8946935. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xie, Q.; Liu, L.; Xiang, H. Enhanced Bioactivity of the Anti-LOX-1 scFv Engineered by Multimerization Strategy. Appl. Biochem. Biotechnol. 2018, 185, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Kume, N.; Shimaoka, T.; Kataoka, H.; Hayashida, K.; Akiyama, Y.; Nagata, I.; Ando, K.; Nobuyoshi, M.; Hanyuu, M.; et al. Expression of SR-PSOX, a Novel Cell-Surface Scavenger Receptor for Phosphatidylserine and Oxidized LDL in Human Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1796–1800. [Google Scholar] [CrossRef] [PubMed]
- Hofnagel, O.; Engel, T.; Severs, N.J.; Robenek, H.; Buers, I. SR-PSOX at sites predisposed to atherosclerotic lesion formation mediates monocyte-endothelial cell adhesion. Atherosclerosis 2011, 217, 371–378. [Google Scholar] [CrossRef]
- Bierhaus, A.; Hofmann, M.A.; Ziegler, R.; Nawroth, P.P. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovasc. Res. 1998, 37, 586–600. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-R.; Li, P.-C.; Feng, B. Advanced glycation end products increase lipids accumulation in macrophages through upregulation of receptor of advanced glycation end products: Increasing uptake, esterification and decreasing efflux of cholesterol. Lipids Health Dis. 2016, 15, 161. [Google Scholar] [CrossRef]
- Zhou, J.; Bai, W.; Liu, Q.; Cui, J.; Zhang, W. Intermittent Hypoxia Enhances THP-1 Monocyte Adhesion and Chemotaxis and Promotes M1 Macrophage Polarization via RAGE. BioMed Res. Int. 2018, 2018, 1650456. [Google Scholar] [CrossRef]
- Soro-Paavonen, A.; Watson, A.M.D.; Li, J.; Paavonen, K.; Koitka, A.; Calkin, A.C.; Barit, D.; Coughlan, M.T.; Drew, B.G.; Lancaster, G.I.; et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 2008, 57, 2461–2469. [Google Scholar] [CrossRef]
- Park, L.; Raman, K.G.; Lee, K.J.; Lu, Y.; Ferran, L.J.; Chow, W.S.; Stern, D.; Schmidt, A.M. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 1998, 4, 1025–1031. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, J.; Zhao, Q.; Zhuang, W.; Ding, J.; Zhang, C.; Gao, H.; Pang, D.W.; Pu, K.; Xie, H.Y. Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angew. Chem. Int. Ed. Engl. 2020, 59, 4068–4074. [Google Scholar] [CrossRef] [PubMed]
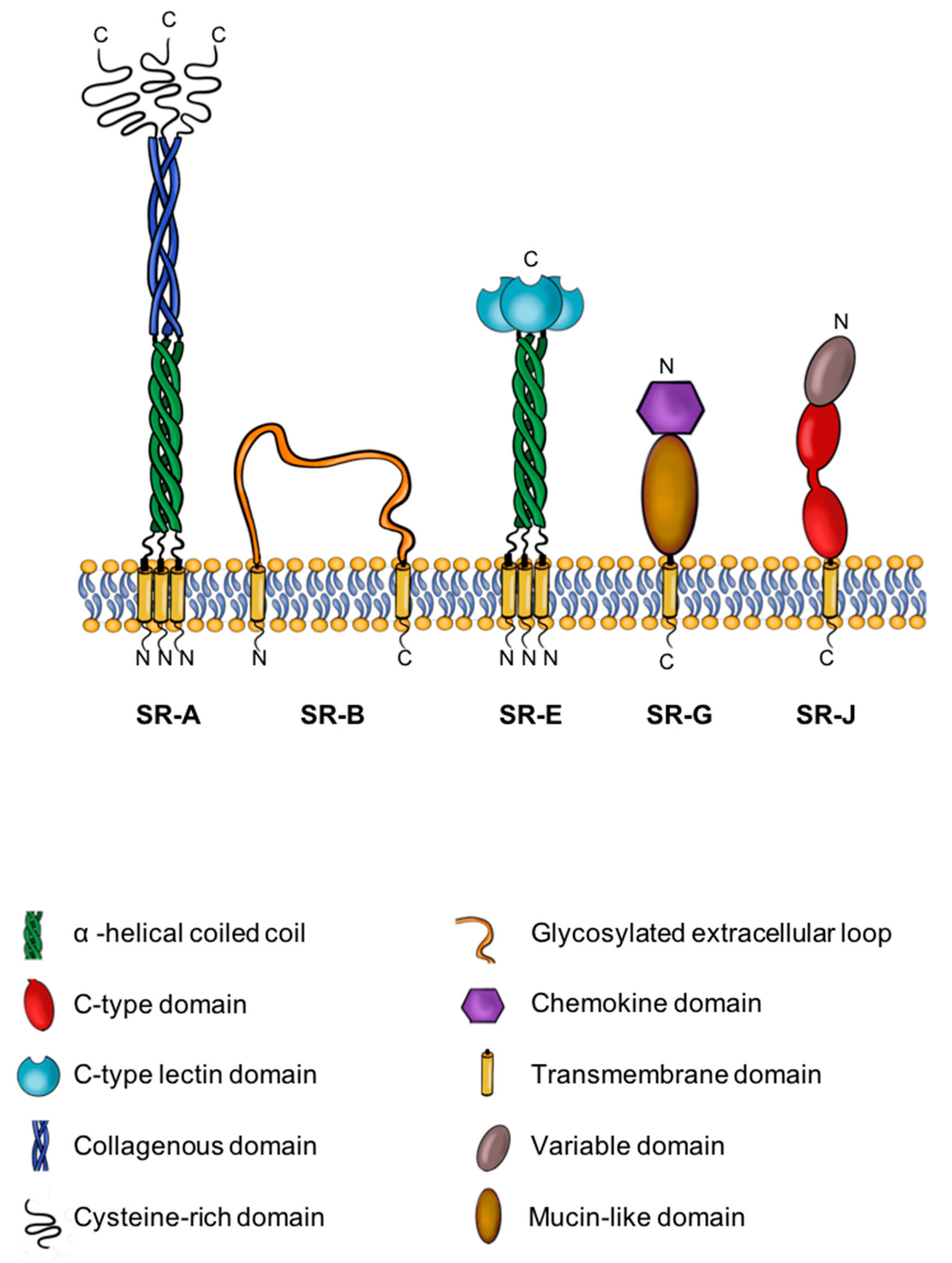
| SR Class | Nomenclature/Isoforms | Expression | Function |
|---|---|---|---|
| SR-A | Macrophage SR |
|
|
| SR-B | SR-B1 SR-B2 (CD36) SR-B3 (LIMP2) |
|
|
| SR-C | Not discussed (plant receptor) | ||
| SR-D | CD68 |
|
|
| SR-E | SR-E1 (LOX-1) SR-E1.1 (LOXIN) SR-E2 (Dectin-1) |
|
|
| SR-F | SR-F1 (SREC-1,SCARF-1) SR-F2 (SREC-2, SCARF-2) MEGF10 |
|
|
| SR-G | SR-G1 (CXCL16, SR-PSOX) |
|
|
| SR-H | SR-H1 (FEEL-1, Stabilin-1, Clever-1) SR-H2 (FEEL-2, Stabilin-2) |
|
|
| SR-I | SR-I1 (CD163, haemoglobin SR) SR-I2 (CD163B) |
|
|
| SR-J | RAGE |
|
|
| Disease State | SR Biomarker | Human Studies |
|---|---|---|
| CAD/ACS | SR-A |
|
| SR-B | ||
| SR-E |
| |
| SR-G |
| |
| SR-J |
| |
| Cardiomyopathy | SR-E |
|
| SR-G | ||
| Stroke | SR-E |
|
| SR-G |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuthbert, G.A.; Shaik, F.; Harrison, M.A.; Ponnambalam, S.; Homer-Vanniasinkam, S. Scavenger Receptors as Biomarkers and Therapeutic Targets in Cardiovascular Disease. Cells 2020, 9, 2453. https://doi.org/10.3390/cells9112453
Cuthbert GA, Shaik F, Harrison MA, Ponnambalam S, Homer-Vanniasinkam S. Scavenger Receptors as Biomarkers and Therapeutic Targets in Cardiovascular Disease. Cells. 2020; 9(11):2453. https://doi.org/10.3390/cells9112453
Chicago/Turabian StyleCuthbert, Gary A., Faheem Shaik, Michael A. Harrison, Sreenivasan Ponnambalam, and Shervanthi Homer-Vanniasinkam. 2020. "Scavenger Receptors as Biomarkers and Therapeutic Targets in Cardiovascular Disease" Cells 9, no. 11: 2453. https://doi.org/10.3390/cells9112453
APA StyleCuthbert, G. A., Shaik, F., Harrison, M. A., Ponnambalam, S., & Homer-Vanniasinkam, S. (2020). Scavenger Receptors as Biomarkers and Therapeutic Targets in Cardiovascular Disease. Cells, 9(11), 2453. https://doi.org/10.3390/cells9112453







