Smart Nanofibers with Natural Extracts Prevent Senescence Patterning in a Dynamic Cell Culture Model of Human Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanofibers Electrospinning and Combination with Myrtus Communis Extracts
2.2. Evaluation of Release Kinetic (from PCL Nanofiber of Myrtle Extract)
2.3. Cell Isolation and Culturing
2.4. Setting Up of Bioreactor and Cell Culture Conditions
2.5. Evaluation of Cell Viability
2.6. Senescence-Associated β-Galactosidase Staining
2.7. Gene Expression of Stemness and Cell Cycle Genes
2.8. Electronic Microscope Morphological Analysis
2.9. Statistical Analysis
3. Results
3.1. Nanofibers Features
3.2. NanoPCL-M Controls the Release of Myrtle Extracts
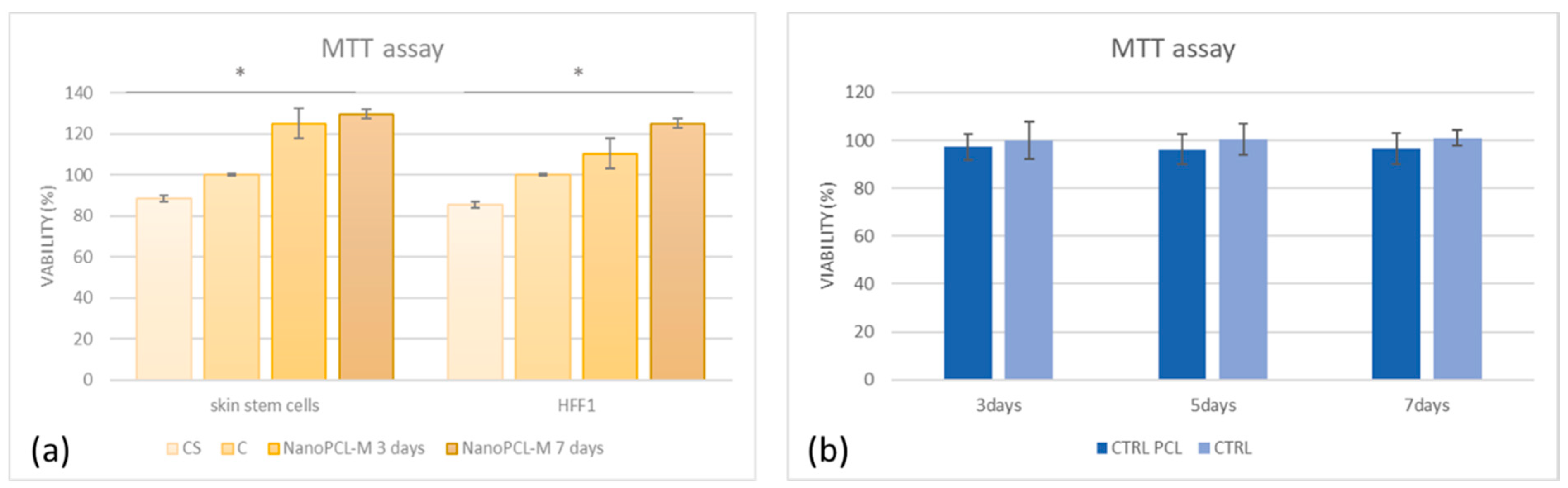
3.3. NanoPCL-M Pretreatment Preserves Cell Viability under UV Stress
3.4. NanoPCL-M is able to Counteract UV-Induced Cell Senescence
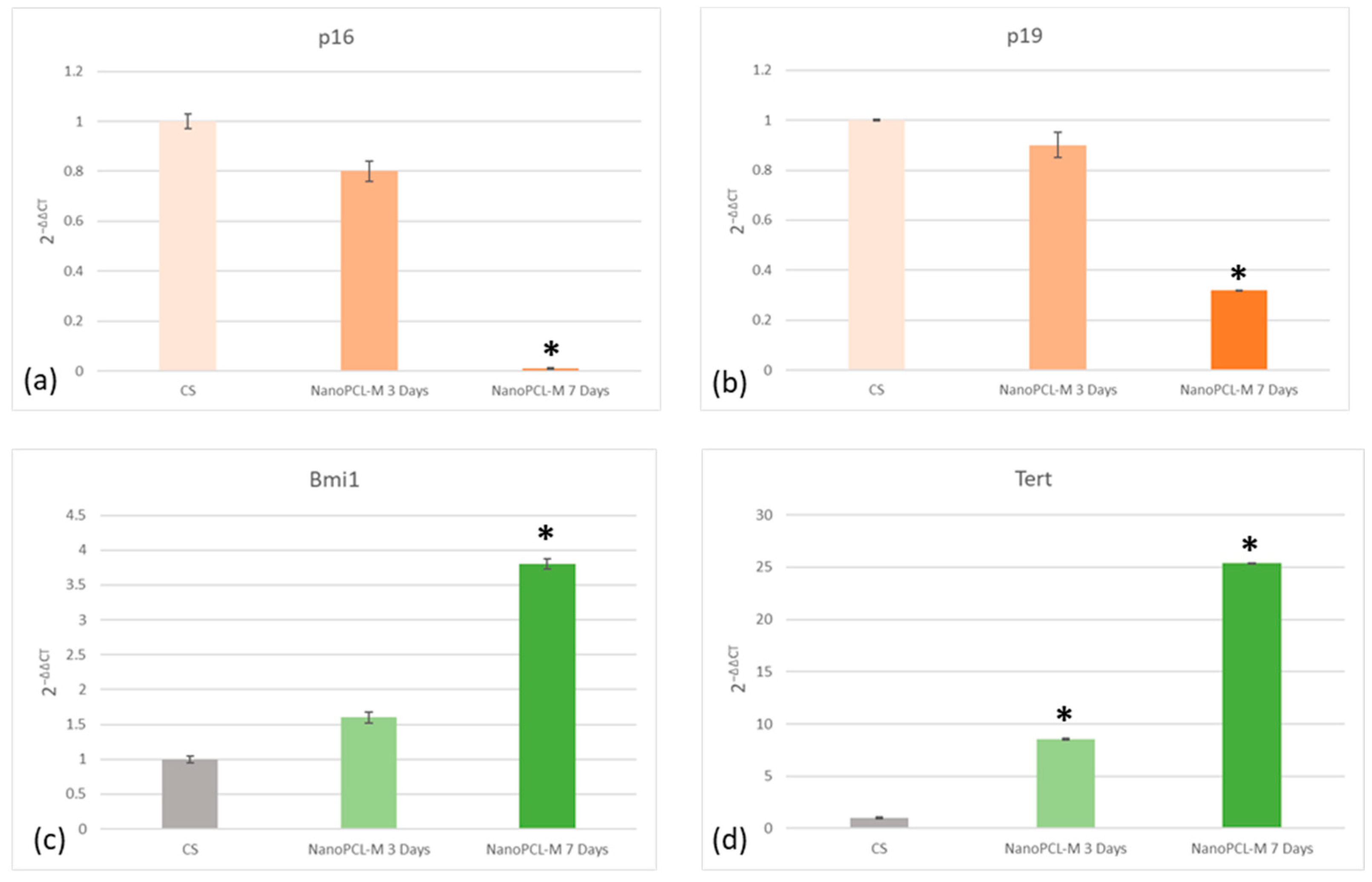
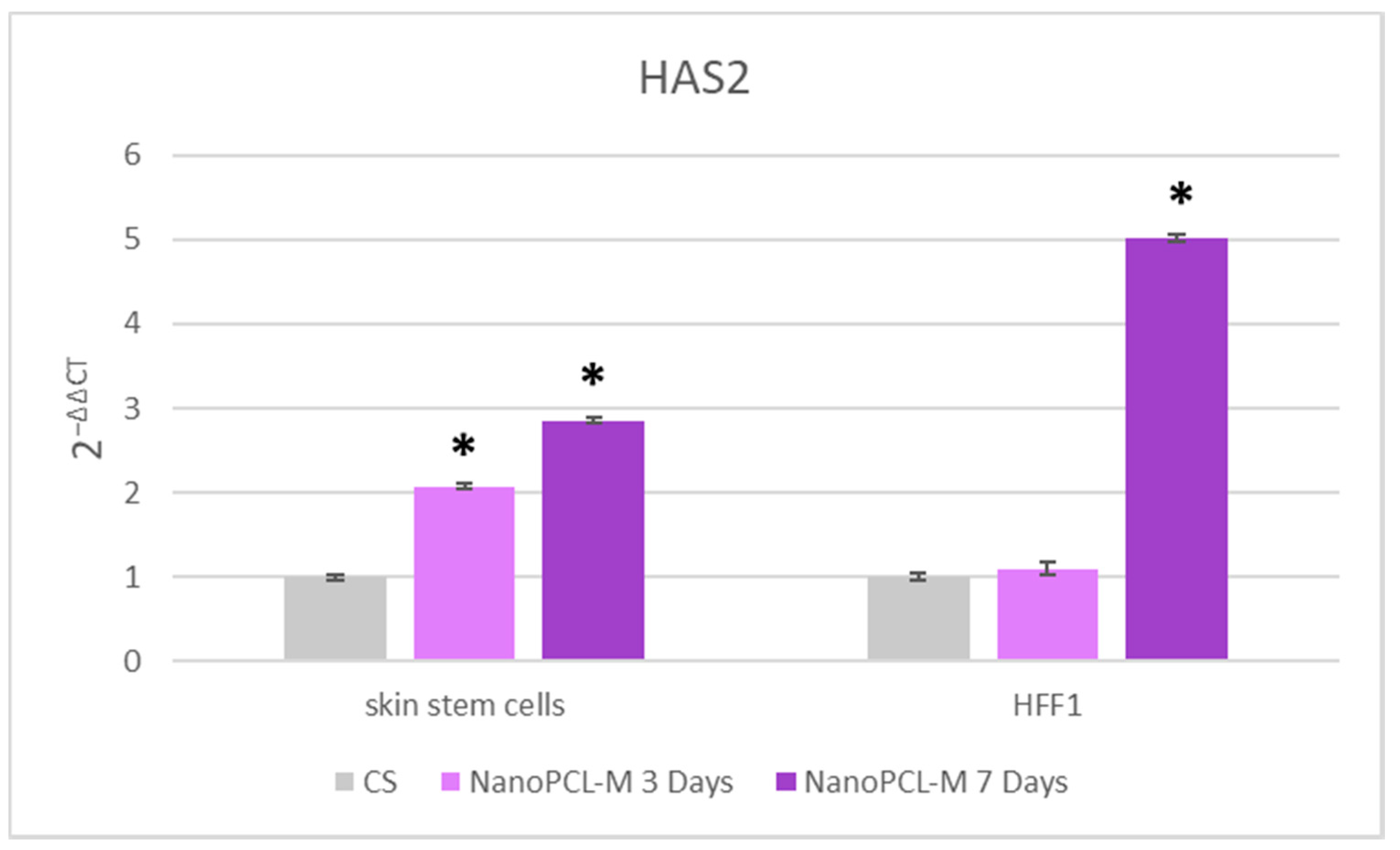
3.5. NanoPCL-M Maintains a Molecular Program of Youngness
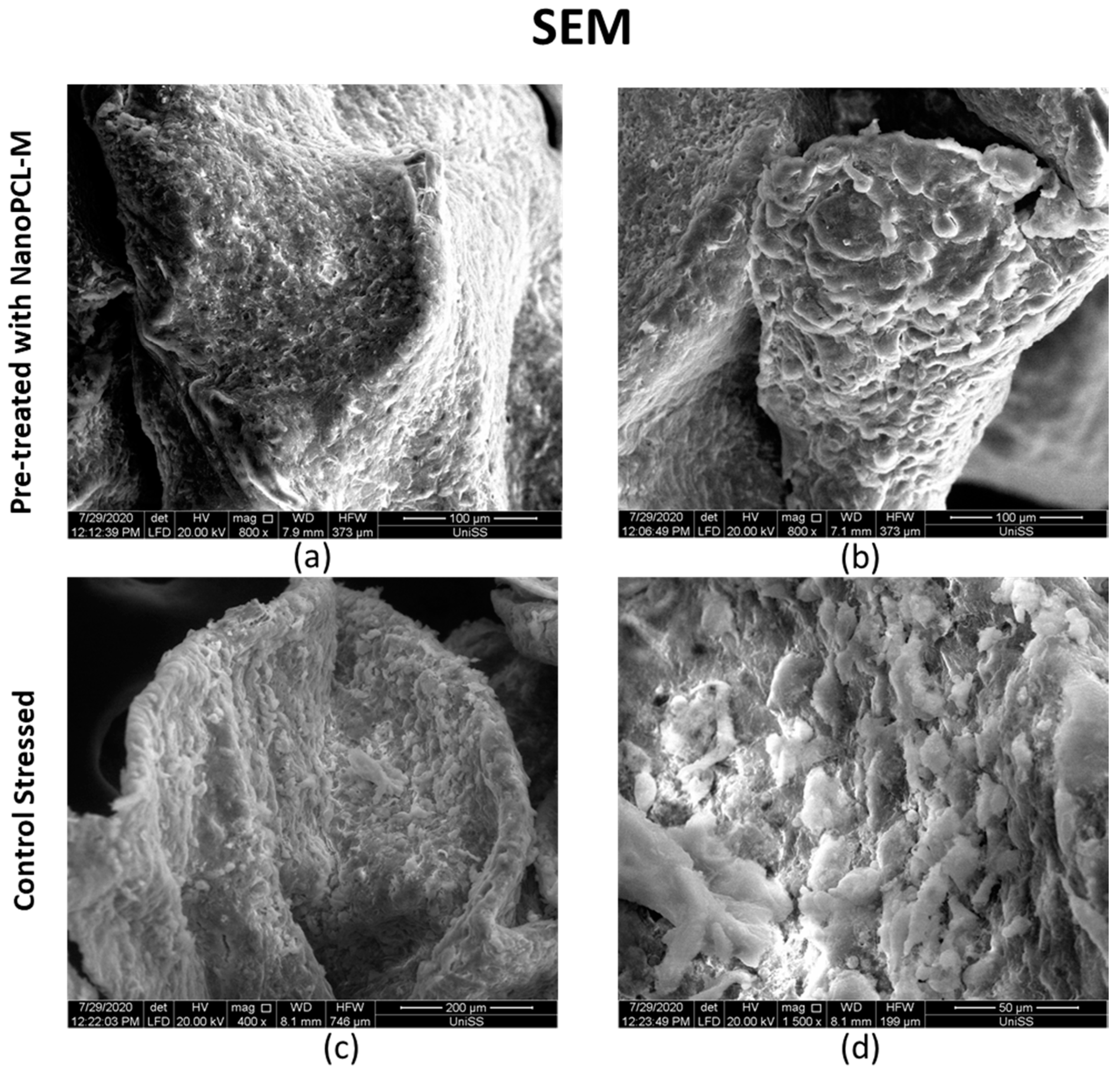
3.6. NanoPCL-M Pretreatment Preserve Keratinocytes’ Main Features also after Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, E.K.; Kim, H.O.; Park, Y.M.; Park, C.J.; Yu, D.S.; Lee, J.Y. Prevalence and risk factors of depression in geriatric patients with dermatological diseases. Ann. Dermatol. 2013, 25, 278–284. [Google Scholar] [CrossRef]
- Draelos, Z.D. Cosmetics and skin care products. A historical perspective. Dermatol. Clin. 2000, 18, 557–559. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential role of natural compounds against skin aging. Curr. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Amler, E.; Filova, E.; Buzgo, M.; Prosecka, E.; Rampichova, M.; Necas, A.; Nooeaid, P.; Boccaccini, A.R. Functionalized nanofibers as drug-delivery systems for osteochondral regeneration. Nanomedicine 2014, 9, 1083–1094. [Google Scholar] [CrossRef]
- Chakraborty, S.; Liao, I.C.; Adler, A.; Leong, K.W. Electrohydrodynamics: A facile technique to fabricate drug delivery systems. Adv. Drug Deliv. Rev. 2009, 61, 1043–1054. [Google Scholar] [CrossRef]
- Chew, S.Y.; Hufnagel, T.C.; Lim, C.T.; Leong, K.W. Mechanical properties of single electrospun drug-encapsulated nanofibres. Nanotechnology 2006, 17, 3880–3891. [Google Scholar] [CrossRef]
- Charles, D.J. Myrtle. In Antioxidant Properties of Spices, Herbs and Other Sources; Springer: New York, NY, USA, 2013; pp. 409–410. [Google Scholar]
- Olga, G.; Stavros, L.; Ioanna, C.; John, T. Revaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes. Eur. Food Res. Technol. 2008, 226, 583–590. [Google Scholar]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef]
- Evans, W.C. Trease and Evans’ Pharmacognosy, 15th ed.; W.B. Sanders: Nottingham, UK, 2002; p. 477. [Google Scholar]
- Mendes, M.M.; Gazarini, L.C.; Rodrigues, M.L. Acclimation of Myrtus communis to contrasting Mediterranean light environments-effects on structure and chemical composition of foliage and plant water relations. Env. Exp. Bot. 2001, 45, 165–178. [Google Scholar] [CrossRef]
- Elfellah, M.S.; Akhter, M.H.; Khan, M.T. Anti-hyperglycaemic effect of an extract of Myrtus communis in streptozotocin-induced diabetes in mice. J. Ethnopharmacol. 1984, 11, 275–281. [Google Scholar] [CrossRef]
- Cruciani, S.; Santaniello, S.; Garroni, G.; Fadda, A.; Balzano, F.; Bellu, E.; Sarais, G.; Fais, G.; Mulas, M.; Maioli, M. Myrtus Polyphenols, from Antioxidants to Anti-Inflammatory Molecules: Exploring a Network Involving Cytochromes P450 and Vitamin D. Molecules 2019, 24, 1515. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, S.; Amini Boroujeni, N.; Ahmadi, H.; Hassanshahian, M. Antibacterial Activity of Some Plant Extracts Against Extended- Spectrum Beta-Lactamase Producing Escherichia coli Isolates. Jundishapur J. Microbiol. 2015, 8, e15434. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Di Paola, R.; Mazzon, E.; Genovese, T.; Caminiti, R.; Bramanti, P.; Pergola, C.; Koeberle, A.; Werz, O.; Sautebin, L.; et al. Myrtucommulone from Myrtus communis exhibits potent anti-inflammatory effectiveness in vivo. J. Pharmacol. Exp. Ther. 2009, 329, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Amira, S.; Dade, M.; Schinella, G.; Ríos, J.L. Anti-inflammatory, anti-oxidant, and apoptotic activities of four plant species used in folk medicine in the Mediterranean basin. Pak. J. Pharm. Sci. 2012, 25, 65–72. [Google Scholar]
- Cruciani, S.; Santaniello, S.; Fadda, A.; Sale, L.; Sarais, G.; Sanna, D.; Mulas, M.; Ginesu, G.C.; Cossu, M.L.; Serra, P.A.; et al. Extracts from Myrtle Liqueur Processing Waste Modulate Stem Cells Pluripotency under Stressing Conditions. Biomed Res. Int. 2019, 2019, 5641034. [Google Scholar] [CrossRef]
- Fiorini-Puybaret, C.; Aries, M.-F.; Fabre, B.; Mamatas, S.; Luc, J.; Degouy, A.; Ambonati, M.; Mejean, C.; Poli, F. Pharmacological Properties of Myrtacine® and Its Potential Value in Acne Treatment. Planta Med. 2011, 77, 1582–1589. [Google Scholar] [CrossRef]
- Lu, C.; Fuchs, E. Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb. Perspect. Med. 2014, 4, a015222. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Trautinger, F. Mechanisms of photodamage of the skin and its functional consequences for skin ageing. Clin. Exp. Dermatol. 2001, 26, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am. J. Pathol. 2004, 165, 741–751. [Google Scholar] [CrossRef]
- Maioli, M.; Rinaldi, S.; Pigliaru, G.; Santaniello, S.; Basoli, V.; Castagna, A.; Fontani, V.; Ventura, C. REAC technology and hyaluron synthase 2, an interesting network to slow down stem cell senescence. Sci. Rep. 2016, 6, 28682. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Sheng, M.H.; Wasnik, S.; Baylink, D.J.; Lau, K.W. Effect of aging on stem cells. World J. Exp. Med. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Maioli, M.; Pigliaru, G.; Castagna, A.; Santaniello, S.; Basoli, V.; Fontani, V.; Ventura, C. Stem cell senescence. Effects of REAC technology on telomerase-independent and telomerase-dependent pathways. Sci. Rep. 2014, 4, 6373. [Google Scholar] [CrossRef]
- Park, I.K.; Morrison, S.J.; Clarke, M.F. Bmi1, stem cells, and senescence regulation. J. Clin. Invest. 2004, 113, 175–179. [Google Scholar] [CrossRef]
- Wang, Y.; Lauer, M.E.; Anand, S.; Mack, J.A.; Maytin, E.V. Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress. J. Biol. Chem. 2014, 289, 32253–32265. [Google Scholar] [CrossRef]
- Bellu, E.; Garroni, G.; Balzano, F.; Satta, R.; Montesu, M.A.; Kralovic, M.; Fedacko, J.; Cruciani, S.; Maioli, M. Isolating stem cells from skin: Designing a novel highly efficient non-enzymatic approach. Physiol. Res. 2019, 68, S385–S388. [Google Scholar] [CrossRef]
- Torreggiani, E.; Rossini, M.; Bononi, I.; Pietrobon, S.; Mazzoni, E.; Iaquinta, M.R.; Feo, C.; Rotondo, J.C.; Rizzo, P.; Tognon, M.; et al. Protocol for the long-term culture of human primary keratinocytes from the normal colorectal mucosa. J. Cell Physiol. 2019, 234, 9895–9905. [Google Scholar] [CrossRef]
- Wei, J.C.J.; Edwards, G.A.; Martin, D.J.; Huang, H.; Crichton, M.L.; Kendall, M.A.F. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for micro-medical device translation: From mice, rats, rabbits, pigs to humans. Sci. Rep. 2017, 7, 15885. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, S.; Garroni, G.; Ginesu, G.C.; Fadda, A.; Ventura, C.; Maioli, M. Unravelling Cellular Mechanisms of Stem Cell Senescence: An Aid from Natural Bioactive Molecules. Biology 2020, 9, 57. [Google Scholar] [CrossRef]
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Delitala, A.; Lotti Margotti, M.; Bagella, L.; Fontani, V.; Ventura, C. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age (Dordr) 2014, 36, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Maioli, M.; Contini, G.; Santaniello, S.; Bandiera, P.; Pigliaru, G.; Sanna, R.; Rinaldi, S.; Delitala, A.P.; Montella, A.; Bagella, L.; et al. Amniotic fluid stem cells morph into a cardiovascular lineage: Analysis of a chemically induced cardiac and vascular commitment. Drug Des. Devel. 2013, 7, 1063–1073. [Google Scholar]
- Maioli, M.; Basoli, V.; Santaniello, S.; Cruciani, S.; Delitala, A.P.; Pinna, R.; Milia, E.; Grillari-Voglauer, R.; Fontani, V.; Rinaldi, S.; et al. Osteogenesis from Dental Pulp Derived Stem Cells: A Novel Conditioned Medium Including Melatonin within a Mixture of Hyaluronic, Butyric, and Retinoic Acids. Stem Cells Int. 2016, 2056416. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, S.P.; Costa, R.; Silva, M.M.; Marcelino, P.; Brito, C.; Alves, P.M. Three-dimensional co-culture of human hepatocytes and mesenchymal stem cells: Improved functionality in long-term bioreactor cultures. J. Tissue Eng. Regen. Med. 2017, 11, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, S.; Santaniello, S.; Montella, A.; Ventura, C.; Maioli, M. Orchestrating stem cell fate: Novel tools for regenerative medicine. World J. Stem Cells 2019, 11, 464–475. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, D.; Tinaburri, L.; Dellambra, E. The Role of p16INK4a Pathway in Human Epidermal Stem Cell Self-Renewal, Aging and Cancer. Int. J. Mol. Sci. 2017, 18, 1591. [Google Scholar] [CrossRef]
- Rando, T.A. Stem cells, ageing and the quest for immortality. Nature 2006, 441, 1080–1086. [Google Scholar] [CrossRef]
- Klapper, W.; Parwaresch, R.; Krupp, G. Telomere biology in human aging and aging syndromes. Mech. Ageing Dev. 2001, 122, 695–712. [Google Scholar] [CrossRef]
- Tzellos, T.G.; Klagas, I.; Vahtsevanos, K.; Triaridis, S.; Printza, A.; Kyrgidis, A.; Karakiulakis, G.; Zouboulis, C.C.; Papakonstantinou, E. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp. Dermatol. 2009, 18, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Itahana, K.; Zou, Y.; Itahana, Y.; Martinez, J.L.; Beausejour, C.; Jacobs, J.J.; Van Lohuizen, M.; Band, V.; Campisi, J.; Dimri, G.P. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol. Cell Biol. 2003, 23, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Frankart, A.; Malaisse, J.; De Vuyst, E.; Minner, F.; de Rouvroit, C.L.; Poumay, Y. Epidermal morphogenesis during progressive in vitro 3D reconstruction at the air-liquid interface. Exp. Derm. 2012, 21, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-based composite scaffold matrices for tissue engineering applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Fathi-Azarbayjani, A.; Qun, L.; Chan, Y.W.; Chan, S.Y. Novel vitamin and gold-loaded nanofiber facial mask for topical delivery. Aaps. Pharm. Sci. Tech. 2010, 11, 1164–1170. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellu, E.; Garroni, G.; Cruciani, S.; Balzano, F.; Serra, D.; Satta, R.; Montesu, M.A.; Fadda, A.; Mulas, M.; Sarais, G.; et al. Smart Nanofibers with Natural Extracts Prevent Senescence Patterning in a Dynamic Cell Culture Model of Human Skin. Cells 2020, 9, 2530. https://doi.org/10.3390/cells9122530
Bellu E, Garroni G, Cruciani S, Balzano F, Serra D, Satta R, Montesu MA, Fadda A, Mulas M, Sarais G, et al. Smart Nanofibers with Natural Extracts Prevent Senescence Patterning in a Dynamic Cell Culture Model of Human Skin. Cells. 2020; 9(12):2530. https://doi.org/10.3390/cells9122530
Chicago/Turabian StyleBellu, Emanuela, Giuseppe Garroni, Sara Cruciani, Francesca Balzano, Diletta Serra, Rosanna Satta, Maria Antonia Montesu, Angela Fadda, Maurizio Mulas, Giorgia Sarais, and et al. 2020. "Smart Nanofibers with Natural Extracts Prevent Senescence Patterning in a Dynamic Cell Culture Model of Human Skin" Cells 9, no. 12: 2530. https://doi.org/10.3390/cells9122530
APA StyleBellu, E., Garroni, G., Cruciani, S., Balzano, F., Serra, D., Satta, R., Montesu, M. A., Fadda, A., Mulas, M., Sarais, G., Bandiera, P., Torreggiani, E., Martini, F., Tognon, M., Ventura, C., Beznoska, J., Amler, E., & Maioli, M. (2020). Smart Nanofibers with Natural Extracts Prevent Senescence Patterning in a Dynamic Cell Culture Model of Human Skin. Cells, 9(12), 2530. https://doi.org/10.3390/cells9122530









