Cerebrospinal Fluid Biomarkers in Relation to MRZ Reaction Status in Primary Progressive Multiple Sclerosis
Abstract
:1. Introduction
1.1. Patients
1.2. CSF and Serum Routine Parameters
1.3. BAFF, CXCL-13, GFAP, CHI3L1, NfL, sBCMA, and sTACI
1.4. Statistical Analysis
2. Results
2.1. MRZ Reaction
2.2. Clinical Features
2.3. CSF Routine Parameters
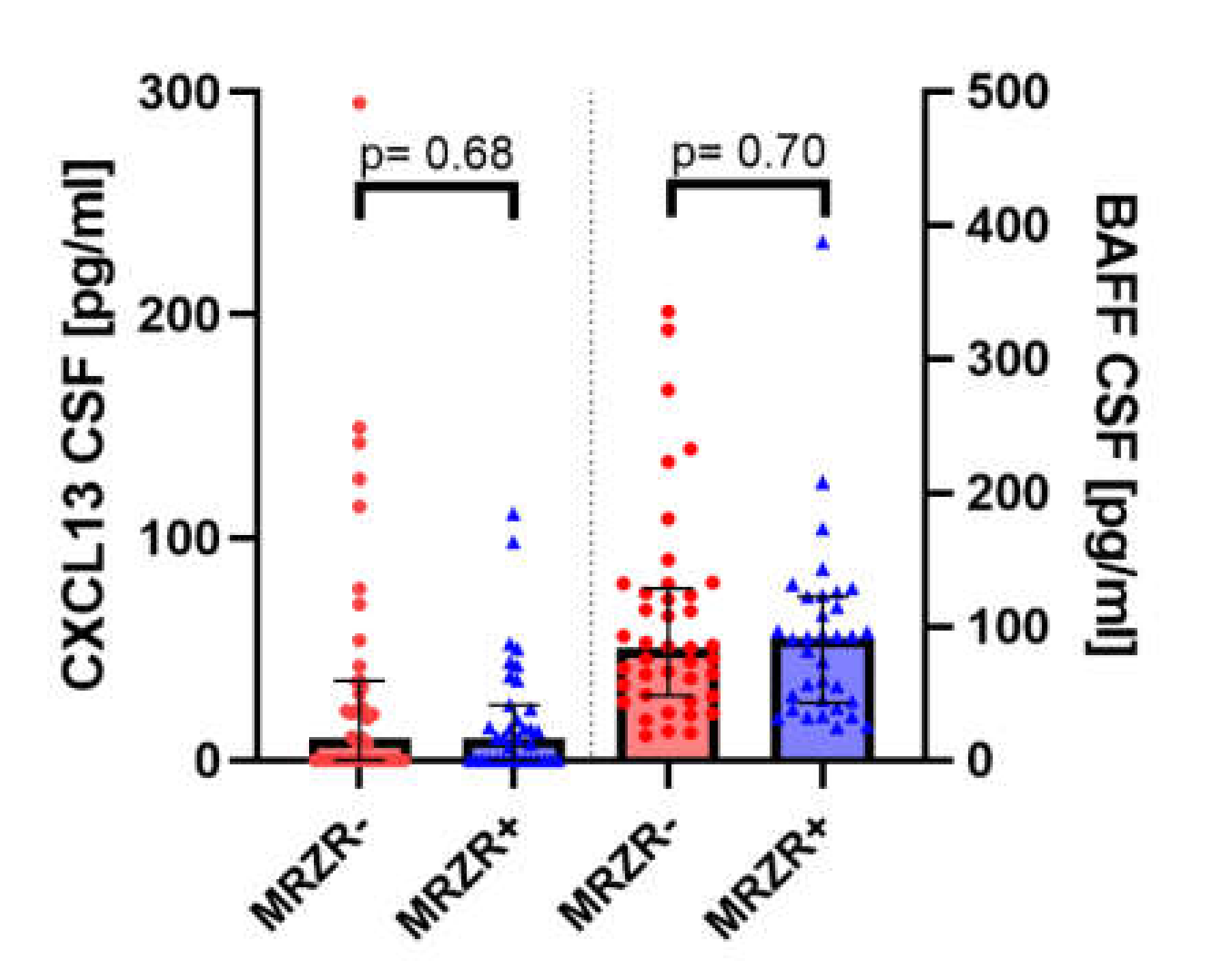
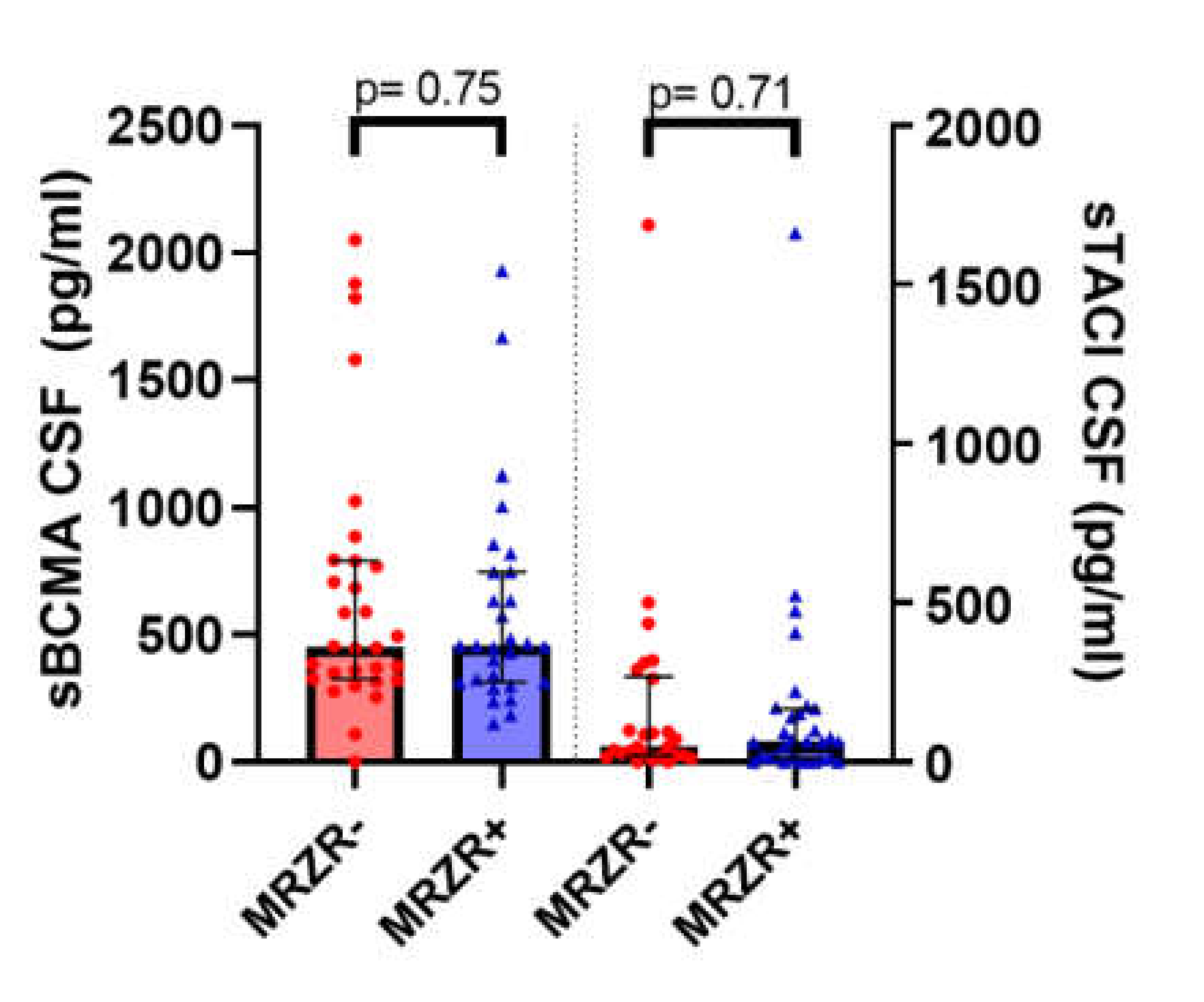
2.4. B Cell Biomarkers in CSF
2.5. Biomarkers of Glial Activation and Neuroaxonal Damage in CSF and Serum
2.6. Correlation Analysis
3. Discussion
3.1. CSF Biomarkers for MS Diagnosis
3.2. CSF Biomarkers as Indicators for Clinical Disease Severity and Disease Course in PPMS
3.3. CSF Biomarkers in Relation to MRZR
3.4. Limitations
3.5. Conclusions Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Ethic Statements
Abbreviations
| AI | Antibody index |
| AIs | Antibody indices |
| APRIL | A proliferation-inducing ligand |
| BAFF | B cell-activating factor |
| BCMA | B cell maturation antigen |
| CHI3L1 | Chitinase-3-like protein 1 |
| CSF | Cerebrospinal fluid |
| CXCL-13 | Chemokine CXC ligand 13 |
| EDSS | Expanded Disability Status Scale |
| ELISA | Enzyme-linked immunosorbent assay |
| GFAP | Glial fibrillary acidic protein |
| IgG/A/M | Immunoglobulin G/A/M |
| IgGloc | Intrathecal synthesis of IgG |
| LP | Lumbar puncture |
| MRZ | Measles, rubella, and varicella zoster virus |
| MRZR | MRZ reaction |
| MRZR+ | Patients with a positive MRZR result defined as at least two positive MRZ antibody indices |
| MRZR- | Patients with a negative MRZR result defined as no more than one positive MRZ antibody index |
| MS | Multiple sclerosis |
| NfL | Neurofilament light chain |
| n.s. | Not statistically significantly different |
| OCB | Oligoclonal IgG bands |
| OCB+ | Patients with at least two positive oligoclonal IgG bands in the CSF |
| OCB- | Patients with not more than one oligoclonal IgG band in the CSF |
| PPMS | Primary progressive multiple sclerosis |
| QAlb | Albumin quotient |
| RRMS | Relapsing–remitting multiple sclerosis |
| sBCMA | Soluble B cell maturation antigen |
| SD | Standard deviation |
| SIMOA | Single molecule array |
| sTACI | Soluble transmembrane activator and CAML interactor |
| TACI | Transmembrane activator and CAML interactor |
References
- Antel, J.; Antel, S.; Caramanos, Z.; Arnold, D.L.; Kuhlmann, T. Primary progressive multiple sclerosis: Part of the MS disease spectrum or separate disease entity? Acta Neuropathol. 2012, 123, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.H.; Leary, S.M. Primary-progressive multiple sclerosis. Lancet Neurol. 2007, 6, 903–912. [Google Scholar] [CrossRef]
- Owens, G.P.; Bennett, J.L.; Gilden, D.H.; Burgoon, M.P. The B cell response in multiple sclerosis. Neurol. Res. 2006, 28, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, M.; Derfuss, T.; Hohlfeld, R.; Meinl, E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat. Rev. Neurol. 2012, 8, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.J.; Pröbstel, A.K.; Zamvil, S.S. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat. Rev. Neurosci. 2019, 20, 728–745. [Google Scholar] [CrossRef]
- Stahnke, A.M.; Holt, K.M. Ocrelizumab: A New B-cell Therapy for Relapsing Remitting and Primary Progressive Multiple Sclerosis. Ann. Pharmacother. 2018, 52, 473–483. [Google Scholar] [CrossRef]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; De Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
- Pender, M.P. The pathogenesis of primary progressive multiple sclerosis: Antibody-mediated attack and no repair? J. Clin. Neurosci. 2004, 11, 689–692. [Google Scholar] [CrossRef] [Green Version]
- Abdelhak, A.; Weber, M.S.; Tumani, H. Primary progressive multiple sclerosis: Putting together the Puzzle. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Gasperi, C.; Salmen, A.; Antony, G.; Bayas, A.; Heesen, C.; Kümpfel, T.; Linker, R.A.; Paul, F.; Stangel, M.; Tackenberg, B.; et al. Association of Intrathecal Immunoglobulin G Synthesis with Disability Worsening in Multiple Sclerosis. JAMA Neurol. 2019, 76, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Eichhorn, P.; Franciotta, D.; Petereit, H.F.; Akman-Demir, G.; Wick, M.; Wildemann, B. The MRZ reaction as a highly specific marker of multiple sclerosis: Re-evaluation and structured review of the literature. J. Neurol. 2017, 264, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Felgenhauer, K.; Reiber, H. The diagnostic significance of antibody specificity indices in multiple sclerosis and herpes virus induced diseases of the nervous system. Clin. Investig. 1992, 70, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H.; Ungefehr, S.; Jacobi, C. The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult. Scler. 1998, 4, 111–117. [Google Scholar] [CrossRef]
- Abdelhak, A.; Hottenrott, T.; Mayer, C.; Hintereder, G.; Zettl, U.K.; Stich, O.; Tumani, H. CSF profile in primary progressive multiple sclerosis: Re-exploring the basics. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Hottenrott, T.; Dersch, R.; Berger, B.; Rauer, S.; Huzly, D.; Stich, O. The MRZ reaction in primary progressive multiple sclerosis. Fluids Barriers CNS 2017, 14. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [Green Version]
- Lublin, F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014, 72, 1–5. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- Hottenrott, T.; Dersch, R.; Berger, B.; Rauer, S.; Eckenweiler, M.; Huzly, D.; Stich, O. The intrathecal, polyspecific antiviral immune response in neurosarcoidosis, acute disseminated encephalomyelitis and autoimmune encephalitis compared to multiple sclerosis in a tertiary hospital cohort. Fluids Barriers CNS 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H.; Felgenhauer, K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin. Chim. Acta 1987, 163, 319–328. [Google Scholar] [CrossRef]
- Andersson, M.; Alvarez-Cermeñio, J.; Bernardi, G.; Cogato, I.; Fredman, P.; Frederiksen, J.; Fredrikson, S.; Gallo, P.; Grimaldi, L.M.; Grønning, M.; et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensus report. J. Neurol. Neurosurg. Psychiatry 1994, 57, 897–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelhak, A.; Hottenrott, T.; Morenas-Rodríguez, E.; Suárez-Calvet, M.; Zettl, U.K.; Haass, C.; Meuth, S.G.; Rauer, S.; Otto, M.; Tumani, H.; et al. Glial Activation Markers in CSF and Serum From Patients With Primary Progressive Multiple Sclerosis: Potential of Serum GFAP as Disease Severity Marker? Front. Neurol. 2019, 10, 280. [Google Scholar] [CrossRef]
- Brecht, I.; Weissbrich, B.; Braun, J.; Toyka, K.V.; Weishaupt, A.; Buttmann, M. Intrathecal, polyspecific antiviral immune response in oligoclonal band negative multiple sclerosis. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Feki, S.; Gargouri, S.; Mejdoub, S.; Dammak, M.; Hachicha, H.; Hadiji, O.; Feki, L.; Hammami, A.; Mhiri, C.; Karray, H.; et al. The intrathecal polyspecific antiviral immune response (MRZ reaction): A potential cerebrospinal fluid marker for multiple sclerosis diagnosis. J. Neuroimmunol. 2018, 321, 66–71. [Google Scholar] [CrossRef]
- Hottenrott, T.; Schorb, E.; Fritsch, K.; Dersch, R.; Berger, B.; Huzly, D.; Rauer, S.; Tebartz van Elst, L.; Endres, D.; Stich, O. The MRZ reaction and a quantitative intrathecal IgG synthesis may be helpful to differentiate between primary central nervous system lymphoma and multiple sclerosis. J. Neurol. 2018, 265, 1106–1114. [Google Scholar] [CrossRef]
- Brettschneider, J.; Tumani, H.; Kiechle, U.; Muche, R.; Richards, G.; Lehmensiek, V.; Ludolph, A.C.; Otto, M. IgG antibodies against measles, rubella, and varicella zoster virus predict conversion to multiple sclerosis in clinically isolated syndrome. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Tumani, H.; Deisenhammer, F.; Giovannoni, G.; Gold, R.; Hartung, H.P.; Hemmer, B.; Hohlfeld, R.; Otto, M.; Stangel, M.; Wildemann, B.; et al. Revised McDonald criteria: The persisting importance of cerebrospinal fluid analysis. Ann. Neurol. 2011, 70, 520. [Google Scholar] [CrossRef]
- Tumani, H.; Tourtellotte, W.W.; Peter, J.B.; Felgenhauer, K. The Optic Neuritis Study Group Acute optic neuritis: Combined immunological markers and magnetic resonance imaging predict subsequent development of multiple sclerosis. J. Neurol. Sci. 1998, 155, 44–49. [Google Scholar] [CrossRef]
- Khademi, M.; Kockum, I.; Andersson, M.L.; Iacobaeus, E.; Brundin, L.; Sellebjerg, F.; Hillert, J.; Piehl, F.; Olsson, T. Cerebrospinal fluid CXCL13 in multiple sclerosis: A suggestive prognostic marker for the disease course. Mult. Scler. J. 2011, 17, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Pawlitzki, M.; Schreiber, S.; Bittner, D.; Kreipe, J.; Leypoldt, F.; Rupprecht, K.; Carare, R.O.; Meuth, S.G.; Vielhaber, S.; Körtvélyessy, P. CSF Neurofilament Light Chain Levels in Primary Progressive MS: Signs of Axonal Neurodegeneration. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalatha, T.; Hatzifilippou, E.; Arnaoutoglou, M.; Balogiannis, S.; Koutsouraki, E. Glial and Neuroaxonal Biomarkers in a Multiple Sclerosis (MS) Cohort. Hell. J. Nucl. Med. 2019, 22 (Suppl. 2), 113–121. [Google Scholar]
- Petereit, H.F.; Reske, D. Expansion of antibody reactivity in the cerebrospinal fluid of multiple sclerosis patients—Follow-up and clinical implications. Cerebrospinal Fluid Res. 2005, 2. [Google Scholar] [CrossRef] [Green Version]
- Krumbholz, M.; Theil, D.; Cepok, S.; Hemmer, B.; Kivisäkk, P.; Ransohoff, R.M.; Hofbauer, M.; Farina, C.; Derfuss, T.; Hartle, C.; et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 2006, 129, 200–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowarik, M.C.; Cepok, S.; Sellner, J.; Grummel, V.; Weber, M.S.; Korn, T.; Berthele, A.; Hemmer, B. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J. Neuroinflamm. 2012, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, K.; Zhong, X.; Qiu, W.; Dai, Y.; Wu, A.; Hu, X. Cerebrospinal fluid BAFF and APRIL levels in neuromyelitis optica and multiple sclerosis patients during relapse. J. Clin. Immunol. 2012, 32, 1007–1011. [Google Scholar] [CrossRef]
- Ragheb, S.; Li, Y.; Simon, K.; Vanhaerents, S.; Galimberti, D.; De Riz, M.; Fenoglio, C.; Scarpini, E.; Lisak, R. Multiple sclerosis: BAFF and CXCL13 in cerebrospinal fluid. Mult. Scler. J. 2011, 17, 819–829. [Google Scholar] [CrossRef]
- Puthenparampil, M.; Stropparo, E.; Zywicki, S.; Bovis, F.; Cazzola, C.; Federle, L.; Grassivaro, F.; Rinaldi, F.; Perini, P.; Sormani, M.P.; et al. Wide Cytokine Analysis in Cerebrospinal Fluid at Diagnosis Identified CCL-3 as a Possible Prognostic Factor for Multiple Sclerosis. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- MacKay, F.; Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 2009, 9, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.A.; Hoffmann, F.S.; Kuhn, P.H.; Cheng, Q.; Chu, Y.; Schmidt-Supprian, M.; Hauck, S.M.; Schuh, E.; Krumbholz, M.; Rübsamen, H.; et al. γ-secretase directly sheds the survival receptor BCMA from plasma cells. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.S.; Kuhn, P.-H.; Laurent, S.A.; Hauck, S.M.; Berer, K.; Wendlinger, S.A.; Krumbholz, M.; Khademi, M.; Olsson, T.; Dreyling, M.; et al. The Immunoregulator Soluble TACI Is Released by ADAM10 and Reflects B Cell Activation in Autoimmunity. J. Immunol. 2015, 194, 542–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Perotin, S.; Castillo-Villalba, J.; Cubas-Nuñez, L.; Gasque, R.; Hervas, D.; Gomez-Mateu, J.; Alcala, C.; Perez-Miralles, F.; Gascon, F.; Dominguez, J.A.; et al. Combined Cerebrospinal Fluid Neurofilament Light Chain Protein and Chitinase-3 Like-1 Levels in Defining Disease Course and Prognosis in Multiple Sclerosis. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Mañé-Martínez, M.A.; Olsson, B.; Bau, L.; Matas, E.; Cobo-Calvo, Á.; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martínez-Yélamos, S.; Zetterberg, H. Glial and neuronal markers in cerebrospinal fluid in different types of multiple sclerosis. J. Neuroimmunol. 2016, 299, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Meinl, E.; Thaler, F.S.; Lichtenthaler, S.F. Shedding of BAFF/APRIL Receptors Controls B Cells. Trends Immunol. 2018, 39, 673–676. [Google Scholar] [CrossRef]
- Mahler, M.R.; Søndergaard, H.B.; Buhelt, S.; von Essen, M.R.; Romme Christensen, J.; Enevold, C.; Sellebjerg, F. Multiplex assessment of cerebrospinal fluid biomarkers in multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 45. [Google Scholar] [CrossRef]
- Huss, A.; Mojib-Yezdani, F.; Bachhuber, F.; Fangerau, T.; Lewerenz, J.; Otto, M.; Tumani, H.; Senel, M. Association of cerebrospinal fluid kappa free light chains with the intrathecal polyspecific antiviral immune response in multiple sclerosis. Clin. Chim. Acta 2019, 498, 148–153. [Google Scholar] [CrossRef]
- Coulombe, P.A.; Wong, P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat. Cell Biol. 2004, 6, 699–706. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Khalil, M. Neurofilaments as biomarkers in multiple sclerosis. Mult. Scler. J. 2012, 18, 552–556. [Google Scholar] [CrossRef]
- Gentil, B.J.; Tibshirani, M.; Durham, H.D. Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res. 2015, 360, 609–620. [Google Scholar] [CrossRef]
- Semra, Y.K.; Seidi, O.A.; Sharief, M.K. Heightened intrathecal release of axonal cytoskeletal proteins in multiple sclerosis is associated with progressive disease and clinical disability. J. Neuroimmunol. 2002, 122, 132–139. [Google Scholar] [CrossRef]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017, 81, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Novakova, L.; Zetterberg, H.; Sundström, P.; Axelsson, M.; Khademi, M.; Gunnarsson, M.; Malmeström, C.; Svenningsson, A.; Olsson, T.; Piehl, F.; et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017, 89, 2230–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, M.A.M.; Olsson, B.; Bau, L.; Matas, E.; Calvo, Á.C.; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martínez-Yélamos, S.; Zetterberg, H. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult. Scler. J. 2015, 21, 550–561. [Google Scholar] [CrossRef] [Green Version]
- Burman, J.; Raininko, R.; Blennow, K.; Zetterberg, H.; Axelsson, M.; Malmeström, C. YKL-40 is a CSF biomarker of intrathecal inflammation in secondary progressive multiple sclerosis. J. Neuroimmunol. 2016, 292, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Nakamura, Y.; Michalak, Z.; Isobe, N.; Barro, C.; Leppert, D.; Matsushita, T.; Hayashi, F.; Yamasaki, R.; Kuhle, J.; et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology 2019, 93, E1299–E1311. [Google Scholar] [CrossRef]
- Robinson-Agramonte, M.; Reiber, H.; Cabrera-Gomez, J.A.; Galvizu, R. Intrathecal polyspecific immune response to neurotropic viruses in multiple sclerosis: A comparative report from Cuban patients. Acta Neurol. Scand. 2007, 115, 312–318. [Google Scholar] [CrossRef]
| MRZR+ PPMS (n = 37) | MRZR- PPMS (n = 44) | Comparison Statistics | |
|---|---|---|---|
| Sex, females in % | 56.3 | 52.8 | n.s. (data available for n = 81) |
| Mean age in years at the time of symptom onset (range; SD) | 43.7 (24–62; 10.3) | 42.8 (15–60; 10.2) | n.s. (n = 77; missing data: n = 4) |
| Age in years at the time of LP | 51.6 (32–78; 11.8) | 50.0 (25–70; 10.1) | n.s. (n = 81) |
| Disease duration in years at the time of LP | 6.6 (1–39; 7.9) | 8.0 (1–27; 7.5) | n.s. (n = 77; missing data: n = 4) |
| EDSS at the time of LP | 4.8 (2.0–8.5; 1.6) | 4.7 (1.5–8.0; 1.8) | n.s. (n = 73; missing data: n = 8) |
| EDSS at last follow-up | 5.6 (4.0–7.0; 1.2) | 5.7 (1.5–8.0; 2.2) | n.s. (n = 20; missing data: n = 61) |
| Individual EDSS progression between LP and last follow-up | 1.0 (0–2.5; 1.0) | 1.0 (−0.5–4.5; 1.6) | n.s. (n = 20; missing data: n = 61) |
| Frequency of a clinical progressive disease within the last two years prior to LP in % | 87.5 | 88.6 | n.s. (n = 67; missing data: n = 14) |
| MRZR+ PPMS (n = 37) | MRZR- PPMS (n = 44) | Comparison Statistics | |
|---|---|---|---|
| Mean leucocyte count in cells/µL (range; SD) | 3.0 (0–11; 2.4) | 5.7 (0–43; 8.5) | n.s. (data available for n = 68; missing data: n = 13) |
| Total protein in mg/L | 522.0 (278.0–1200.0; 220.5) | 520.2 (151.0–1410.0; 220.2) | n.s. (n = 68; missing data: n = 13) |
| QAlb × 10−3 | 6.8 (2.9–17.8; 3.2) | 6.2 (2.7–20.1; 3.0) | n.s. (n = 68; missing data: n = 13) |
| Frequency of an elevated QAlb in % | 38.1 | 31.8 | n.s. (n = 68; missing data: n = 13) |
| Intrathecal synthesis of IgG (IgGloc) | 14.2 (0–50.6; 18.0) | 24.5 (0–149.8; 37.5) | n.s. (n = 32; missing data: n = 49) |
| Frequency of an intrathecal IgG synthesis in % | 42.9 | 40.9 | n.s. (n = 43; missing data: n = 38) |
| Frequency of an intrathecal IgA synthesis in % | 9.5 | 0 | n.s. (n = 43; missing data: n = 38) |
| Frequency of an intrathecal IgM synthesis in % | 14.3 | 22.7 | n.s. (n = 43; missing data: n = 38) |
| IgG index | 0.88 (0.5–1.40; 0.32) | 1.03 (0.40–2.60; 0.52) | n.s. (n = 32; missing data: n = 49) |
| Frequency of positive OCB in % | 89.2 | 95.5 | n.s. (n = 81) |
| Lactate in mmol/L | 1.9 (1.4–2.9; 0.5) | 1.6 (1.2–2.3; 0.3) | n.s. (n = 25; missing data: n = 56) |
| MRZR+ PPMS (n = 37) | MRZR- PPMS (n = 44) | Comparison Statistics | |
|---|---|---|---|
| Mean CSF concentration of BAFF in pg/mL (range; SD) | 92.7 (24.7–387.5; 68.8) | 103.6 (18.5–335.5; 77.7) | n.s. (data available for n = 75; missing data: n = 6) |
| CXCL-13 in pg/mL | 18.6 (0–111.0; 26.8) | 33.4 (0–295.0; 58.3) | n.s. (data available for n = 76; missing data: n = 5) |
| sBCMA in pg/mL | 587.7 (149.9–1931.5; 414–8) | 658.8 (0–2051.5; 524.2) | n.s. (data available for n = 59; missing data: n = 22) |
| sTACI in pg/mL | 155.7 (0–1662.5; 311.5) | 175.3 (0–1686.6; 339.9) | n.s. (data available for n = 57; missing data: n = 24) |
| MRZR+ PPMS (n = 37) | MRZR- PPMS (n = 44) | Comparison Statistics | |
|---|---|---|---|
| Mean concentration of CHI3L1 in CSF in ng/mL (range; SD) | 236.4 (87.8–453.2; 108.0) | 219.8 (71.8–525.0; 112.6) | n.s. (data available for n = 78; missing data: n = 3) |
| GFAP in CSF in pg/mL | 9807 (1572–21,760; 5420) | 9053 (2480–24,120; 4087) | n.s. (n = 79; missing data: n = 2) |
| GFAP in serum in pg/mL | 142.4 (67.0–391.0; 76.7) | 149.0 (35.6–289.0; 62.5) | n.s. (n = 68; missing data: n = 13) |
| NfL in CSF in pg/mL | 1842 (598–5384; 1124) | 1696 (322–12,720; 2090) | p = 0.04 (n = 78; missing data: n = 3) |
| NfL in serum in pg/mL | 34.8 (8.4–291.5; 55.3) | 26.1 (5.7–147.6; 26.7) | n.s. (n = 68; missing data: n = 3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, T.; Abdelhak, A.; Bose, T.; Meinl, E.; Otto, M.; Zettl, U.K.; Dersch, R.; Tumani, H.; Rauer, S.; Huss, A. Cerebrospinal Fluid Biomarkers in Relation to MRZ Reaction Status in Primary Progressive Multiple Sclerosis. Cells 2020, 9, 2543. https://doi.org/10.3390/cells9122543
Robinson T, Abdelhak A, Bose T, Meinl E, Otto M, Zettl UK, Dersch R, Tumani H, Rauer S, Huss A. Cerebrospinal Fluid Biomarkers in Relation to MRZ Reaction Status in Primary Progressive Multiple Sclerosis. Cells. 2020; 9(12):2543. https://doi.org/10.3390/cells9122543
Chicago/Turabian StyleRobinson, Tilman, Ahmed Abdelhak, Tanima Bose, Edgar Meinl, Markus Otto, Uwe K. Zettl, Rick Dersch, Hayrettin Tumani, Sebastian Rauer, and André Huss. 2020. "Cerebrospinal Fluid Biomarkers in Relation to MRZ Reaction Status in Primary Progressive Multiple Sclerosis" Cells 9, no. 12: 2543. https://doi.org/10.3390/cells9122543





