Systematic Review of Stem-Cell-Based Therapy of Burn Wounds: Lessons Learned from Animal and Clinical Studies
Abstract
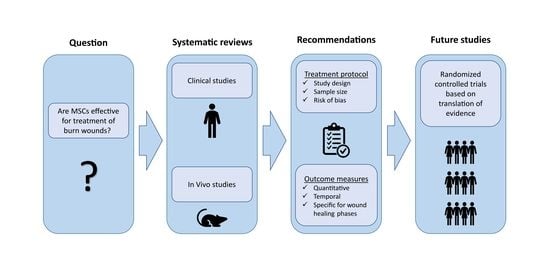
:1. Introduction
2. Methods
2.1. Search Strategies
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Assessment of Continuity of Evidence
3. Results
3.1. Bias Assessment
3.2. Study Characteristics
3.2.1. Population
3.2.2. Intervention
3.2.3. Comparison
3.2.4. Outcome
3.3. Outcome Assessment Parameters
3.3.1. Wound Healing
3.3.2. Inflammation
3.3.3. Proliferation
Neovascularization
Granulation
Re-Epithelialization
3.3.4. Remodeling/Scarring
3.4. Pre-Clinical to Clinical Continuity of Evidence
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lang, T.C.; Zhao, R.; Kim, A.; Wijewardena, A.; Vandervord, J.; Xue, M.; Jackson, C.J. A Critical Update of the Assessment and Acute Management of Patients with Severe Burns. Adv. Wound Care 2019, 8, 607–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spronk, I.; Polinder, S.; Haagsma, J.A.; Nieuwenhuis, M.; Pijpe, A.; van der Vlies, C.H.; Middelkoop, E.; van Baar, M.E. Patient-reported scar quality of adults after burn injuries: A five-year multicenter follow-up study. Wound Repair Regen. 2019, 27, 406–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, V.K. Burn wound: How it differs from other wounds. Indian J. Plast. Surg. 2012, 45, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Maranda, E.L.; Rodriguez-Menocal, L.; Badiavas, E. Role of Mesenchymal Stem Cells in Dermal Repair in Burns and Diabetic Wounds. Curr. Stem Cell Res. Ther. 2017, 12, 61–70. [Google Scholar] [CrossRef]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- Guerrouahen, B.S.; Sidahmed, H.; Al Sulaiti, A.; Al Khulaifi, M.; Cugno, C. Enhancing Mesenchymal Stromal Cell Immunomodulation for Treating Conditions Influenced by the Immune System. Stem Cells Int. 2019, 2019, 7219297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, J.G.; Riis, S.E.; Frøbert, O.; Yang, S.; Kastrup, J.; Zachar, V.; Simonsen, U.; Fink, T. Activation of Protease-Activated Receptor 2 Induces VEGF Independently of HIF-1. PLoS ONE 2012, 7, e46087. [Google Scholar] [CrossRef] [Green Version]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Caseiro, A.R.; Pedrosa, S.S.; Ivanova, G.; Branquinho, M.V.; Almeida, A.; Faria, F.; Amorim, I.; Pereira, T.; Maurício, A.C. Mesenchymal Stem/Stromal Cells metabolomic and bioactive factors profiles: A comparative analysis on the umbilical cord and dental pulp derived Stem/Stromal Cells secretome. PLoS ONE 2019, 14, e0221378. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N.; Willis, E.; Chan, V.T.; Muffley, L.A.; Isik, F.F.; Gibran, N.S.; Hocking, A.M. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp. Cell Res. 2010, 316, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riis, S.; Newman, R.; Ipek, H.; Andersen, J.I.; Kuninger, D.; Boucher, S.; Vemuri, M.C.; Pennisi, C.P.; Zachar, V.; Fink, T. Hypoxia enhances the wound-healing potential of adipose-derived stem cells in a novel human primarykeratinocyte-based scratch assay. Int. J. Mol. Med. 2017, 39, 587–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, F.M.; Riis, S.E.; Andersen, J.I.; Lesage, R.; Fink, T.; Pennisi, C.P.; Zachar, V. Discrete adipose-derived stem cell subpopulations may display differential functionality after in vitro expansion despite convergence to a common phenotype distribution. Stem Cell Res. Ther. 2016, 7, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riis, S.; Stensballe, A.; Emmersen, J.; Pennisi, C.P.; Birkelund, S.; Zachar, V.; Fink, T. Mass spectrometry analysis of adipose-derived stem cells reveals a significant effect of hypoxia on pathways regulating extracellular matrix. Stem Cell Res. Ther. 2016, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Hyldig, K.; Riis, S.; Pennisi, C.P.; Zachar, V.; Fink, T. Implications of extracellular matrix production by adipose tissue-derived stem cells for development of wound healing therapies. Int. J. Mol. Sci. 2017, 18, 1167. [Google Scholar] [CrossRef] [Green Version]
- Riis, S.; Zachar, V.; Boucher, S.; Vemuri, M.C.; Pennisi, C.P.; Fink, T. Critical steps in the isolation and expansion of adipose-derived stem cells for translational therapy. Expert Rev. Mol. Med. 2015, 17, e11. [Google Scholar] [CrossRef]
- Bruun Mathiasen, A.; Jørgensen, E.; Ali Qayyum Rigshospitalet, A.; Ali Qayyum, A.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J.; Copenhagen, D.M.S. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous bone-marrow derived Mesenchymal Stromal Cells in chr Rationale and design of the first randomized, double-blind, placebo-contro. Am. Heart J. 2012, 164, 285–291. [Google Scholar] [CrossRef]
- Haack-Sørensen, M.; Juhl, M.; Follin, B.; Harary Søndergaard, R.; Kirchhoff, M.; Kastrup, J.; Ekblond, A. Development of large-scale manufacturing of adipose-derived stromal cells for clinical applications using bioreactors and human platelet lysate. Scand. J. Clin. Lab. Investig. 2018, 78, 293–300. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Klersy, C.; Leffler, D.A.; Rogers, R.; Bennett, D.; Corazza, G.R. Systematic review with meta-analysis: Safety and efficacy of local injections of mesenchymal stem cells in perianal fistulas. JGH Open 2019, 3, 249–260. [Google Scholar] [CrossRef]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: Final 4-year follow-up of the MSC-HF trial. Eur. J. Heart Fail. 2019, 36, 1744–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Gang, X.; Sun, C.; Wang, G. Mesenchymal Stem Cells Improve Healing of Diabetic Foot Ulcer. J. Diabetes Res. 2017, 2017, 9328347. [Google Scholar] [CrossRef] [PubMed]
- Kavala, A.A.; Turkyilmaz, S. Autogenously derived regenerative cell therapy for venous leg ulcers. Arch. Med. Sci.-Atheroscler. Dis. 2018, 3, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group, T.P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Study Quality Assessment Tools | National Heart, Lung, and Blood Institute (NHLBI). Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 24 June 2020).
- Oryan, A.; Alemzadeh, E.; Mohammadi, A.A.; Moshiri, A. Healing potential of injectable Aloe vera hydrogel loaded by adipose-derived stem cell in skin tissue-engineering in a rat burn wound model. Cell Tissue Res. 2019, 377, 215–227. [Google Scholar] [CrossRef]
- Atalay, S.; Coruh, A.; Deniz, K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns 2014, 40, 1375–1383. [Google Scholar] [CrossRef]
- Eyuboglu, A.A.; Uysal, C.A.; Ozgun, G.; Coskun, E.; Markal Ertas, N.; Haberal, M. The effect of adipose derived stromal vascular fraction on stasis zone in an experimental burn model. Burns 2018, 44, 386–396. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Scutaru, T.T.; Ghetu, N.; Carasevici, E.; Lupascu, C.D.; Ferariu, D.; Pieptu, D.; Coman, C.-G.; Danciu, M. The Effects of Adipose-Derived Stem Cell-Differentiated Adipocytes on Skin Burn Wound Healing in Rats. J. Burn Care Res. 2017, 38, 1–10. [Google Scholar] [CrossRef]
- Loder, S.; Peterson, J.R.; Agarwal, S.; Eboda, O.; Brownley, C.; DeLaRosa, S.; Ranganathan, K.; Cederna, P.; Wang, S.C.; Levi, B. Wound healing after thermal injury is improved by fat and adipose-derived stem cell isografts. J. Burn Care Res. 2015, 36, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Shokrgozar, M.A.; Fattahi, M.; Bonakdar, S.; Ragerdi Kashani, I.; Majidi, M.; Haghighipour, N.; Bayati, V.; Sanati, H.; Saeedi, S.N. Healing potential of mesenchymal stem cells cultured on a collagen-based scaffold for skin regeneration. Iran. Biomed. J. 2012, 16, 68–76. [Google Scholar] [PubMed]
- Kaita, Y.; Tarui, T.; Yoshino, H.; Matsuda, T.; Yamaguchi, Y.; Nakagawa, T.; Asahi, M.; Ii, M. Sufficient therapeutic effect of cryopreserved frozen adipose-derived regenerative cells on burn wounds. Regen. Ther. 2019, 10, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Bliley, J.M.; Argenta, A.; Satish, L.; McLaughlin, M.M.; Dees, A.; Tompkins-Rhoades, C.; Marra, K.G.; Rubin, J.P. Administration of adipose-derived stem cells enhances vascularity, induces collagen deposition, and dermal adipogenesis in burn wounds. Burns 2016, 42, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Gholipourmalekabadi, M.; Seifalian, A.M.; Urbanska, A.M.; Omrani, M.D.; Hardy, J.G.; Madjd, Z.; Hashemi, S.M.; Ghanbarian, H.; Milan, P.B.; Mozafari, M.; et al. 3D Protein-Based Bilayer Arti fi cial Skin for the Guided Scarless Healing of Third-Degree Burn Wounds in Vivo. Biomacromolecules 2018, 19, 2409–2422. [Google Scholar] [CrossRef] [Green Version]
- Motamed, S.; Taghiabadi, E.; Molaei, H.; Sodeifi, N.; Hassanpour, S.E.; Shafieyan, S.; Azargashb, E.; Farajzadeh-Vajari, F.; Aghdami, N.; Bajouri, A. Cell-based skin substitutes accelerate regeneration of extensive burn wounds in rats. Am. J. Surg. 2017, 214, 762–769. [Google Scholar] [CrossRef]
- Abbas, O.L.; Özatik, O.; Gönen, Z.B.; Öğüt, S.; Özatik, F.Y.; Salkın, H.; Musmul, A. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Dental Pulp as Sources of Cell Therapy for Zone of Stasis Burns. J. Investig. Surg. 2019, 32, 477–490. [Google Scholar] [CrossRef]
- Caliari-Oliveira, C.; Yaochite, J.N.U.; Ramalho, L.N.Z.; Palma, P.V.B.; Carlos, D.; de Queiróz Cunha, F.; De Souza, D.A.; Frade, M.A.C.; Covas, D.T.; Malmegrim, K.C.R.; et al. Xenogeneic Mesenchymal Stromal Cells Improve Wound Healing and Modulate the Immune Response in an Extensive Burn Model. Cell Transplant. 2016, 25, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Hosni Ahmed, H.; Rashed, L.A.; Mahfouz, S.; Elsayed Hussein, R.; Alkaffas, M.; Mostafa, S.; Abusree, A. Can mesenchymal stem cells pretreated with platelet-rich plasma modulate tissue remodeling in a rat with burned skin? Biochem. Cell Biol. 2017, 95, 537–548. [Google Scholar] [CrossRef]
- Singer, D.D.; Singer, A.J.; Gordon, C.; Brink, P. The effects of rat mesenchymal stem cells on injury progression in a rat model. Acad. Emerg. Med. 2013, 20, 398–402. [Google Scholar] [CrossRef]
- Revilla, G.; Darwin, E.; Yanwirasti; Rantam, F.A. Effect of Allogeneic Bone Marrow-mesenchymal Stem Cells (BM-MSCs) to Accelerate Burn Healing of Rat on the Expression of Collagen Type I and Integrin α2β1. Pakistan J. Biol. Sci. PJBS 2016, 19, 345–351. [Google Scholar] [CrossRef]
- Oh, E.J.; Lee, H.W.; Kalimuthu, S.; Kim, T.J.; Kim, H.M.; Baek, S.H.; Zhu, L.; Oh, J.M.; Son, S.H.; Chung, H.Y.; et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J. Control. Release 2018, 279, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xia, B.; Lu, X.B.; Zhang, Z.J.; Li, Z.; Li, W.L.; Xiong, A.B.; Deng, L.; Tan, M.Y.; Huang, Y.C. Grafting of mesenchymal stem cell-seeded small intestinal submucosa to repair the deep partial-thickness burns. Connect. Tissue Res. 2016, 57, 388–397. [Google Scholar] [CrossRef]
- Xue, L.; Xu, Y.B.; Xie, J.L.; Tang, J.M.; Shu, B.; Chen, L.; Qi, S.H.; Liu, X.S. Effects of human bone marrow mesenchymal stem cells on burn injury healing in a mouse model. Int. J. Clin. Exp. Pathol. 2013, 6, 1327–1336. [Google Scholar]
- Zhang, J.; La, X.; Fan, L.; Li, P.; Yu, Y.; Huang, Y.; Ding, J.; Xing, Y. Immunosuppressive effects of mesenchymal stem cell transplantation in rat burn models. Int. J. Clin. Exp. Pathol. 2015, 8, 5129–5136. [Google Scholar] [PubMed]
- Pourfath, M.R.; Behzad-Behbahani, A.; Hashemi, S.S.; Derakhsahnfar, A.; Taheri, M.N.; Salehi, S. Monitoring wound healing of burn in rat model using human Wharton’s jelly mesenchymal stem cells containing cGFP integrated by lentiviral vectors. Iran. J. Basic Med. Sci. 2018, 21, 70–76. [Google Scholar] [PubMed]
- Gholipour-Kanani, A.; Bahrami, S.H.; Joghataie, M.T.; Samadikuchaksaraei, A.; Ahmadi-Taftie, H.; Rabbani, S.; Kororian, A.; Erfani, E. Tissue engineered poly(caprolactone)-chitosan-poly (vinyl alcohol) nanofibrous scaffolds for burn and cutting wound healing. IET Nanobiotechnol. 2014, 8, 123–131. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, S.; Fu, X. Autologous transplantation of bone marrow-derived mesenchymal stem cells: A promising therapeutic strategy for prevention of skin-graft contraction. Clin. Exp. Dermatol. 2012, 37, 497–500. [Google Scholar] [CrossRef]
- Arkoulis, N.; Watson, S.; Weiler-Mithoff, E. Stem cell enriched dermal substitutes for the treatment of late burn contractures in patients with major burns. Burns 2018, 44, 724–726. [Google Scholar] [CrossRef]
- Abo-Elkheir, W.; Hamza, F.; Elmofty, A.M.; Emam, A.; Abdl-Moktader, M.; Elsherefy, S.; Gabr, H. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: A case-control prospective study. Am. J. Stem Cells 2017, 6, 23–35. [Google Scholar]
- Chamuleau, S.A.J.; Van Der Naald, M.; Climent, A.M.; Kraaijeveld, A.O.; Wever, K.E.; Duncker, D.J.; Fernández-Avilés, F.; Bolli, R. Translational research in cardiovascular repair a call for a paradigm shift. Circ. Res. 2018, 122, 310–318. [Google Scholar] [CrossRef]
- Mogil, J.S.; Macleod, M.R. No publication without confirmation. Nature 2017, 542, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Ragan, B.G.; Park, J.H. Issues in outcomes research: An overview of randomization techniques for clinical trials. J. Athl. Train. 2008, 43, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, K.K.; Attri, J.P.; Singh, A.; Kaur, H.; Kaur, G. Basic concepts for sample size calculation: Critical step for any clinical trials! Saudi J. Anaesth. 2016, 10, 328–331. [Google Scholar] [CrossRef] [PubMed]

| Database | Search Strategy |
|---|---|
| PubMed | (Burn [MeSH] OR “Thermal injury” OR “second degree burn” OR “third degree burn” OR “skin burn” OR “burn trauma” OR “burn damage” OR “burn wound” OR “burn injur *”OR “burn patient *” OR “burn lesion” OR “burn complication” OR “deep burn” OR “thermal burn” OR “deep partial thickness” OR “full thickness”) AND (Mesenchymal stromal cells [MeSH] OR “Adipose mesenchymal stem cell *” OR “adipose derived mesenchymal cell *”) AND (“Adipose stromal cell *” OR “Adipose tissue-derived stem cell *” OR “adipose-derived adult stem cell *” OR “adipose derived regenerative cell *”) |
| Embase | (Exp/burn OR Exp/thermal injury) AND (Exp/mesenchymal stem cell OR Exp/mesenchymal stromal cell OR “Adipose mesenchymal stem cell *”, “adipose derived mesenchymal cell *”) OR (Exp/adipose derived stem cell OR (“adipose derived stem cell” OR “adipose stromal cell *” OR adipose tissue-derived stem cell *” OR “adipose-derived adult stem cell *” OR “adipose derived regenerative cell *”) |
| Sequence Generation | Allocation Concealment | Blinding of Personnel | Blinding of Personnel Assessors | Incomplete Outcome Data | Selective Reporting | Other Bias | Random Housing | Baseline Characteristics | Random Outcome Assessment | |
|---|---|---|---|---|---|---|---|---|---|---|
| Oryan et al. [27] | ||||||||||
| Atalay et al. [28] | ||||||||||
| Eyuboglu et al. [29] | ||||||||||
| Chen et al. [30] | ||||||||||
| Loder et al. [31] | ||||||||||
| Shokrgazor et al. [32] | ||||||||||
| Kaita et al. [33] | ||||||||||
| Bliley et al. [34] | ||||||||||
| Gholipourmalekabadi et al. [35] | ||||||||||
| Motamed et al. [36] | ||||||||||
| Abbas et al. [37] | ||||||||||
| Caliari-Oliveira et al. [38] | ||||||||||
| Ahmed et al. [39] | ||||||||||
| Singer et al. [40] | ||||||||||
| Revilla et al. [41] | ||||||||||
| Oh et al. [42] | ||||||||||
| Guo et al. [43] | ||||||||||
| Xue et al. [44] | ||||||||||
| Zhang et al. [45] | ||||||||||
| Pourfath et al. [46] | ||||||||||
| Gholipour-Kanani et al. [47] |
| (A) A visual representation of the risk of bias in human case series studies. | ||||||||||||
| Study Question/Objective | Study Population | Consecutive Cases | Comparable Subjects | Description of Intervention | Outcome Measures | Follow-Up Length | Statistical Analysis | Well Described Results | ||||
| Xu et al., 2012 [48] | ||||||||||||
| Arkilous et al., 2018 [49] | ||||||||||||
| (B) A visual representation of the risk of bias in human case–control studies. | ||||||||||||
| Objective | Study Population | Sample Size Justification | Controls | Use of Inclusion/Exclusion Criteria | Case Definition | Random Selection | Concurrent Controls | Exposure | Exposure Measurement | Blinding | Statistical Analysis | |
| Abo-Elkheir et al., 2017 [50] | NA | NA | NA | |||||||||
| Study | Population | Intervention | Comparison | Outcome (Global) | ||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Species | Type | Origin | Delivery | Dose | |||
| Oryan et al., 2019 [27] | 12 | R | ASC | Murine | Injection | 1 × 106 | ASC + Aloe Vera Aloe Vera Aloe Vera + DBM DBM | Positive, significantly better than other groups |
| Atalay et al., 2014 [28] | 20 | R | ASC * | Murine | Injection | 4 × 106 | ASC Control | Positive |
| Eyuboglu et al., 2018 [29] | 20 | R | ASC * | Murine | Injection | 4 × 106 | ASC Control | Positive |
| Chen et al., 2017 [30] | 6 | R | ASC | Murine | Injection | 1 × 106 | ASC Control | Positive |
| Loder et al., 2015 [31] | 20 | M | ASC | Murine | Injection | 1 × 106 | ASC AT AT + ASC Sham | Positive, significantly better than non-stem cell groups |
| Shokrgazor et al., 2012 [32] | 10 | R | ASC | Murine | Graft | 5 × 105 | ASC Control | Positive |
| Kaita et al., 2019 [33] | 18 | M | ASC | Human | Graft | 5 × 104 | Fresh Frozen Control | Positive |
| Bliley et al., 2016 [34] | 24 | M | ASC | Human | Injection | 6.8 × 106 | ASC Control | Positive, but limited |
| Gholipourmalekabadi et al., 2018 [35] | 45 | M | ASC | Human | Graft | 1 × 104 | HAM HAM + ASC Control | Positive. More significant in HAM + ASC |
| Motamed et al., 2017 [36] | 24 | R | ASC | Human | Graft | 5 × 105 | HAM HAM + ASC Control | Positive. More significant in HAM + ASC |
| Abbas et al., 2018 [37] | 40 | R | ASC, BM-MSC, DPSC | Human | Injection | 1 × 106 | BM-MSC ASC DPSC Control | Positive, no difference between choice of stem cells |
| Caliari-Oliveira et al., 2016 [38] | 54 | R | BM-MSC | Murine | Injection | 5 × 106 | MSC Control | Positive |
| Ahmed et al., 2017 [39] | 36 | R | BM-MSC | Murine | Injection | 1 × 106 | MSC Control Sham | Positive |
| Singer et al., 2013 [40] | 20 | R | BM-MSC | Murine | Injection | 1 × 106 | MSC Control | Positive |
| Revilla et al., 2016 [41] | 12 | R | BM-MSC | Murine | Injection | 2 × 106 | MSC Control | Positive |
| Oh et al., 2018 [42] | 30 | M | BM-MSC | Murine | Injection | 5 × 105 | MSC Control Sham | Positive |
| Guo et al., 2016 [43] | 48 | R | BM-MSC | Murine | Graft | 5 × 105 | SIS SIS + MSC Control | Positive. More significant in SIS + MSC |
| Xue et al., 2013 [44] | 60 | M | BM-MSC | Human | Injection | 1 × 106 | MSC Control | Positive |
| Zhang et al., 2015 [45] | 84 | R | UC-MSC | Human | Injection | 2 × 106 | MSC Control | Positive |
| Pourfath et al., 2018 [46] | 24 | R | UC-MSC | Human | Spray | 5 × 105 | MSC Control | Positive |
| Gholipour-Kanani et al., 2014 [47] | 12 | R | UC-MSC | Human | Graft | 4 × 104 | MSC Control | Positive |
| Study | Population | Intervention | Comparison | Outcome (Global) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Species | Type | Origin | Delivery | Dose | |||||
| Xu et al., 2012 [48] | 1 | H | BM-MSC | Autologous | Injection | 2.1 × 106/mL | BM-MSC + Decellularized allogeneic dermal matrix + Skin graft | Decellularized allogeneic dermal matrix + Skin graft | Positive | |
| Arkoulis et al., 2018 [49] | 2 | H | ASC * | Autologous | Topical | 46,400 /cm2 | Pre-intervention | Post-intervention | Positive | |
| Abo-Elkheir et al., 2017 [50] | 60 | H | BM-MSC | Autologous | Injection | 1 × 105/mL/cm2 × 2 | BM-MSC + dressing with gentamicinointment | UC-MSC + dressing with gentamicinointment | Standard treatment | Positive |
| UC-MSC | Allogeneic | |||||||||
| Wound Healing | Inflammation | Proliferation | Remodeling/Scarring | |||
|---|---|---|---|---|---|---|
| Neovascularization | Granulation | Re-Epithelialization | ||||
| Oryan et al., 2019 [27] | ||||||
| Atalay et al., 2014 [28] | ||||||
| Eyuboglu et al., 2018 [29] | ||||||
| Chen et al., 2017 [30] | ||||||
| Loder et al., 2015 [31] | ||||||
| Shokrgazor et al., 2012 [32] | ||||||
| Kaita et al., 2019 [33] | ||||||
| Bliley et al., 2016 [34] | ||||||
| Gholipourmalekabadi et al., 2018 [35] | ||||||
| Motamed et al., 2017 [36] | ||||||
| Abbas et al., 2018 [37] | ||||||
| Caliari-Oliveira et al., 2016 [38] | ||||||
| Ahmed et al., 2017 [39] | ||||||
| Singer et al., 2013 [40] | ||||||
| Revilla et al., 2016 [41] | ||||||
| Oh et al., 2018 [42] | ||||||
| Guo et al., 2016 [43] | ||||||
| Xue et al., 2013 [44] | ||||||
| Zhang et al., 2015 [45] | ||||||
| Pourfath et al., 2018 [46] | ||||||
| Gholipour-Kanani et al., 2014 [47] | ||||||
| Wound Healing | Inflammation | Proliferation | Remodeling/Scarring | |
|---|---|---|---|---|
| Xu et al., 2012 [48] | ||||
| Arkoulis et al., 2018 [49] | ||||
| Abo-Elkheir et al., 2017 [50] |
| Study | Wound Healing | Inflammation | Proliferation | Remodeling/Scarring | ||
|---|---|---|---|---|---|---|
| Neovascularization | Granulation | Re-Epithelialization | ||||
| Oryan et al., 2019 [27] | Wound area, Rate of wound closure (NI) | Inflammation markers (visual inspection, NI). Inflammatory cell infiltration (H, BI). IL-1b, TGF-β1, bFGF (qPCR, BI) | Capillary density (H, BI) | Collagen structure (SEM, BI). Number of fibroblasts and fibrocytes (H, BI). Collagen level (Hydroxyproline, BI) | Epidermal formation (H, BI) | Connective tissue arrangement (H, BI) |
| Atalay et al., 2014 [28] | Polymorphonuclear and mononuclear inflammatory infiltrate score (H, BI) | VEGF index (VEGF; H + I, BI) | Cell proliferation index (PCNA; H + I, BI) | |||
| Eyuboglu et al., 2018 [29] | Area of necrosis (NI) | Neutrophil score (H, BI) | Capillary count (Angiography + H, BI). Vascular density grading (H, BI). Endothelial count (vWF; H + I, BI) | Epithelial thickness (H, BI) | Fibrosis gradient (Masson’s trichrome, BI) | |
| Chen et al., 2017 [30] | Wound area, Rate of wound closure (NI) | Lymphocytic inflammatory infiltration (H, BI) | Epithelial regeneration (H, BI) | Pathologic dermal fibrosis (H, BI) | ||
| Loder et al., 2015 [31] | Wound area closure (NI). Wound depth, Rate of wound closure (BI) | Endothelial count (CD-31; H + I, BI) | Proliferation (Ki67; H + I, BI) | |||
| Shokrgazor et al., 2012 [32] | Wound area (NI) | Epidermal formation (H, BI) | ||||
| Kaita et al., 2019 [33] | Wound area, Rate of wound closure (NI) | Neovascularization (IB4; I, BI) | Collagen production (Picro-Sirus Red, Col I/III; H + WB + qPCR, BI) | Skin thickness ratio (Masson’s trichrome, BI) | ||
| Bliley et al., 2016 [34] | Wound area, Rate of wound closure, Wound area, Time to healing (NI) | Vascularity (CD31; H + I, BI) | Collagen production (Picro-Sirius Red, Masson’s trichrome; H) (Col I, Col III; qPCR, BI) | Wound contraction (α-SMA; qPCR, BI) | Collagen production (Col I and III; qPCR, BI) | |
| Gholipourmalekabadi et al., 2018 [35] | Wound area, Rate of wound closure, Wound area (NI). Wound-healing scoring (H, BI) | Acute inflammatory cells (H, BI). Localized Inflammatory Response (MIP2, TNFα1, and TGFβ1; qPCR, BI) | Capillary density (CD31; I, BI). Neovascularization score (CD31, VEGF- α1, VEGFR2; I, BI). Neovascularization rate (IL-1b, bFGF, VEGF-α1, VEGFR2; qPCR, BI) | Deposition of the extracellular matrix (H, BI). Collagen deposition score (Masson’s trichrome, BI). Density of Col I, III, and IV (I, BI). | Hair follicle formation (H, BI). Re-epithelialization (H, BI), Epidermal Thickness Index. | Maturation (Masson’s trichrome, BI). Scar formation (Col I, III, and IV; I) (Col I, III, IV, MIP-2, TGFβ1, TNFα1, MMP-1, MMP-2; qPCR, BI). Scar Elevation Index. |
| Motamed et al., 2017 [36] | Wound area, Rate of wound closure (NI) | Acute inflammatory cells (polymorphonuclear cells, eosinophils; H + Masson’s trichrome, BI), Chronic inflammatory cells (histocytes, lymphocytes, plasma cells; H + Masson’s trichrome, BI) | Epidermal and dermal structures, re-epithelialization, epithelium thickness, rete-ridges, dermal appendages (H + Masson’s trichrome, BI) | Pathologic dermal fibrosis (H + Masson’s trichrome, BI) | ||
| Abbas et al., 2018 [37] | Area of necrosis (NI) | Inflammatory Cell Infiltration (myeloperoxidase activity, BI) | Microvascular density (CD31; H + I, BI) | |||
| Caliari-Oliveira et al., 2016 [38] | Wound area Rate of wound closure (NI) | Bacterial contamination (swabs, NI). Total polymorphonuclear inflammatory cells score (H, BI). Neutrophils accumulation (myeloperoxidase assay, BI). CD4+ T-cells and CD8+ T-cells (Flow, BI). IL-10, IL-6, TGF-β, CINC-1 (ELISA, BS) | Vascularization score (H, BI) | Granulation tissue thickness score (H, BI) | Collagen fiber score (H, BI) | |
| Ahmed et al., 2017 [39] | Acute inflammatory cells (H, BI). IL-10, TNF-α, TGF-β (ELISA, BS) | Capillaries (H, BI), TGF-β (ELISA, BS). PDGF (I, BI). ANG-1, ANG-2 (qPCR, BI) | TGF-β (ELISA, BS). PDGF (I, BI). Vimentin (qPCR, BI) | Epithelialization (H, BS), TGF-β (ELISA, BS). MMP-1, TIMP-2 (qPCR, BI) | TGF-β (ELISA, BS) | |
| Singer et al., 2013 [40] | Area of necrosis (NI) | |||||
| Revilla et al., 2016 [41] | Wound appearance (NI) | Collagen type I fiber thickness, Integrin a2b1 (H + I, BI) | ||||
| Oh et al., 2018 [42] | Inflammatory cell infiltration (Masson’s trichrome, BI) | Collagen production (Masson’s trichrome, BI). TGF-β1 and VEGF (WB, BI) | ||||
| Guo et al., 2016 [43] | Wound area, Rate of wound healing (NI). Wound maturity score (H, BI) | Capillary density (vWF; I, BI) | Granulation score (Collagen; Masson’s trichrome, BI) | Neoepithelium length (H, BI). Epidermal cell proliferation (Ki-67; I, BI) | ||
| Xue et al., 2013 [44] | Wound area, Rate of wound healing (NI) | Capillary density (H, BI). VEGF, Ang-1/2, CD31 (qPCR, WB, BI). | ||||
| Zhang et al., 2015 [45] | Wound healing rate and time (NI) | WBC (count, BS). CRP (nephelometric immunoassay method, BS). IFN-γ, TNF-α, IL-6, IL-10 (ELISA, BS) | Capillary density (H, BI) | Granulation tissue amount (H, BI). Number of fibroblasts (H, BI) | ||
| Pourfath et al., 2018 [46] | Granulation (H, BI) | Re-epithelialization (H, BI) | ||||
| Gholipour-Kanani et al., 2014 [47] | Wound area (NI). Total wound healing score (H, BI) | Inflammation markers (visual inspection, NI). Inflammatory cell infiltration (H, BI) | Collagen regeneration and granulation tissue thickness (H, BI) | Epithelial regeneration, appendage (H, BI) | ||
| Study | Wound Healing | Inflammation | Proliferation | Remodeling/Scarring |
|---|---|---|---|---|
| Xu et al., 2012 [48] | Overall healing (visual) | Infection (visual) | Contracture (visual) | |
| Arkoulis et al., 2018 [49] | Contracture (visual) | |||
| Abo-Elkheir et al., 2017 [50] | Type of burn Onset, cause, mechanism, site, wound percentage (Lund and Browder), area of wound, depth of wound, rate of healing | Infection (visual) | Hypertrophic scars, keloid, contracture, and pigmentation (visual) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriksen, J.L.; Sørensen, N.B.; Fink, T.; Zachar, V.; Porsborg, S.R. Systematic Review of Stem-Cell-Based Therapy of Burn Wounds: Lessons Learned from Animal and Clinical Studies. Cells 2020, 9, 2545. https://doi.org/10.3390/cells9122545
Henriksen JL, Sørensen NB, Fink T, Zachar V, Porsborg SR. Systematic Review of Stem-Cell-Based Therapy of Burn Wounds: Lessons Learned from Animal and Clinical Studies. Cells. 2020; 9(12):2545. https://doi.org/10.3390/cells9122545
Chicago/Turabian StyleHenriksen, Josefine Lin, Nana Brandborg Sørensen, Trine Fink, Vladimir Zachar, and Simone Riis Porsborg. 2020. "Systematic Review of Stem-Cell-Based Therapy of Burn Wounds: Lessons Learned from Animal and Clinical Studies" Cells 9, no. 12: 2545. https://doi.org/10.3390/cells9122545
APA StyleHenriksen, J. L., Sørensen, N. B., Fink, T., Zachar, V., & Porsborg, S. R. (2020). Systematic Review of Stem-Cell-Based Therapy of Burn Wounds: Lessons Learned from Animal and Clinical Studies. Cells, 9(12), 2545. https://doi.org/10.3390/cells9122545