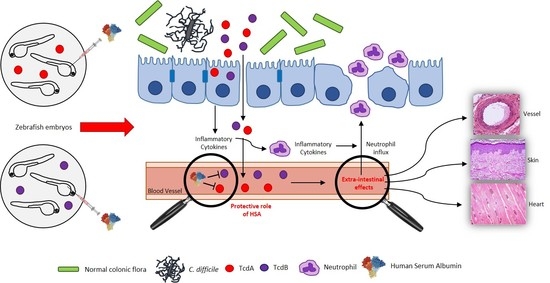
Extra-Intestinal Effects of C. difficile Toxin A and B: An In Vivo Study Using the Zebrafish Embryo Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Care and Use Statement
2.2. Embryos Preparation and Treatment
2.3. Histology of the Skin
2.4. Protein Extraction and Western Blot
2.5. RNA Extraction and Real-Time Quantitative PCR (qRT-PCR)
2.6. Sudan Black Staining
2.7. Cell Culture Conditions
2.8. MTT and LDH Assay
2.9. Immunostaining
2.10. Image Analysis
2.11. Statistical Analysis
2.12. Data Availability and Statement
3. Results
3.1. Toxins Effects on Zebrafish Vitality
3.2. Toxins Effects on the Zebrafish Cardiac System
3.3. Toxins Effects on the Zebrafish Vascular System
3.4. C. difficile Toxins Activate Zebrafish Immune System and Inflammation
3.5. TcdA Induces Skin Alteration in Zebrafish Embryos
3.6. Human Serum Albumin Protects Zebrafish Embryos towards TcdA Intoxication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care—Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile Infection. N. Engl. J. Med. 2015, 373, 287–288. [Google Scholar] [CrossRef]
- Kuehne, S.A.; Cartman, S.T.; Heap, J.T.; Kelly, M.L.; Cockayne, A.; Minton, N.P. The role of toxin A and toxin B in Clostridium difficile infection. Nat. Cell Biol. 2010, 467, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Zhang, H.; Cai, C.; Zhu, S.; Zhou, Y.; Yang, X.; He, R.; Li, C.; Guo, S.; Li, S.; et al. Chondroitin sulfate proteoglycan 4 functions as the cellular receptor for Clostridium difficile toxin B. Cell Res. 2015, 25, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, S.; Ascenzi, P.; Siarakas, S.; Petrosillo, N.; Di Masi, A. Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects. Toxins 2016, 8, 134. [Google Scholar] [CrossRef]
- Garey, K.W.; Sethi, S.; Yadav, Y.; Dupont, H. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J. Hosp. Infect. 2008, 70, 298–304. [Google Scholar] [CrossRef]
- Di Bella, S.; Di Masi, A.; Turla, S.; Ascenzi, P.; Gouliouris, T.; Petrosillo, N. The Protective Role of Albumin in Clostridium difficile Infection: A Step Toward Solving the Puzzle. Infect. Control. Hosp. Epidemiol. 2015, 36, 1478–1479. [Google Scholar] [CrossRef]
- Kumarappa, V.S.; Patel, H.; Shah, A.; Baddoura, W.; Debari, V.A. Temporal changes in serum albumin and total protein in patients with hospital-acquired Clostridium difficile infection. Ann. Clin. Lab. Sci. 2014, 44. [Google Scholar]
- Tabak, Y.P.; Johannes, R.S.; Sun, X.; Nunez, C.M.; McDonald, L.C. Predicting the risk for hospital-onset Clostridium difficile infection (HO-CDI) at the time of inpatient admission: HO-CDI risk score. Infect. Control. Hosp. Epidemiol. 2015, 36, 695–701. [Google Scholar] [CrossRef]
- Walker, A.S.; Eyre, D.W.; Wyllie, D.H.; Dingle, K.E.; Griffiths, D.; Shine, B.; Oakley, S.; O’Connor, L.; Finney, J.; Vaughan, A.; et al. Relationship between bacterial strain type, host biomarkers and mortality in Clostridium difficile infection. Clin. Infect. Dis. 2013, 56, 1589–1600. [Google Scholar] [CrossRef]
- Di Masi, A.; Leboffe, L.; Polticelli, F.; Tonon, F.; Zennaro, C.; Caterino, M.; Stano, P.; Fischer, S.; Hägele, M.; Müller, M.; et al. Human Serum Albumin Is an Essential Component of the Host Defense Mechanism against Clostridium difficile Intoxication. J. Infect. Dis. 2018, 218, 1424–1435. [Google Scholar] [CrossRef]
- Yu, H.; Chen, K.; Wu, J.; Yang, Z.; Shi, L.; Barlow, L.L.; Aronoff, D.M.; Garey, K.W.; Savidge, T.C.; Von Rosenvinge, E.C.; et al. Identification of Toxemia in Patients with Clostridium difficile Infection. PLoS ONE 2015, 10, e0124235. [Google Scholar] [CrossRef]
- Jacob, S.S.; Sebastian, J.C.; Hiorns, D.; Jacob, S.; Mukerjee, P.K. Clostridium difficile and acute respiratory distress syndrome. Heart Lung 2004, 33, 265–268. [Google Scholar] [CrossRef]
- Qualman, S.J.; Petric, M.; Karmali, M.A.; Smith, C.R.; Hamilton, S.R. Clostridium Difficile Invasion and Toxin Circulation in Fatal Pediatric Pseudomembranous Colitis. Am. J. Clin. Pathol. 1990, 94, 410–416. [Google Scholar] [CrossRef]
- Tsourous, G.I.; Raftopoulos, L.G.; Kafe, E.E.; Manoleris, E.K.; Makaritsis, K.P.; Pinis, S.G. A case of pseudomembranous colitis presenting with massive ascites. Eur. J. Intern Med. 2007, 18, 328–330. [Google Scholar] [CrossRef]
- Mattila, P.S.; Arkkila, P.; Tarkka, E.; Tissari, P.; Anttila, V.-J. Extraintestinal Clostridium difficile Infections. Clin. Infect. Dis. 2013, 57, e148–e153. [Google Scholar] [CrossRef]
- Garcáa-Lechuz, J.; Hernangómez, S.; Juan, R.S.; Peláez, T.; Alcalá, L.; Bouza, E.; Lechuz, J.M.G. Extra-intestinal infections caused by Clostridium difficile. Clin. Microbiol. Infect. 2001, 7, 453–457. [Google Scholar] [CrossRef]
- Hamm, E.E.; Voth, D.E.; Ballard, J.D. Identification of Clostridium difficile toxin B cardiotoxicity using a zebrafish embryo model of intoxication. Proc. Natl. Acad. Sci. USA 2006, 103, 14176–14181. [Google Scholar] [CrossRef]
- Miller, J.D.; Neely, M.N. Zebrafish as a model host for streptococcal pathogenesis. Acta Trop. 2004, 91, 53–68. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish; University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- Delov, V.; Muth-Köhne, E.; Schäfers, C.; Fenske, M. Transgenic fluorescent zebrafish Tg(fli1:EGFP)y1 for the identification of vasotoxicity within the zFET. Aquat. Toxicol. 2014, 150, 189–200. [Google Scholar] [CrossRef]
- Tonon, F.; Zennaro, C.; Dapas, B.; Carraro, M.; Mariotti, M.; Grassi, M. Rapid and cost-effective xenograft hepatocellular carcinoma model in Zebrafish for drug testing. Int. J. Pharm. 2016, 515, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Zennaro, C.; Tonon, F.; Zarattini, P.; Clai, M.; Corbelli, A.; Carraro, M.; Marchetti, M.; Ronda, L.; Paredi, G.; Rastaldi, M.P.; et al. The renal phenotype of allopurinol-treated HPRT-deficient mouse. PLoS ONE 2017, 12, e0173512. [Google Scholar] [CrossRef]
- Novoa, B.; Bowman, T.V.; Zon, L.I.; Figueras, A. LPS response and tolerance in the zebrafish (Danio rerio). Fish Shellfish. Immunol. 2009, 26, 326–331. [Google Scholar] [CrossRef]
- Hanke, N.; Staggs, L.; Schroder, P.; Litteral, J.; Fleig, S.; Kaufeld, J.; Pauli, C.; Haller, H.; Schiffer, M. “Zebrafishing” for Novel Genes Relevant to the Glomerular Filtration Barrier. BioMed Res. Int. 2013, 2013, 658270. [Google Scholar] [CrossRef]
- Zennaro, C.; Mariotti, M.; Carraro, M.; Pasqualetti, S.; Corbelli, A.; Armelloni, S.; Li, M.; Ikehata, M.; Clai, M.; Artero, M.; et al. Podocyte developmental defects caused by adriamycin in zebrafish embryos and larvae: A novel model of glomerular damage. PLoS ONE 2014, 9, e98131. [Google Scholar] [CrossRef]
- Miura, G.I.; Yelon, D. A guide to analysis of cardiac phenotypes in the zebrafish embryo. Methods Cell Biol. 2011, 101, 161–180. [Google Scholar] [PubMed]
- Sergeeva, I.A.; Christoffels, V.M. Regulation of expression of atrial and brain natriuretic peptide, biomarkers for heart development and disease. Biochim. Biophys. Acta 2013, 1832, 2403–2413. [Google Scholar] [CrossRef]
- Koenig, A.L.; Baltrunaite, K.; Bower, N.I.; Rossi, A.; Stainier, D.Y.; Hogan, B.M.; Sumanas, S. Vegfa signaling promotes zebrafish intestinal vasculature development through endothelial cell migration from the posterior cardinal vein. Dev. Biol. 2016, 411, 115–127. [Google Scholar] [CrossRef]
- Huang, J.; Kelly, C.P.; Bakirtzi, K.; Gálvez, J.A.V.; Lyras, D.; Mileto, S.J.; Larcombe, S.; Xu, H.; Yang, X.; Shields, K.S.; et al. Clostridium difficile toxins induce VEGF-A and vascular permeability to promote disease pathogenesis. Nat. Microbiol. 2018, 4, 269–279. [Google Scholar] [CrossRef]
- Solomon, K. The host immune response to Clostridium difficile infection. Ther. Adv. Infect. Dis. 2013, 1, 19–35. [Google Scholar]
- Carroll, K.C.; Bartlett, J.G. Biology of Clostridium difficile: Implications for Epidemiology and Diagnosis. Annu. Rev. Microbiol. 2011, 65, 501–521. [Google Scholar] [CrossRef]
- Goonetilleke, A.; Harris, J.B. Clostridial neurotoxins. J. Neurol. Neurosurg. Psychiatry 2004, 75, 35–39. [Google Scholar] [CrossRef]
- Stevens, D.L.; Troyer, B.E.; Merrick, D.T.; Mitten, J.E.; Olson, R.D. Lethal effects and cardiovascular effects of purified alpha- and theta-toxins from Clostridium perfringens. J. Infect. Dis. 1988, 157, 272–279. [Google Scholar] [CrossRef]
- Naiditch, M.J.; Bower, A.G. Diphtheria: A study of 1,433 cases observed during a ten-year period at the Los Angeles County Hospital. Am. J. Med. 1954, 17, 229–245. [Google Scholar] [CrossRef]
- Trujillo, M.H.; Castillo, A.; Espana, J.; Manzo, A.; Zerpa, R. Impact of intensive care management on the prognosis of tetanus. Analysis of 641 cases. Chest 1987, 92, 63–65. [Google Scholar] [CrossRef]
- Suffredini, D.A.; Sampath-Kumar, H.; Li, Y.; Ohanjanian, L.; Remy, K.E.; Cui, X.; Eichacker, P.Q. Does Bacillus anthracis Lethal Toxin Directly Depress Myocardial Function? A Review of Clinical Cases and Preclinical Studies. Toxins 2015, 7, 5417–5434. [Google Scholar] [CrossRef]
- Alhamdi, Y.; Neill, D.R.; Abrams, S.T.; Malak, H.A.; Yahya, R.; Barrett-Jolley, R.; Wang, G.; Kadioglu, A.; Toh, C.-H. Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection. PLoS Pathog. 2015, 11, e1004836. [Google Scholar] [CrossRef]
- Bolz, D.D.; Li, Z.; McIndoo, E.R.; Tweten, R.K.; Bryant, A.E.; Stevens, D.L. Cardiac myocyte dysfunction induced by streptolysin O is membrane pore and calcium dependent. Shock 2015, 43, 178–184. [Google Scholar] [CrossRef]
- Musher, D.M.; Rueda, A.M.; Kaka, A.S.; Mapara, S.M. The Association between Pneumococcal Pneumonia and Acute Cardiac Events. Clin. Infect. Dis. 2007, 45, 158–165. [Google Scholar] [CrossRef]
- Buggey J, ElAmm CA: Myocarditis and cardiomyopathy. Curr. Opin. Cardiol. 2018, 33, 341–346. [CrossRef]
- Van Bruggen, N.; Thibodeaux, H.; Palmer, J.T.; Lee, W.P.; Fu, L.; Cairns, B.; Tumas, D.; Gerlai, R.; Williams, S.-P.; Campagne, M.V.L.; et al. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J. Clin. Investig. 1999, 104, 1613–1620. [Google Scholar] [CrossRef]
- Fernandez, C.E.; Bakovic, M.; Karra, R. Endothelial Contributions to Zebrafish Heart Regeneration. J. Cardiovasc. Dev. Dis. 2018, 5, 56. [Google Scholar] [CrossRef]
- Harvie, E.A.; Huttenlocher, A. Neutrophils in host defense: New insights from zebrafish. J. Leukoc. Biol. 2015, 98, 523–537. [Google Scholar] [CrossRef]
- Kobayashi, Y. The role of chemokines in neutrophil biology. Front. Biosci. 2008, 13, 2400–2407. [Google Scholar] [CrossRef]
- Yu, H.; Chen, K.; Sun, Y.; Carter, M.; Garey, K.W.; Savidge, T.C.; Devaraj, S.; Tessier, M.E.; Von Rosenvinge, E.C.; Kelly, C.P.; et al. Cytokines Are Markers of the Clostridium difficile-Induced Inflammatory Response and Predict Disease Severity. Clin. Vaccine Immunol. 2017, 24, e00037-17. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonon, F.; Di Bella, S.; Grassi, G.; Luzzati, R.; Ascenzi, P.; di Masi, A.; Zennaro, C. Extra-Intestinal Effects of C. difficile Toxin A and B: An In Vivo Study Using the Zebrafish Embryo Model. Cells 2020, 9, 2575. https://doi.org/10.3390/cells9122575
Tonon F, Di Bella S, Grassi G, Luzzati R, Ascenzi P, di Masi A, Zennaro C. Extra-Intestinal Effects of C. difficile Toxin A and B: An In Vivo Study Using the Zebrafish Embryo Model. Cells. 2020; 9(12):2575. https://doi.org/10.3390/cells9122575
Chicago/Turabian StyleTonon, Federica, Stefano Di Bella, Gabriele Grassi, Roberto Luzzati, Paolo Ascenzi, Alessandra di Masi, and Cristina Zennaro. 2020. "Extra-Intestinal Effects of C. difficile Toxin A and B: An In Vivo Study Using the Zebrafish Embryo Model" Cells 9, no. 12: 2575. https://doi.org/10.3390/cells9122575
APA StyleTonon, F., Di Bella, S., Grassi, G., Luzzati, R., Ascenzi, P., di Masi, A., & Zennaro, C. (2020). Extra-Intestinal Effects of C. difficile Toxin A and B: An In Vivo Study Using the Zebrafish Embryo Model. Cells, 9(12), 2575. https://doi.org/10.3390/cells9122575