Rho GTPases Signaling in Zebrafish Development and Disease
Abstract
1. Introduction
2. Rac1 in Zebrafish Development
2.1. Rac1 and Cell Motility in Different Cell Types
2.2. Rac1 in Cell Rearrangement and Survival
2.3. Rac1 in Pathology
3. Cdc42 in Zebrafish Development
3.1. Cdc42 in Cell Movement and Rearrangement
3.1.1. Gastrulation, Cell Migration
3.1.2. Filopodia and Cilia
3.1.3. Angiogenesis
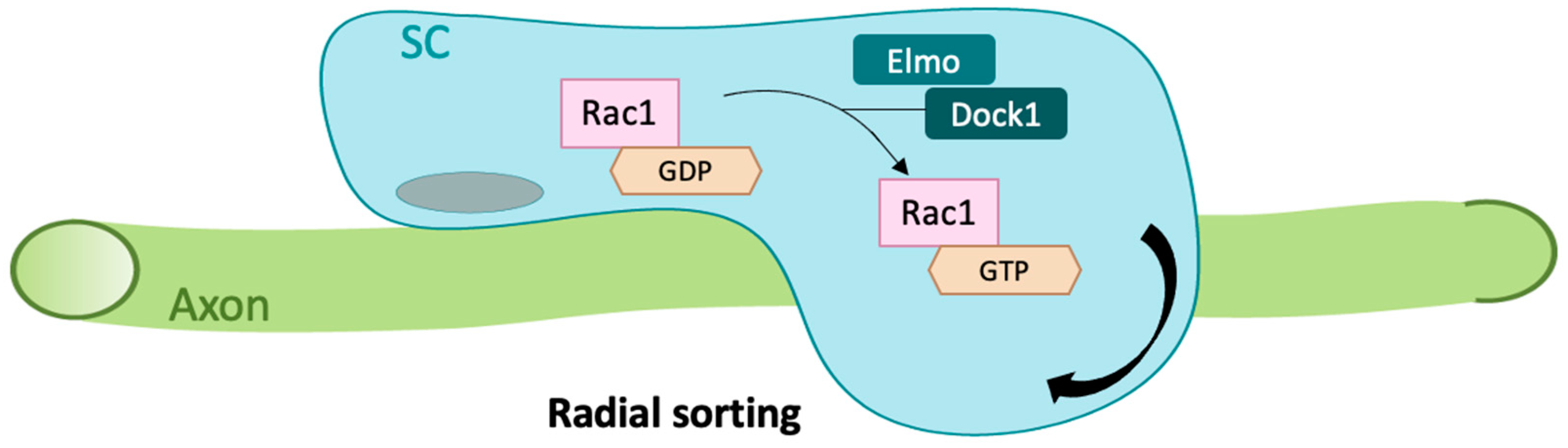
3.2. Cdc42 in Synapses and Regeneration
4. RhoA in Zebrafish Development
4.1. RhoA and Cell Dynamics
4.2. RhoA and Apoptosis
4.3. RhoA in Pathology and Regeneration
5. Zebrafish Tools to Study Small GTPases
| Transgenic/Mutant Lines | Observation | Citation | |
|---|---|---|---|
| Rac | |||
| Tg(MPO:mCherry-PA-Rac1)uw | Rac1 photoactivation in neutrophils | Rescues protrusion defects in neutrophils following PI(3)K inhibition | [21] |
| Tg(UAS:GFP-f2a-Rac1XX) | UAS driven expression of WT, DN or CA forms of Rac1 | XX = WT (au70), T17N (au71), Q61L (au72) | [110] |
| Tg(Tol2LUAS-GFP-DARac1-PATol2R) | UAS driven expression of a Dominant Active form of Rac1 | Accelerates gliomagenesis in DAAkt1 fish | [41] |
| Tg(mnx1:mcherry-PA-Rac1) | Rac1 photoactivation in motor neurons | Rescues motor axon guidance defects in plod3−/− | [33] |
| Tg(UAS:EGFP-DN-RAC1) | UAS driven expression of a DN form of Rac1 | [47] | |
| Tg(UAS:EGFP-CA-RAC1) | UAS driven expression of a CA form of Rac1 | [47] | |
| Rac2−/− | Loss of function mutation | Motility defects in neutrophils and macrophages | [49] |
| Tg(mpeg:mcherry-2A-rac2) | Rac2 expression in macrophages | [49] | |
| Tg(mpx:mcherry-2A-rac1) | Rac1 expression in neutrophils | [49] | |
| Tg(mpx:mcherry-2A-rac2) | Rac2 expression in neutrophils | [111] | |
| Tg(mpx:mcherry-2A-rac2D57N) | Dominant Negative Rac2 mutation in neutrophils | Defective neutrophil wound response | [111] |
| Tg(mitfa-V12Rac1GFP) | Constitutive Active Rac1 expression in melanocytes | Accelerates melanoma progression | [43] |
| Cdc42 | |||
| Tg(UAS: RaichuEV-Cdc42 NC), Negative Control | UAS driven expression of a Negative Control Cdc42 FRET biosensor | [77] | |
| Tg(UAS:mCherry-f2a-myc-Cdc42XX) | UAS driven expression of WT, DN or CA forms of Cdc42 | XX = WT (au66), T17N (au67), Q61L (au68), F37A (au69) | [110] |
| Tg(UAS:RaichuEV-Cdc42) | UAS driven expression of a Cdc42 FRET-based biosensor | [77] | |
| Tg(UAS:Myr-GFP-ACK42), Cdc42 inhibitor | UAS driven expression of a Cdc42 inhibitor FRET biosensor | [77] | |
| RhoA | |||
| Tg(UAS:mCherry-f2a-myc-RhoAXX) | UAS driven expression of WT, DN or CA forms of RhoA | XX = WT (au73), T19N (au74), Q63L (au75) | [110] |
| Tg(fabp10:rtTA2s-M2:TRE2:mCherry-rhoA) | WT RhoA expression in the liver | [98] | |
| Tg(fabp10:rtTA2s-M2:TRE2:mCherry-rhoAT19N) | DN RhoA expression in the liver | Accelerates death in KrasG12V fish with an increase in liver outgrowth and tumorigenesis | [98] |
| Tg(fabp10:rtTA2s-M2:TRE2:mCherry-rhoAG14V) | CA RhoA expression in the liver | Negatively regulates Kras oncogenic signaling | [98] |
| Plasmids | Citation | |
|---|---|---|
| Rac | ||
| pCS2-Rac1T17N | Rac1 DN expression | [112] |
| Tol2-MPO-mCherry-PA-Rac1-polyA | Photoactivated form of Rac1 in neutrophils | [21] |
| pTol2-Sox10:Rac1V12-P2A-mCherry | CA form of Rac1 in sox10 expressing cells (including Schwann cells) | [37] |
| mbp:RacV12-P2A-mCherry-CaaX | CA form of Rac1 in mbp expressing cells (Schwann cells and Oligodendrocytes) | [37] |
| WT-Rac1 | WT Rac1 expression | [6] |
| CA-Rac1 | CA Rac1 expression | [4,5,6,8] |
| Tol2-nxr-UAS-PA Rac1 mcherry | UAS driven expression of a photoactivatable form of Rac1 | [31] |
| DN-Rac1 | DN Rac1 expression | [4,5,6,7,9,10,71] |
| pCS2-rac1l | Rac1l expression | [39] |
| Rac-FRET-nos1-3′UTR | Rac FRET biosensor | [19,20] |
| DNRac-nos1-3′UTR | DN form of Rac FRET biosensor | [19,20] |
| FynTag RFP-T-T2A-Rac1 | Rac1 expression | [35] |
| FynTag RFP-T-T2A-dnRac1 | DN Rac1 expression | [35] |
| pCS2-TagRFP-PBD | Rac1 activity | [10] |
| psox10: PA-Rac1-mcherry | Photoactivatable form of Rac1 in sox10 expressing cells | [32] |
| Rac1 (P29S) | Mutant form of Rac1 (Melanoma) | [44] |
| Rac1- p.C18Y | Mutant form of Rac1 (microcephaly) | [45] |
| Rac1- p.N39S | Mutant form of Rac1 (microcephaly) | [45] |
| Raichu-Rac | Rac FRET biosensor | [18,109] |
| Cdc42 | ||
| pCS2-cdc42 | WT Cdc42 expression | [77] |
| Cdc42 T17N | DN form of Cdc42 | [7,9,71,77] |
| Cdc42 G12V | CA form of Cdc42 | [71,77] |
| Tol2 UAS:Cdc42-G12V-EGFP | UAS driven expression of a CA form of Cdc42 | [83] |
| Tol2 UAS:Cdc42- T17N-EGFP | UAS driven expression of a DN form of Cdc42 | [83] |
| Raichu-Cdc42 | Cdc42 FRET biosensor | [18,109] |
| RhoA | ||
| RhoA biosensor | RhoA FRET biosensor | [18,113] |
| RhoAT19N | DN form of RhoA | [98] |
| RhoAV14 | CA form of RhoA | [6,98] |
| WT-RhoA mRNA | WT RhoA expression | [6] |
| RhoA Q63L mRNA | CA form of RhoA | [106] |
| Morpholinos/gRNA/Drug Inhibitors or Activators | Sequence | Citation |
|---|---|---|
| Rac | ||
| siRac1ss, | CCCUAACACUCCAAUAAUUtt | [34] |
| Rac1as, | AAUUAUUGGAGUGUUAGGGtt | [34] |
| siRNAcontrol1ss, | UAGCCCACACCACGAUAGAtt | [34] |
| siRNAcontrol1as, | UCUAUCGUGUGGGCUAtt | [34] |
| siRNAcontrol2ss, | UCGACCAGGGCGGAUGUGtt | [34] |
| siRNAcontrol2as, | CACUAUCCGCCCUGGUCGAtt | [34] |
| NCS23766 (Rac1 inhibitor) | [23,44,48] | |
| Rac1 inhibitor I | [48] | |
| Rac1 inhibitor II | [48] | |
| TB-MO rac1l | 5′-CCACACACTTGATGGCCTGCATGAC-3′ | [39] |
| TB-MO rac1 | 5′-CCACACACTTTATGGCCTGCATCTG-3′ | [39] |
| TB-MO rac1 | 5′-GCCTGCATGGCAGCGAATGTCCCG-3′ | [39,91] |
| Activator of Rho/Rac/Cdc42 I | [46] | |
| Activator of Rac/Cdc42 II | [46] | |
| Cdc42 | ||
| Cdc42 gRNA#2 | GGGAGACGACTTCTTAACAGTGG | [108] |
| ML141 (Cdc42/Rac1 inhibitor) | [80] | |
| ACK42 | Cdc42-specific inhibitor, a cDNA encoding minimal Cdc42 binding domain of human ACK1 (amino acid 504–545) | [77] |
| siCDC42ss, | GGGUAAAACCUGUCUAUUAtt | [34] |
| siCDC42as, | UAAUAGACAGGUUUUACCCtt | [34] |
| RhoA | ||
| RhoA MO1 | 5′-TCCGTCGCCTCTCTTATGTCCGATA-3′ | [90] |
| RhoA MO2 | 5′-CTAGCCGTTTTGTTTTAGTCCAACG-3′ | [90] |
| Antibodies | Reference | Citation |
|---|---|---|
| Rac | ||
| Anti Rac1 | Upstate biotechnology | [38] |
| Anti-Rac1 | Millipore 05-389 | [114] |
| Anti-Rac1 | Abcam | [84] |
| Anti-Rac1 | Santa Cruz Biotechnology (C14, sc-217) | [89] |
| Cdc42 | ||
| Mouse monoclonal anti-Cdc42 (610929) | BD Transduction Laboratories) | [71] |
| Anti-Cdc42 | (Lifespan Biologicals) | [84] |
| Anti-Cdc42 | BD | [77] |
| RhoA | ||
| Anti-RhoA | Santa Cruz Biotechnology (119, sc-179) | [89] |
| Anti-RhoA-GTP | [89] |
| Primers for qPCR | Sequence | Citation |
|---|---|---|
| Rac | ||
| Rac1-F | GGTGAATCTGGGCTTATGGG | [47] |
| Rac1-R | TCAGGATACCACTTTGCACG | [47] |
| Rac2-F | ACTCTCCTACCC GCAGACG | [111] |
| Rac2-R | CACCTCTGGGTACCACTTGGC | [111] |
| Cdc42 | ||
| Zeb cdc42-F | 5′-GACAGTAGCCCTGTAAATGGTTG-3′ | [59] |
| Zeb cdc42-R | 5′-GTTAGAAAGTTCCCTGCTTGAGAG-3′ | [59] |
| RhoA | ||
| qrhoaa_F | TCCTGAGGTTTACGTTCCCA | [49] |
| qrhoaa_R | TGGCCAGCTGTATCCCATAG | [49] |
| qrhoab_F | ACAGGCTTCGTCCTCTTTCA | [49] |
| qrhoab_R | AATGTTTGACCTCAGGCGTC | [49] |
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Aspenstrom, P.; Ruusala, A.; Pacholsky, D. Taking Rho GTPases to the next level: The cellular functions of atypical Rho GTPases. Exp. Cell Res. 2007, 313, 3673–3679. [Google Scholar] [CrossRef] [PubMed]
- Riou, P.; Villalonga, P.; Ridley, A.J. Rnd proteins: Multifunctional regulators of the cytoskeleton and cell cycle progression. Bioessays 2010, 32, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Salas-Vidal, E.; Meijer, A.H.; Cheng, X.; Spaink, H.P. Genomic annotation and expression analysis of the zebrafish Rho small GTPase family during development and bacterial infection. Genomics 2005, 86, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, J.; Kramer, C.; Pothof, J.; Quaedvlieg, N.E.; Spaink, H.P.; Hammerschmidt, M. Has2 is required upstream of Rac1 to govern dorsal migration of lateral cells during zebrafish gastrulation. Development 2004, 131, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Shao, M.; Shi, D.L. Rac1 signalling coordinates epiboly movement by differential regulation of actin cytoskeleton in zebrafish. Biochem. Biophys. Res. Commun. 2017, 490, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Muerdter, C.P.; Knickerbocker, A.D.; Walsh, R.M.; Zepeda-Rivera, M.A.; Depner, K.H.; Sangesland, M.; Cisneros, T.B.; Kim, J.Y.; Sanchez-Vazquez, P.; et al. Cdc42 GTPase and Rac1 GTPase act downstream of p120 catenin and require GTP exchange during gastrulation of zebrafish mesoderm. Dev. Dyn. 2012, 241, 1545–1561. [Google Scholar] [CrossRef]
- Dumortier, J.G.; Martin, S.; Meyer, D.; Rosa, F.M.; David, N.B. Collective mesendoderm migration relies on an intrinsic directionality signal transmitted through cell contacts. Proc. Natl. Acad. Sci. USA 2012, 109, 16945–16950. [Google Scholar] [CrossRef]
- Dumortier, J.G.; David, N.B. The TORC2 component, Sin1, controls migration of anterior mesendoderm during zebrafish gastrulation. PLoS ONE 2015, 10, e0118474. [Google Scholar] [CrossRef]
- Giger, F.A.; David, N.B. Endodermal germ-layer formation through active actin-driven migration triggered by N-cadherin. Proc. Natl. Acad. Sci. USA 2017, 114, 10143–10148. [Google Scholar] [CrossRef]
- Woo, S.; Housley, M.P.; Weiner, O.D.; Stainier, D.Y. Nodal signaling regulates endodermal cell motility and actin dynamics via Rac1 and Prex1. J. Cell Biol. 2012, 198, 941–952. [Google Scholar] [CrossRef]
- Migeotte, I.; Grego-Bessa, J.; Anderson, K.V. Rac1 mediates morphogenetic responses to intercellular signals in the gastrulating mouse embryo. Development 2011, 138, 3011–3020. [Google Scholar] [CrossRef] [PubMed]
- Araya, C.; Tawk, M.; Girdler, G.C.; Costa, M.; Carmona-Fontaine, C.; Clarke, J.D. Mesoderm is required for coordinated cell movements within zebrafish neural plate in vivo. Neural Dev. 2014, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulou, E.; Galea, G.L.; Rolo, A.; Greene, N.D.; Copp, A.J. Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development 2017, 144, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Tawk, M.; Araya, C.; Lyons, D.A.; Reugels, A.M.; Girdler, G.C.; Bayley, P.R.; Hyde, D.R.; Tada, M.; Clarke, J.D. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature 2007, 446, 797–800. [Google Scholar] [CrossRef]
- Rolo, A.; Savery, D.; Escuin, S.; de Castro, S.C.; Armer, H.E.; Munro, P.M.; Mole, M.A.; Greene, N.D.; Copp, A.J. Regulation of cell protrusions by small GTPases during fusion of the neural folds. Elife 2016, 5, e13273. [Google Scholar] [CrossRef]
- Ohata, S.; Uga, H.; Okamoto, H.; Katada, T. Small GTPase R-Ras participates in neural tube formation in zebrafish embryonic spinal cord. Biochem. Biophys. Res. Commun. 2018, 501, 786–790. [Google Scholar] [CrossRef]
- Thomas, P.S.; Kim, J.; Nunez, S.; Glogauer, M.; Kaartinen, V. Neural crest cell-specific deletion of Rac1 results in defective cell-matrix interactions and severe craniofacial and cardiovascular malformations. Dev. Biol. 2010, 340, 613–625. [Google Scholar] [CrossRef]
- Matthews, H.K.; Marchant, L.; Carmona-Fontaine, C.; Kuriyama, S.; Larrain, J.; Holt, M.R.; Parsons, M.; Mayor, R. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 2008, 135, 1771–1780. [Google Scholar] [CrossRef]
- Kardash, E.; Reichman-Fried, M.; Maitre, J.L.; Boldajipour, B.; Papusheva, E.; Messerschmidt, E.M.; Heisenberg, C.P.; Raz, E. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat. Cell Biol. 2010, 12, 47–53. [Google Scholar] [CrossRef]
- Xu, H.; Kardash, E.; Chen, S.; Raz, E.; Lin, F. Gbetagamma signaling controls the polarization of zebrafish primordial germ cells by regulating Rac activity. Development 2012, 139, 57–62. [Google Scholar] [CrossRef]
- Yoo, S.K.; Deng, Q.; Cavnar, P.J.; Wu, Y.I.; Hahn, K.M.; Huttenlocher, A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev. Cell 2010, 18, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Palmby, T.R.; Gavard, J.; Amornphimoltham, P.; Zheng, Y.; Gutkind, J.S. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 2008, 22, 1829–1838. [Google Scholar] [CrossRef]
- Paatero, I.; Sauteur, L.; Lee, M.; Lagendijk, A.K.; Heutschi, D.; Wiesner, C.; Guzman, C.; Bieli, D.; Hogan, B.M.; Affolter, M.; et al. Junction-based lamellipodia drive endothelial cell rearrangements in vivo via a VE-cadherin-F-actin based oscillatory cell-cell interaction. Nat. Commun. 2018, 9, 3545. [Google Scholar] [CrossRef]
- Epting, D.; Wendik, B.; Bennewitz, K.; Dietz, C.T.; Driever, W.; Kroll, J. The Rac1 regulator ELMO1 controls vascular morphogenesis in zebrafish. Circ. Res. 2010, 107, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Schaker, K.; Bartsch, S.; Patry, C.; Stoll, S.J.; Hillebrands, J.L.; Wieland, T.; Kroll, J. The bipartite rac1 Guanine nucleotide exchange factor engulfment and cell motility 1/dedicator of cytokinesis 180 (elmo1/dock180) protects endothelial cells from apoptosis in blood vessel development. J. Biol. Chem. 2015, 290, 6408–6418. [Google Scholar] [CrossRef]
- Chen, L.; Liao, G.; Waclaw, R.R.; Burns, K.A.; Linquist, D.; Campbell, K.; Zheng, Y.; Kuan, C.Y. Rac1 controls the formation of midline commissures and the competency of tangential migration in ventral telencephalic neurons. J. Neurosci. 2007, 27, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Melendez, J.; Campbell, K.; Kuan, C.Y.; Zheng, Y. Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly. Dev. Biol. 2009, 325, 162–170. [Google Scholar] [CrossRef]
- Hua, Z.L.; Emiliani, F.E.; Nathans, J. Rac1 plays an essential role in axon growth and guidance and in neuronal survival in the central and peripheral nervous systems. Neural Dev. 2015, 10, 21. [Google Scholar] [CrossRef]
- Kassai, H.; Terashima, T.; Fukaya, M.; Nakao, K.; Sakahara, M.; Watanabe, M.; Aiba, A. Rac1 in cortical projection neurons is selectively required for midline crossing of commissural axonal formation. Eur. J. Neurosci. 2008, 28, 257–267. [Google Scholar] [CrossRef]
- Leone, D.P.; Srinivasan, K.; Brakebusch, C.; McConnell, S.K. The rho GTPase Rac1 is required for proliferation and survival of progenitors in the developing forebrain. Dev. Neurobiol. 2010, 70, 659–678. [Google Scholar] [CrossRef]
- Zhang, Y.; Nichols, E.L.; Zellmer, A.M.; Guldner, I.H.; Kankel, C.; Zhang, S.; Howard, S.S.; Smith, C.J. Generating intravital super-resolution movies with conventional microscopy reveals actin dynamics that construct pioneer axons. Development 2019, 146. [Google Scholar] [CrossRef]
- Nichols, E.L.; Smith, C.J. Pioneer axons employ Cajal’s battering ram to enter the spinal cord. Nat. Commun. 2019, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Wang, A.Y.; Boulanger-Weill, J.; Santoriello, C.; Foianini, S.; Lichtman, J.W.; Zon, L.I.; Arlotta, P. Long-Range Optogenetic Control of Axon Guidance Overcomes Developmental Boundaries and Defects. Dev. Cell 2020, 53, 577–588 e577. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, T.; Morita, K.; Omizu, S.; Kato, S.; Koriyama, Y. Mechanisms of RhoA inactivation and CDC42 and Rac1 activation during zebrafish optic nerve regeneration. Neurochem. Int. 2018, 112, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, T.K.; Dogan, T.; Hocking, J.C.; Scholz, R.P.; Mooz, J.; Anderson, C.L.; Karreman, C.; Meyer zu Heringdorf, D.; Schmidt, G.; Ruonala, M.; et al. IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J. 2012, 31, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.W.; Hsu, L.S. Zebrafish diras1 Promoted Neurite Outgrowth in Neuro-2a Cells and Maintained Trigeminal Ganglion Neurons In Vivo via Rac1-Dependent Pathway. Mol. Neurobiol. 2016, 53, 6594–6607. [Google Scholar] [CrossRef] [PubMed]
- Mikdache, A.; Fontenas, L.; Albadri, S.; Revenu, C.; Loisel-Duwattez, J.; Lesport, E.; Degerny, C.; Del Bene, F.; Tawk, M. Elmo1 function, linked to Rac1 activity, regulates peripheral neuronal numbers and myelination in zebrafish. Cell. Mol. Life Sci. 2020, 77, 161–177. [Google Scholar] [CrossRef]
- Tep, C.; Kim, M.L.; Opincariu, L.I.; Limpert, A.S.; Chan, J.R.; Appel, B.; Carter, B.D.; Yoon, S.O. Brain-derived neurotrophic factor (BDNF) induces polarized signaling of small GTPase (Rac1) protein at the onset of Schwann cell myelination through partitioning-defective 3 (Par3) protein. J. Biol. Chem. 2012, 287, 1600–1608. [Google Scholar] [CrossRef]
- Epting, D.; Slanchev, K.; Boehlke, C.; Hoff, S.; Loges, N.T.; Yasunaga, T.; Indorf, L.; Nestel, S.; Lienkamp, S.S.; Omran, H.; et al. The Rac1 regulator ELMO controls basal body migration and docking in multiciliated cells through interaction with Ezrin. Development 2015, 142, 174–184. [Google Scholar] [CrossRef]
- Haga, R.B.; Ridley, A.J. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases 2016, 7, 207–221. [Google Scholar] [CrossRef]
- Jung, I.H.; Leem, G.L.; Jung, D.E.; Kim, M.H.; Kim, E.Y.; Kim, S.H.; Park, H.C.; Park, S.W. Glioma is formed by active Akt1 alone and promoted by active Rac1 in transgenic zebrafish. Neuro Oncol. 2013, 15, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Tsai, J.C.; Tseng, Y.T.; Wu, M.S.; Liu, W.S.; Lam, H.I.; Yu, J.H.; Nozell, S.E.; Benveniste, E.N. Small G protein Rac GTPases regulate the maintenance of glioblastoma stem-like cells in vitro and in vivo. Oncotarget 2017, 8, 18031–18049. [Google Scholar] [CrossRef] [PubMed]
- Dalton, L.E.; Kamarashev, J.; Barinaga-Rementeria Ramirez, I.; White, G.; Malliri, A.; Hurlstone, A. Constitutive RAC activation is not sufficient to initiate melanocyte neoplasia but accelerates malignant progression. J. Investig. Dermatol. 2013, 133, 1572–1581. [Google Scholar] [CrossRef]
- Araiza-Olivera, D.; Feng, Y.; Semenova, G.; Prudnikova, T.Y.; Rhodes, J.; Chernoff, J. Suppression of RAC1-driven malignant melanoma by group A PAK inhibitors. Oncogene 2018, 37, 944–952. [Google Scholar] [CrossRef]
- Reijnders, M.R.F.; Ansor, N.M.; Kousi, M.; Yue, W.W.; Tan, P.L.; Clarkson, K.; Clayton-Smith, J.; Corning, K.; Jones, J.R.; Lam, W.W.K.; et al. RAC1 Missense Mutations in Developmental Disorders with Diverse Phenotypes. Am. J. Hum. Genet. 2017, 101, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Gee, H.Y.; Sadowski, C.E.; Aggarwal, P.K.; Porath, J.D.; Yakulov, T.A.; Schueler, M.; Lovric, S.; Ashraf, S.; Braun, D.A.; Halbritter, J.; et al. FAT1 mutations cause a glomerulotubular nephropathy. Nat. Commun. 2016, 7, 10822. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Lee, M.S.; Zhou, W. Dosage-dependent role of Rac1 in podocyte injury. Am. J. Physiol. Renal. Physiol. 2016, 310, F777–F784. [Google Scholar] [CrossRef]
- Gee, H.Y.; Saisawat, P.; Ashraf, S.; Hurd, T.W.; Vega-Warner, V.; Fang, H.; Beck, B.B.; Gribouval, O.; Zhou, W.; Diaz, K.A.; et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J. Clin. Investig. 2013, 123, 3243–3253. [Google Scholar] [CrossRef]
- Rosowski, E.E.; Deng, Q.; Keller, N.P.; Huttenlocher, A. Rac2 Functions in Both Neutrophils and Macrophages To Mediate Motility and Host Defense in Larval Zebrafish. J. Immunol. 2016, 197, 4780–4790. [Google Scholar] [CrossRef]
- Williams, D.A.; Tao, W.; Yang, F.; Kim, C.; Gu, Y.; Mansfield, P.; Levine, J.E.; Petryniak, B.; Derrow, C.W.; Harris, C.; et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood 2000, 96, 1646–1654. [Google Scholar]
- Hsu, A.Y.; Wang, D.; Gurol, T.; Zhou, W.; Zhu, X.; Lu, H.Y.; Deng, Q. Overexpression of microRNA-722 fine-tunes neutrophilic inflammation by inhibiting Rac2 in zebrafish. Dis. Models Mech. 2017, 10, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, L.; Parrini, M.C.; Mao, X.; Lopez, M.; Wu, C.; Marks, P.W.; Davidson, L.; Kwiatkowski, D.J.; Kirchhausen, T.; et al. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr. Biol. 2000, 10, 758–765. [Google Scholar] [CrossRef]
- Farhan, H.; Hsu, V.W. Cdc42 and Cellular Polarity: Emerging Roles at the Golgi. Trends Cell Biol. 2016, 26, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Woods, B.; Lew, D.J. Polarity establishment by Cdc42: Key roles for positive feedback and differential mobility. Small GTPases 2019, 10, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Tay, H.G.; Ng, Y.W.; Manser, E. A vertebrate-specific Chp-PAK-PIX pathway maintains E-cadherin at adherens junctions during zebrafish epiboly. PLoS ONE 2010, 5, e10125. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Ramirez, S.P.; Toledo-Jacobo, L.; Henson, J.H.; Shuster, C.B. Cdc42 controls primary mesenchyme cell morphogenesis in the sea urchin embryo. Dev. Biol. 2018, 437, 140–151. [Google Scholar] [CrossRef]
- Penzo-Mendez, A.; Umbhauer, M.; Djiane, A.; Boucaut, J.C.; Riou, J.F. Activation of Gbetagamma signaling downstream of Wnt-11/Xfz7 regulates Cdc42 activity during Xenopus gastrulation. Dev. Biol. 2003, 257, 302–314. [Google Scholar] [CrossRef]
- Yeh, C.M.; Liu, Y.C.; Chang, C.J.; Lai, S.L.; Hsiao, C.D.; Lee, S.J. Ptenb mediates gastrulation cell movements via Cdc42/AKT1 in zebrafish. PLoS ONE 2011, 6, e18702. [Google Scholar] [CrossRef][Green Version]
- Tahara, N.; Bessho, Y.; Matsui, T. Celf1 is required for formation of endoderm-derived organs in zebrafish. Int. J. Mol. Sci. 2013, 14, 18009–18023. [Google Scholar] [CrossRef]
- Wong, E.W.; Cheng, C.Y. Polarity proteins and cell-cell interactions in the testis. Int. Rev. Cell Mol. Biol. 2009, 278, 309–353. [Google Scholar] [CrossRef]
- Murphy, A.M.; Montell, D.J. Cell type-specific roles for Cdc42, Rac, and RhoL in Drosophila oogenesis. J. Cell Biol. 1996, 133, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Welchman, D.P.; Mathies, L.D.; Ahringer, J. Similar requirements for CDC-42 and the PAR-3/PAR-6/PKC-3 complex in diverse cell types. Dev. Biol. 2007, 305, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, W.; Xiang, Y.; Zhang, W.; Zhang, H.; Jia, K.; Yi, M. Maternal miR-202-5p is required for zebrafish primordial germ cell migration by protecting small GTPase Cdc42. J. Mol. Cell Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Diz-Munoz, A.; Romanczuk, P.; Yu, W.; Bergert, M.; Ivanovitch, K.; Salbreux, G.; Heisenberg, C.P.; Paluch, E.K. Steering cell migration by alternating blebs and actin-rich protrusions. BMC Biol. 2016, 14, 74. [Google Scholar] [CrossRef]
- Cvejic, A.; Hall, C.; Bak-Maier, M.; Flores, M.V.; Crosier, P.; Redd, M.J.; Martin, P. Analysis of WASp function during the wound inflammatory response--live-imaging studies in zebrafish larvae. J. Cell Sci. 2008, 121, 3196–3206. [Google Scholar] [CrossRef]
- Jones, R.A.; Feng, Y.; Worth, A.J.; Thrasher, A.J.; Burns, S.O.; Martin, P. Modelling of human Wiskott-Aldrich syndrome protein mutants in zebrafish larvae using in vivo live imaging. J. Cell Sci. 2013, 126, 4077–4084. [Google Scholar] [CrossRef]
- Nobes, C.D.; Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef]
- Stanganello, E.; Hagemann, A.I.; Mattes, B.; Sinner, C.; Meyen, D.; Weber, S.; Schug, A.; Raz, E.; Scholpp, S. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat. Commun. 2015, 6, 5846. [Google Scholar] [CrossRef]
- Malicki, J.J.; Johnson, C.A. The Cilium: Cellular Antenna and Central Processing Unit. Trends Cell Biol. 2017, 27, 126–140. [Google Scholar] [CrossRef]
- Tobin, J.L.; Beales, P.L. Restoration of renal function in zebrafish models of ciliopathies. Pediatric Nephrol. 2008, 23, 2095–2099. [Google Scholar] [CrossRef]
- Choi, S.Y.; Chacon-Heszele, M.F.; Huang, L.; McKenna, S.; Wilson, F.P.; Zuo, X.; Lipschutz, J.H. Cdc42 deficiency causes ciliary abnormalities and cystic kidneys. J. Am. Soc. Nephrol. 2013, 24, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Baek, J.I.; Zuo, X.; Kim, S.H.; Dunaief, J.L.; Lipschutz, J.H. Cdc42 and sec10 Are Required for Normal Retinal Development in Zebrafish. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Heynen, S.R.; Meneau, I.; Caprara, C.; Samardzija, M.; Imsand, C.; Levine, E.M.; Grimm, C. CDC42 is required for tissue lamination and cell survival in the mouse retina. PLoS ONE 2013, 8, e53806. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.I.; Kwon, S.H.; Zuo, X.; Choi, S.Y.; Kim, S.H.; Lipschutz, J.H. Dynamin Binding Protein (Tuba) Deficiency Inhibits Ciliogenesis and Nephrogenesis in Vitro and in Vivo. J. Biol. Chem. 2016, 291, 8632–8643. [Google Scholar] [CrossRef]
- Buchner, D.A.; Su, F.; Yamaoka, J.S.; Kamei, M.; Shavit, J.A.; Barthel, L.K.; McGee, B.; Amigo, J.D.; Kim, S.; Hanosh, A.W.; et al. pak2a mutations cause cerebral hemorrhage in redhead zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 13996–14001. [Google Scholar] [CrossRef]
- Liu, J.; Fraser, S.D.; Faloon, P.W.; Rollins, E.L.; Vom Berg, J.; Starovic-Subota, O.; Laliberte, A.L.; Chen, J.N.; Serluca, F.C.; Childs, S.J. A betaPix Pak2a signaling pathway regulates cerebral vascular stability in zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 13990–13995. [Google Scholar] [CrossRef]
- Wakayama, Y.; Fukuhara, S.; Ando, K.; Matsuda, M.; Mochizuki, N. Cdc42 mediates Bmp-induced sprouting angiogenesis through Fmnl3-driven assembly of endothelial filopodia in zebrafish. Dev. Cell 2015, 32, 109–122. [Google Scholar] [CrossRef]
- Papadopoulos, T.; Korte, M.; Eulenburg, V.; Kubota, H.; Retiounskaia, M.; Harvey, R.J.; Harvey, K.; O’Sullivan, G.A.; Laube, B.; Hulsmann, S.; et al. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 2007, 26, 3888–3899. [Google Scholar] [CrossRef]
- Gerhardt, H.; Ruhrberg, C.; Abramsson, A.; Fujisawa, H.; Shima, D.; Betsholtz, C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev. Dyn. 2004, 231, 503–509. [Google Scholar] [CrossRef]
- Fantin, A.; Lampropoulou, A.; Gestri, G.; Raimondi, C.; Senatore, V.; Zachary, I.; Ruhrberg, C. NRP1 Regulates CDC42 Activation to Promote Filopodia Formation in Endothelial Tip Cells. Cell. Rep. 2015, 11, 1577–1590. [Google Scholar] [CrossRef]
- Imai, F.; Ladle, D.R.; Leslie, J.R.; Duan, X.; Rizvi, T.A.; Ciraolo, G.M.; Zheng, Y.; Yoshida, Y. Synapse Formation in Monosynaptic Sensory-Motor Connections Is Regulated by Presynaptic Rho GTPase Cdc42. J. Neurosci. 2016, 36, 5724–5735. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Wang, H.; Soderling, S.H.; Yasuda, R. Loss of Cdc42 leads to defects in synaptic plasticity and remote memory recall. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, S.; Blechman, J.; Gliksberg, M.; Gordon, L.; Rotkopf, R.; Dadosh, T.; Shimoni, E.; Levkowitz, G. Robo2 regulates synaptic oxytocin content by affecting actin dynamics. Elife 2019, 8, e45650. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; He, Q.; Li, G.; Ma, J.; Zhong, T.P. Rac1-PAK2 pathway is essential for zebrafish heart regeneration. Biochem. Biophys. Res. Commun. 2016, 472, 637–642. [Google Scholar] [CrossRef]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell. Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef]
- Konstantinidis, D.G.; Giger, K.M.; Risinger, M.; Pushkaran, S.; Zhou, P.; Dexheimer, P.; Yerneni, S.; Andreassen, P.; Klingmuller, U.; Palis, J.; et al. Cytokinesis failure in RhoA-deficient mouse erythroblasts involves actomyosin and midbody dysregulation and triggers p53 activation. Blood 2015, 126, 1473–1482. [Google Scholar] [CrossRef]
- Zhang, S.; Konstantinidis, D.G.; Yang, J.Q.; Mizukawa, B.; Kalim, K.; Lang, R.A.; Kalfa, T.A.; Zheng, Y.; Guo, F. Gene targeting RhoA reveals its essential role in coordinating mitochondrial function and thymocyte development. J. Immunol. 2014, 193, 5973–5982. [Google Scholar] [CrossRef]
- Wylie, C. Germ cells. Curr. Opin. Genet. Dev. 2000, 10, 410–413. [Google Scholar] [CrossRef]
- Miranda-Rodriguez, J.R.; Salas-Vidal, E.; Lomeli, H.; Zurita, M.; Schnabel, D. RhoA/ROCK pathway activity is essential for the correct localization of the germ plasm mRNAs in zebrafish embryos. Dev. Biol. 2017, 421, 27–42. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, L.; Korzh, V.; Gong, Z.; Low, B.C. RhoA acts downstream of Wnt5 and Wnt11 to regulate convergence and extension movements by involving effectors Rho kinase and Diaphanous: Use of zebrafish as an in vivo model for GTPase signaling. Cell. Signal. 2006, 18, 359–372. [Google Scholar] [CrossRef]
- Habas, R.; Dawid, I.B.; He, X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes. Dev. 2003, 17, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Takesono, A.; Moger, J.; Farooq, S.; Cartwright, E.; Dawid, I.B.; Wilson, S.W.; Kudoh, T. Solute carrier family 3 member 2 (Slc3a2) controls yolk syncytial layer (YSL) formation by regulating microtubule networks in the zebrafish embryo. Proc. Natl. Acad. Sci. USA 2012, 109, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gan, S.; Xie, G.; Li, L.; Chen, C.; Ding, X.; Han, M.; Xiang, S.; Zhang, J. KCTD10 is critical for heart and blood vessel development of zebrafish. Acta Biochim. Biophys. Sin. 2014, 46, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Fromigue, O.; Hay, E.; Modrowski, D.; Bouvet, S.; Jacquel, A.; Auberger, P.; Marie, P.J. RhoA GTPase inactivation by statins induces osteosarcoma cell apoptosis by inhibiting p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell differentiation. Cell. Death. Differ. 2006, 13, 1845–1856. [Google Scholar] [CrossRef]
- Gomez, J.; Martinez, C.; Giry, M.; Garcia, A.; Rebollo, A. Rho prevents apoptosis through Bcl-2 expression: Implications for interleukin-2 receptor signal transduction. Eur. J. Immunol. 1997, 27, 2793–2799. [Google Scholar] [CrossRef]
- Zhu, S.; Korzh, V.; Gong, Z.; Low, B.C. RhoA prevents apoptosis during zebrafish embryogenesis through activation of Mek/Erk pathway. Oncogene 2008, 27, 1580–1589. [Google Scholar] [CrossRef]
- Fernandez-Medarde, A.; Santos, E. Ras in cancer and developmental diseases. Genes. Cancer 2011, 2, 344–358. [Google Scholar] [CrossRef]
- Chew, T.W.; Liu, X.J.; Liu, L.; Spitsbergen, J.M.; Gong, Z.; Low, B.C. Crosstalk of Ras and Rho: Activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafish models. Oncogene 2014, 33, 2717–2727. [Google Scholar] [CrossRef]
- Khosravi-Far, R.; Solski, P.A.; Clark, G.J.; Kinch, M.S.; Der, C.J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell. Biol. 1995, 15, 6443–6453. [Google Scholar] [CrossRef]
- Sahai, E.; Olson, M.F.; Marshall, C.J. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001, 20, 755–766. [Google Scholar] [CrossRef]
- Vidal, A.; Millard, S.S.; Miller, J.P.; Koff, A. Rho activity can alter the translation of p27 mRNA and is important for RasV12-induced transformation in a manner dependent on p27 status. J. Biol. Chem. 2002, 277, 16433–16440. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Land, H. Tumor suppressor p53 restricts Ras stimulation of RhoA and cancer cell motility. Nat. Struct. Mol. Biol. 2007, 14, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Y.; Zhao, Y.; Cao, D.; Ai, X.; Zeng, A.; Gou, M.; Cai, L.; Yang, H.; Zhao, C. RhoA-stimulated intra-capillary morphology switch facilitates the arrest of individual circulating tumor cells. Int. J. Cancer 2018, 142, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; Gibbs, K.M.; Davila, J.; Campbell, N.; Sung, S.; Todorova, T.I.; Otsuka, S.; Sabaawy, H.E.; Hart, R.P.; Schachner, M. MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur. J. Neurosci. 2011, 33, 1587–1597. [Google Scholar] [CrossRef]
- Hu, J.; Selzer, M.E. RhoA as a target to promote neuronal survival and axon regeneration. Neural. Regen. Res. 2017, 12, 525–528. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, L.; Xu, C.; Chu, L.; Gao, L.; Wu, R.; Liu, Y.; Liu, T.; Sun, X.J.; Ren, R.; et al. ARHGEF12 regulates erythropoiesis and is involved in erythroid regeneration after chemotherapy in acute lymphoblastic leukemia patients. Haematologica 2020, 105, 925–936. [Google Scholar] [CrossRef]
- Sugihara, K.; Nakatsuji, N.; Nakamura, K.; Nakao, K.; Hashimoto, R.; Otani, H.; Sakagami, H.; Kondo, H.; Nozawa, S.; Aiba, A.; et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene 1998, 17, 3427–3433. [Google Scholar] [CrossRef]
- Charlton-Perkins, M.; Almeida, A.D.; MacDonald, R.B.; Harris, W.A. Genetic control of cellular morphogenesis in Muller glia. Glia 2019, 67, 1401–1411. [Google Scholar] [CrossRef]
- Itoh, R.E.; Kurokawa, K.; Ohba, Y.; Yoshizaki, H.; Mochizuki, N.; Matsuda, M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 2002, 22, 6582–6591. [Google Scholar] [CrossRef]
- Hanovice, N.J.; McMains, E.; Gross, J.M. Using GAL4-Inducible Transgenics to Modulate Rho GTPase Activity in Zebrafish. Methods Mol. Biol. 2018, 1821, 359–370. [Google Scholar] [CrossRef]
- Deng, Q.; Yoo, S.K.; Cavnar, P.J.; Green, J.M.; Huttenlocher, A. Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Dev. Cell 2011, 21, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Tahinci, E.; Symes, K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev. Biol. 2003, 259, 318–335. [Google Scholar] [CrossRef]
- Pertz, O.; Hodgson, L.; Klemke, R.L.; Hahn, K.M. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 2006, 440, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Hanovice, N.J.; McMains, E.; Gross, J.M. A GAL4-inducible transgenic tool kit for the in vivo modulation of Rho GTPase activity in zebrafish. Dev. Dyn. 2016, 245, 844–853. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boueid, M.-J.; Mikdache, A.; Lesport, E.; Degerny, C.; Tawk, M. Rho GTPases Signaling in Zebrafish Development and Disease. Cells 2020, 9, 2634. https://doi.org/10.3390/cells9122634
Boueid M-J, Mikdache A, Lesport E, Degerny C, Tawk M. Rho GTPases Signaling in Zebrafish Development and Disease. Cells. 2020; 9(12):2634. https://doi.org/10.3390/cells9122634
Chicago/Turabian StyleBoueid, Marie-José, Aya Mikdache, Emilie Lesport, Cindy Degerny, and Marcel Tawk. 2020. "Rho GTPases Signaling in Zebrafish Development and Disease" Cells 9, no. 12: 2634. https://doi.org/10.3390/cells9122634
APA StyleBoueid, M.-J., Mikdache, A., Lesport, E., Degerny, C., & Tawk, M. (2020). Rho GTPases Signaling in Zebrafish Development and Disease. Cells, 9(12), 2634. https://doi.org/10.3390/cells9122634






