Establishment and Characterization of a Sclerosing Spindle Cell Rhabdomyosarcoma Cell Line with a Complex Genomic Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient History
2.2. Histopathology and Immunohistochemistry
2.3. Primary Cell Culture and Propagation of SRH Cells
2.4. Routine Cell Culture
2.5. Ultrastructure Analysis
2.6. Proliferation Kinetics
2.7. Cell Cycle Analysis
2.8. Multicolor Fluorescence In Situ Hybridization (mFISH) and Chromosomal Breakpoint Analysis
2.9. Array-Comparative Genomic Hybridization (CGH) Analysis
2.10. Ingenuity Pathway Analysis
2.11. DNA Preparation
2.12. Mutation Analysis
2.13. RNA Isolation and Quantitative Real Time PCR
2.14. Flow Cytometry Analysis of Cell Surface Markers
2.15. Tumor Sphere Formation Assay
2.16. Osteogenic and Adipogenic Differentiation
2.17. Tumorigenicity in Mice
2.18. Assessment of Cell Line Identity
3. Results
3.1. Histopathology and Immunohistochemistry of the Original Tumor
3.2. Morphology of the SRH Cell Line and Growth Characteristics
3.3. Cytogenetic Analysis by mFISH Karyotyping and Chromosomal Breakpoint Analysis
3.4. Array-CGH and Pathway Analysis
3.5. MYOD1 Mutation and p53 Mutations in SRH Cells
3.6. Myogenic Marker Gene Expression
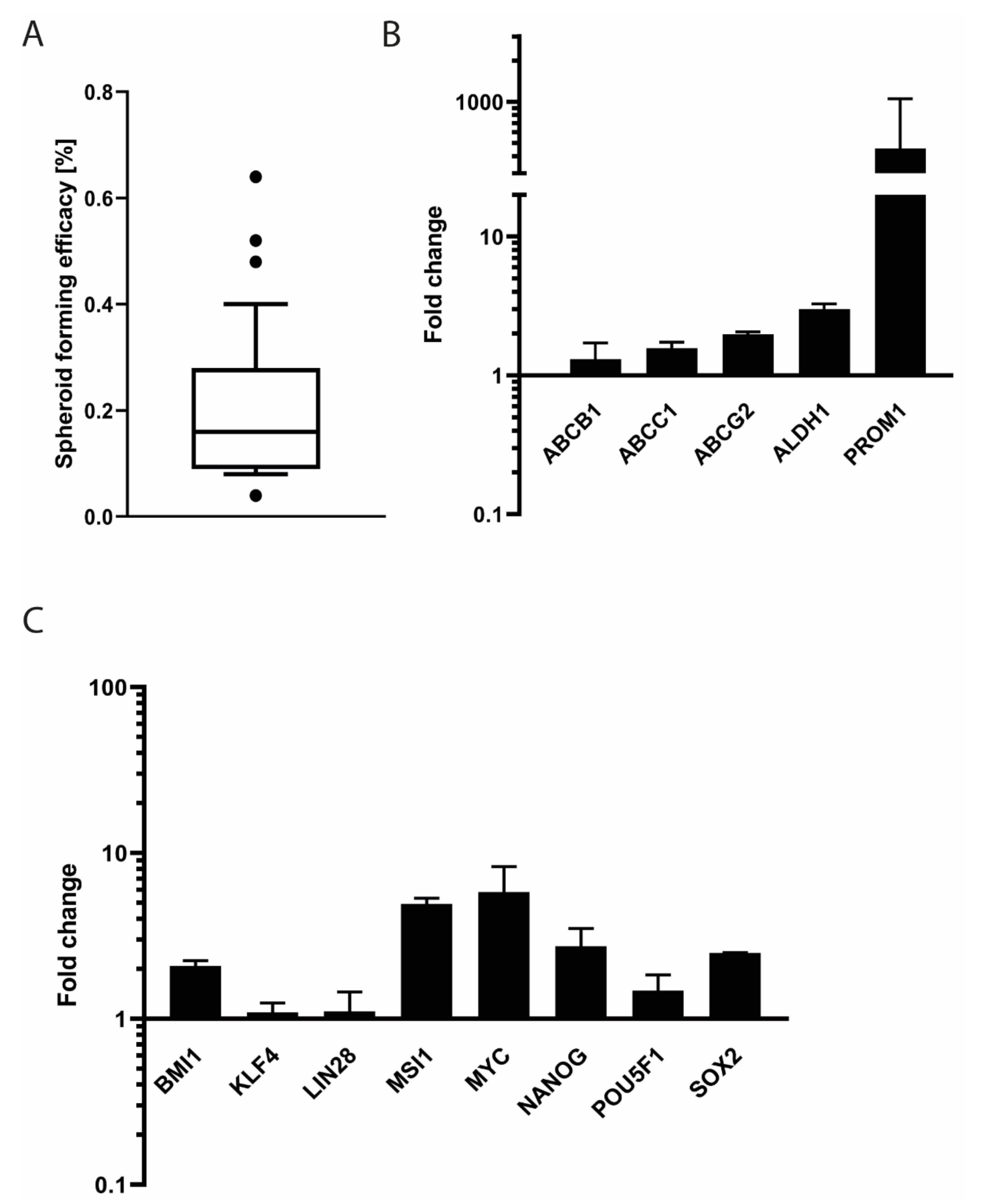
3.7. Stem Cell Features of SRH Cells
3.8. Adipogenic and Osteogenic Differentiation
3.9. Transplantation into Mice
3.10. Cell Line Authentication
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parham, D.M.; Barr, F.G. Classification of Rhabdomyosarcoma and Its Molecular Basis. Adv. Anat. Pathol. 2013, 20, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Amer, K.M.; Thomson, J.E.; Congiusta, D.; Dobitsch, A.; Chaudhry, A.; Li, M.; Chaudhry, A.; Bozzo, A.; Siracuse, B.; Aytekin, M.N.; et al. Epidemiology, Incidence, and Survival of Rhabdomyosarcoma Subtypes: SEER and ICES Database Analysis. J. Orthop. Res. 2019, 37, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- Ducimetière, F.; Lurkin, A.; Ranchère-Vince, D.; Decouvelaere, A.-V.; Péoc’H, M.; Istier, L.; Chalabreysse, P.; Muller, C.; Alberti, L.; Bringuier, P.-P.; et al. Incidence of Sarcoma Histotypes and Molecular Subtypes in a Prospective Epidemiological Study with Central Pathology Review and Molecular Testing. PLoS ONE 2011, 6, e20294. [Google Scholar] [CrossRef] [PubMed]
- Huh, W.W.; Bejar, D.E. Rhabdomyosarcoma in adolescent and young adult patients: Current perspectives. Adolesc. Health Med. Ther. 2014, 5, 115–125. [Google Scholar] [CrossRef]
- Bompas, E.; Campion, L.; Italiano, A.; Le Cesne, A.; Chevreau, C.; Isambert, N.; Toulmonde, M.; Mir, O.; Ray-Coquard, I.; Piperno-Neumann, S.; et al. Outcome of 449 adult patients with rhabdomyosarcoma: An observational ambispective nationwide study. Cancer Med. 2018, 7, 4023–4035. [Google Scholar] [CrossRef]
- Cavazzana, A.O.; Schmidt, D.; Ninfo, V.; Harms, D.; Tollot, M.; Carli, M.; Treuner, J.; Betto, R.; Salviati, G. Spindle Cell Rhabdomyosarcoma. Am. J. Surg. Pathol. 1992, 16, 229–235. [Google Scholar] [CrossRef]
- Rubin, B.P.; Hasserjian, R.P.; Singer, S.; Janecka, I.; Fletcher, J.A.; Fletcher, C.D. Spindle Cell Rhabdomyosarcoma (So-Called) in Adults. Am. J. Surg. Pathol. 1998, 22, 459–464. [Google Scholar] [CrossRef]
- Mentzel, T.; Katenkamp, D. Sclerosing, pseudovascular rhabdomyosarcoma in adults. Clinicopathological and immunohistochemical analysis of three cases. Virchows Arch. 2000, 436, 305–311. [Google Scholar] [CrossRef]
- Zambo, I.; Veselý, K. WHO classification of tumours of soft tissue and bone 2013: The main changes compared to the 3rd edition. Ceskoslovenska Patol. 2014, 50, 64. [Google Scholar]
- Rudzinski, E.R.; Anderson, J.R.; Hawkins, D.S.; Skapek, S.X.; Parham, D.M.; Teot, L.A. The World Health Organization Classification of Skeletal Muscle Tumors in Pediatric Rhabdomyosarcoma: A Report From the Children’s Oncology Group. Arch. Pathol. Lab. Med. 2015, 139, 1281–1287. [Google Scholar] [CrossRef]
- Mosquera, J.M.; Sboner, A.; Zhang, L.; Kitabayashi, N.; Chen, C.-L.; Sung, Y.S.; Wexler, L.H.; Laquaglia, M.P.; Edelman, M.; Sreekantaiah, C.; et al. RecurrentNCOA2gene rearrangements in congenital/infantile spindle cell rhabdomyosarcoma. Geneschromosom. Cancer 2013, 52, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.F.; Fletcher, C.D.M. Spindle cell rhabdomyosarcoma in adults. Am. J. Surg. Pathol. 2005, 29, 1106–1113. [Google Scholar] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours of Soft Tissue and Bone, 5th ed.; IARC Press: Lyon, France, 2020. [Google Scholar]
- Zhao, Z.; Yin, Y.; Zhang, J.; Qi, J.; Zhang, D.; Ma, Y.; Wang, Y.; Li, S.; Zhou, J. Spindle cell/sclerosing rhabdomyosarcoma: Case series from a single institution emphasizing morphology, immunohistochemistry and follow-up. Int. J. Clin. Exp. Pathol. 2015, 8, 13814–13820. [Google Scholar] [PubMed]
- Rekhi, B.; Singhvi, T. Histopathological, immunohistochemical and molecular cytogenetic analysis of 21 spindle cell/sclerosing rhabdomyosarcomas. APMIS 2014, 122, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Tian, Z.; Zhu, Y. Clinicopathologic features and molecular spectrum of spindle cell and sclerosing rhabdomyosarcomas in the head and neck region. Int. J. Clin. Exp. Pathol. 2018, 11, 3436–3444. [Google Scholar] [PubMed]
- Agaram, N.P.; Chen, C.-L.; Zhang, L.; Laquaglia, M.P.; Wexler, L.; Antonescu, C.R. RecurrentMYOD1mutations in pediatric and adult sclerosing and spindle cell rhabdomyosarcomas: Evidence for a common pathogenesis. Geneschromosom. Cancer 2014, 53, 779–787. [Google Scholar] [CrossRef]
- Szuhai, K.; De Jong, D.; Leung, W.Y.; Fletcher, C.D.M.; Hogendoorn, P.C.W. Transactivating mutation of the MYOD1 gene is a frequent event in adult spindle cell rhabdomyosarcoma. J. Pathol. 2014, 232, 300–307. [Google Scholar] [CrossRef]
- Tsai, J.; Changchien, Y.; Lee, J.; Kao, Y.; Li, W.; Liang, C.; Liao, I.; Chang, Y.; Wang, J.; Tsao, C.; et al. The expanding morphological and genetic spectrum ofMYOD1-mutant spindle cell/sclerosing rhabdomyosarcomas: A clinicopathological and molecular comparison of mutated and non-mutated cases. Histopathology 2019, 74, 933–943. [Google Scholar] [CrossRef]
- Soglio, R.B.-D.; Rougemont, A.-L.; Absi, R.; Barrette, S.; Montpetit, A.; Fetni, R.; Fournet, J.-C. SNP genotyping of a sclerosing rhabdomyosarcoma: Reveals highly aneuploid profile and a specific MDM2/HMGA2 amplification. Hum. Pathol. 2009, 40, 1347–1352. [Google Scholar] [CrossRef]
- Kuhnen, C.; Herter, P.; Leuschner, I.; Mentzel, T.; Druecke, D.; Jaworska, M.; Johnen, G. Sclerosing pseudovascular rhabdomyosarcoma—Immunohistochemical, ultrastructural, and genetic findings indicating a distinct subtype of rhabdomyosarcoma. Virchows Arch. 2006, 449, 572–578. [Google Scholar] [CrossRef]
- Croes, R.; Debiec-Rychter, M.; Cokelaere, K.; De Vos, R.; Hagemeijer-Hausman, A.; Sciot, R. Adult sclerosing rhabdomyosarcoma: Cytogenetic link with embryonal rhabdomyosarcoma. Virchows Arch. 2005, 446, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Agaram, N.P.; Laquaglia, M.P.; Alaggio, R.; Zhang, L.; Fujisawa, Y.; Ladanyi, M.; Wexler, L.H.; Antonescu, C.R. MYOD1-mutant spindle cell and sclerosing rhabdomyosarcoma: An aggressive subtype irrespective of age. A reappraisal for molecular classification and risk stratification. Mod. Pathol. 2019, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.H.; Atherton, D.; Reith, J.D.; Islam, N.M.; Bhattacharyya, I.; Cohen, D.M. Rhabdomyosarcoma, Spindle Cell/Sclerosing Variant: A Clinical and Histopathological Examination of this Rare Variant with Three New Cases from the Oral Cavity. Head Neck Pathol. 2017, 11, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.; Satheesha, S.; Albrecht, P.; Bornhauser, B.C.; D’Alessandro, V.; Oesch, S.M.; Rehrauer, H.; Leuschner, I.; Koscielniak, E.; Gengler, C.; et al. CD133 Positive Embryonal Rhabdomyosarcoma Stem-Like Cell Population Is Enriched in Rhabdospheres. PLoS ONE 2011, 6, e19506. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, K.; Uehara, S.; Nishikawa, S.; Kawatsu, M.; Zenitani, M.; Oue, T.; Okuyama, H. Aldehyde Dehydrogenase 1 (ALDH1) Is a Potential Marker for Cancer Stem Cells in Embryonal Rhabdomyosarcoma. PLoS ONE 2015, 10, e0125454. [Google Scholar] [CrossRef]
- Belyea, B.; Kephart, J.G.; Blum, J.; Kirsch, D.G.; Linardic, C.M. Embryonic Signaling Pathways and Rhabdomyosarcoma: Contributions to Cancer Development and Opportunities for Therapeutic Targeting. Sarcoma 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roma, J.; Almazán-Moga, A.; De Toledo, J.S.; Gallego, S. Notch, Wnt, and Hedgehog Pathways in Rhabdomyosarcoma: From Single Pathways to an Integrated Network. Sarcoma 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Noguchi, R.; Tsuchiya, R.; Sei, A.; Sugaya, J.; Iwata, S.; Sugiyama, M.; Yoshida, A.; Kawai, A.; Kondo, T. Establishment and characterization of NCC-ssRMS1-C1: A novel patient-derived spindle-cell/sclerosing rhabdomyosarcoma cell line. Hum. Cell 2020, 33, 886–893. [Google Scholar] [CrossRef]
- Schleicher, S.B.; Zaborski, J.J.; Riester, R.; Zenkner, N.; Handgretinger, R.; Kluba, T.; Traub, F.; Boehme, K.A. Combined application of arsenic trioxide and lithium chloride augments viability reduction and apoptosis induction in human rhabdomyosarcoma cell lines. PLoS ONE 2017, 12, e0178857. [Google Scholar] [CrossRef]
- Boehme, K.A.; Zaborski, J.J.; Riester, R.; Schweiss, S.K.; Hopp, U.; Traub, F.; Kluba, T.; Handgretinger, R.; Schleicher, S.B. Targeting hedgehog signalling by arsenic trioxide reduces cell growth and induces apoptosis in rhabdomyosarcoma. Int. J. Oncol. 2015, 48, 801–812. [Google Scholar] [CrossRef]
- Battula, V.L.; Treml, S.; Bareiss, P.M.; Gieseke, F.; Roelofs, H.; De Zwart, P.; Müller, I.; Schewe, B.; Skutella, T.; Fibbe, W.E.; et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica 2008, 94, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.; Gadzicki, D.; Schlegelberger, B.; Göhring, G. Recurrent involvement of heterochromatic regions in multiple myeloma—A multicolor FISH study. Leuk. Res. 2010, 34, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Kotilingam, D.; Korchin, B.; Liu, J.; Yu, D.; Lazar, A.J.F.; Pollock, R.E.; Lev, D. High prevalence ofp53 exon 4 mutations in soft tissue sarcoma. Cancer 2007, 109, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Köllmer, M.; Buhrman, J.S.; Zhang, Y.; Gemeinhart, R.A. Markers are shared between adipogenic and osteogenic differentiated mesenchymal stem cells. J. Dev. Biol. Tissue Eng. 2013, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wang-Rodriguez, J.; Urquidi, V.; Rivard, A.; Goodison, S. Elevated osteopontin and thrombospondin expression identifies malignant human breast carcinoma but is not indicative of metastatic status. Breast Cancer Res. 2003, 5, R136–R143. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lemma, S.; Avnet, S.; Salerno, M.; Chano, T.; Baldini, N. Identification and Validation of Housekeeping Genes for Gene Expression Analysis of Cancer Stem Cells. PLoS ONE 2016, 11, e0149481. [Google Scholar] [CrossRef]
- Hinson, A.R.P.; Ejones, R.; Crose, L.E.S.; Belyea, B.C.; Barr, F.G.; Linardic, C.M. Human Rhabdomyosarcoma Cell Lines for Rhabdomyosarcoma Research: Utility and Pitfalls. Front. Oncol. 2013, 3, 183. [Google Scholar] [CrossRef]
- Zois, C.E.; Harris, A. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef]
- Henderson, C.A.; Gomez, C.G.; Novak, S.M.; Mi-Mi, L.; Gregorio, C.C. Overview of the Muscle Cytoskeleton. Compr. Physiol. 2017, 7, 891–944. [Google Scholar] [CrossRef]
- Sun, X.; Guo, W.; Shen, J.K.; Mankin, H.J.; Hornicek, F.J.; Duan, Z. Rhabdomyosarcoma: Advances in Molecular and Cellular Biology. Sarcoma 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Forus, A.; Flørenes, V.A.; Maelandsmo, G.M.; Meltzer, P.S.; Fodstad, O.; Myklebost, O. Mapping of amplification units in the q13-14 region of chromosome 12 in human sarcomas: Some amplica do not include MDM. Cell Growth Differ Mol. Biol. J. Am. Assoc. Cancer Res. 1993, 4, 1065–1070. [Google Scholar]
- Ragazzini, P.; Gamberi, G.; Pazzaglia, L.; Serra, M.; Magagnoli, G.; Ponticelli, F.; Ferrari, C.; Ghinelli, C.; Alberghini, M.; Bertoni, F.; et al. Amplification of CDK4, MDM2, SAS and GLI genes in leiomyosarcoma, alveolar and embryonal rhabdomyosarcoma. Histol. Histopathol. 2004, 19, 401–411. [Google Scholar] [PubMed]
- Keller, C.; Guttridge, D.C. Mechanisms of impaired differentiation in rhabdomyosarcoma. FEBS J. 2013, 280, 4323–4334. [Google Scholar] [CrossRef]
- Black, B.L.; Martin, J.F.; Olson, E.N. The MouseMRF4Promoter Istrans-Activated Directly and Indirectly by Muscle-specific Transcription Factors. J. Biol. Chem. 1995, 270, 2889–2892. [Google Scholar] [CrossRef]
- Weintraub, H.; Davis, R.; Tapscott, S.; Thayer, M.; Krause, M.; Benezra, R.; Blackwell, T.K.; Turner, D.; Rupp, R.; Hollenberg, S.; et al. The myoD gene family: Nodal point during specification of the muscle cell lineage. Science 1991, 251, 761–766. [Google Scholar] [CrossRef]
- Shern, J.F.; Chen, L.; Chmielecki, J.; Wei, J.S.; Patidar, R.; Rosenberg, M.; Ambrogio, L.; Auclair, D.; Wang, J.; Song, Y.K.; et al. Comprehensive Genomic Analysis of Rhabdomyosarcoma Reveals a Landscape of Alterations Affecting a Common Genetic Axis in Fusion-Positive and Fusion-Negative Tumors. Cancer Discov. 2014, 4, 216–231. [Google Scholar] [CrossRef]
- Kohlmeyer, J.L.; Gordon, D.J.; Tanas, M.R.; Monga, V.; Dodd, R.D.; Quelle, D.E. CDKs in Sarcoma: Mediators of Disease and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2020, 21, 3018. [Google Scholar] [CrossRef]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef]
- Wong, D.T.W.; Kim, J.J.; Khalid, O.; Sun, H.-H.B.; Kim, Y. Double Edge: CDK2AP1 in cell-cycle regulation and epigenetic regulation. J. Dent. Res. 2011, 91, 235–241. [Google Scholar] [CrossRef]
- Wu, C.L.; Kirley, S.D.; Xiao, H.; Chuang, Y.; Chung, D.C.; Zukerberg, L.R. Cables enhances cdk2 tyrosine 15 phosphorylation by Wee1, inhibits cell growth, and is lost in many human colon and squamous cancers. Cancer Res. 2001, 61, 7325–7332. [Google Scholar] [PubMed]
- Muller, P.A.; Vousden, K.H. Mutant p53 in Cancer: New Functions and Therapeutic Opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Rochette, P.J.; Bastien, N.; Lavoie, J.; Guérin, S.L.; Drouin, R. SW480, a p53 Double-mutant Cell Line Retains Proficiency for Some p53 Functions. J. Mol. Biol. 2005, 352, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Candeias, M.M.; Hagiwara, M.; Matsuda, M. Cancer-specific mutations in p53 induce the translation of Δ160p53 promoting tumorigenesis. EMBO Rep. 2016, 17, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Kashi, V.P.; Hatley, M.E.; Galindo, R.L. Probing for a deeper understanding of rhabdomyosarcoma: Insights from complementary model systems. Nat. Rev. Cancer 2015, 15, 426–439. [Google Scholar] [CrossRef]
- Seki, M.; Nishimura, R.; Yoshida, K.; Shimamura, T.; Shiraishi, Y.; Sato, Y.; Kato, M.; Chiba, K.; Tanaka, H.; Hoshino, N.; et al. Integrated genetic and epigenetic analysis defines novel molecular subgroups in rhabdomyosarcoma. Nat. Commun. 2015, 6, 7557. [Google Scholar] [CrossRef]
- Bs, A.C.T.; Shu, L.; Danks, M.K.; Ms, C.A.P.; Ms, S.S.; Thayer, M.J.; Houghton, P.J.; Harris, L.C. p53 mutation and MDM2 amplification frequency in pediatric rhabdomyosarcoma tumors and cell lines. Med. Pediatr. Oncol. 2000, 35, 96–103. [Google Scholar] [CrossRef]
- Senturk, E.; Manfredi, J.J. Mdm2 and Tumorigenesis: Evolving theories and unsolved mysteries. Genes Cancer 2012, 3, 192–198. [Google Scholar] [CrossRef]
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise Review: The Surface Markers and Identity of Human Mesenchymal Stem Cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bühring, H.-J.; Battula, V.L.; Treml, S.; Schewe, B.; Kanz, L.; Vogel, W. Novel Markers for the Prospective Isolation of Human MSC. Ann. N. Y. Acad. Sci. 2007, 1106, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.L.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hejbøl, E.K.; Hajjaj, M.A.; Nielsen, O.; Schrøder, H.D. Marker Expression of Interstitial Cells in Human Skeletal Muscle: An Immunohistochemical Study. J. Histochem. Cytochem. 2019, 67, 825–844. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-S.; Xin, Z.-C.; Dai, J.; Lue, T.F. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol. Histopathol. 2013, 28, 1109–1116. [Google Scholar] [PubMed]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise Review: Evidence for CD34 as a Common Marker for Diverse Progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef]
- Kacar, A.; Demir, H.A.; Durak, H.; Dervisoglu, S. Spindle cell rhabdomyosarcoma displaying cd34 positivity: A potential diagnostic pitfall; report of two pediatric cases. Turk. J. Pathol. 2013, 29, 221–226. [Google Scholar] [CrossRef]
- Dupas, T.; Rouaud, T.; Rouger, K.; Lieubeau, B.; Cario-Toumaniantz, C.; Fontaine-Pérus, J.; Gardahaut, M.-F.; Auda-Boucher, G. Fetal muscle contains different CD34+ cell subsets that distinctly differentiate into adipogenic, angiogenic and myogenic lineages. Stem Cell Res. 2011, 7, 230–243. [Google Scholar] [CrossRef]
- Wirths, S.; Malenke, E.; Kluba, T.; Rieger, S.; Müller, M.R.; Schleicher, S.; Von Weyhern, C.H.; Nagl, F.; Fend, F.; Vogel, W.; et al. Shared Cell Surface Marker Expression in Mesenchymal Stem Cells and Adult Sarcomas. Stem Cells Transl. Med. 2012, 2, 53–60. [Google Scholar] [CrossRef]
- Trucco, M.; Loeb, D. Sarcoma Stem Cells: Do We Know What We Are Looking for? Sarcoma 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudás, J.; Skvortsov, S.; Ganswindt, U.; Riechelmann, H.; Skvortsova, I.-I. Therapy resistance mediated by cancer stem cells. Semin. Cancer Biol. 2018, 53, 156–167. [Google Scholar] [CrossRef]
- Genadry, K.C.; Pietrobono, S.; Rota, R.; Linardic, C.M. Soft Tissue Sarcoma Cancer Stem Cells: An Overview. Front. Oncol. 2018, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Delgado, P.; Lacerenza, S.; Obrador-Hevia, A.; Lopez-Alvarez, M.; Mondaza-Hernandez, J.L.; Blanco-Alcaina, E.; Sanchez-Bustos, P.; Hindi, N.; Moura, D.S.; Broto, J.M. Cancer Stem Cells in Soft-Tissue Sarcomas. Cells 2020, 9, 1449. [Google Scholar] [CrossRef] [PubMed]
- Satheesha, S.; Manzella, G.; Bovay, A.; A Casanova, E.; Bode, P.-K.; Belle, R.; Feuchtgruber, S.; Jaaks, P.; Dogan, N.; Koscielniak, E.; et al. Targeting hedgehog signaling reduces self-renewal in embryonal rhabdomyosarcoma. Oncogene 2015, 35, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Goodspeed, A.; Heiser, L.M.; Gray, J.W.; Costello, J.C. Tumor-Derived Cell Lines as Molecular Models of Cancer Pharmacogenomics. Mol. Cancer Res. 2016, 14, 3–13. [Google Scholar] [CrossRef]









| Primer | Sequence (5′–3′) |
|---|---|
| TBP for | TGCACAGGAGCCAAGAGTGAA |
| TBP rev | CACATCACAGCTCCCCACCA |
| YWHAZ for | ACCGTTACTTGGCTGAGGTTGC |
| YWHAZ rev | CCCAGTCTGATAGGATGTGTTGG |
| ABCB1 for | GCTGTCAAGGAAGCCAATGCCT |
| ABCB1 rev | TGCAATGGCGATCCTCTGCTTC |
| ABCC1 for | CCGTGTACTCCAACGCTGACAT |
| ABCC1 rev | ATGCTGTGCGTGACCAAGATCC |
| ABCG2 for | GTTCTCAGCAGCTCTTCGGCTT |
| ABCG2 rev | TCCTCCAGACACACCACGGATA |
| ALDH1A1 for | CGGGAAAAGCAATCTGAAGAGGG |
| ALDH1A1 rev | GATGCGGCTATACAACACTGGC |
| BMI1 for | GGTACTTCATTGATGCCACAACC |
| BMI1 rev | CTGGTCTTGTGAACTTGGACATC |
| KLF4 for | CATCTCAAGGCACACCTGCGAA |
| KLF4 rev | TCGGTCGCATTTTTGGCACTGG |
| LIN28 for | CCAGTGGATGTCTTTGTGCACC |
| LIN28 rev | GTGACACGGATGGATTCCAGAC |
| MSI1 for | GCTCAGCCAAAGGAGGTGATGT |
| MSI1 rev | GCGTAGGTTGTGGCTTGGAAAC |
| MYC for | CCTGGTGCTCCATGAGGAGAC |
| MYC rev | CAGACTCTGACCTTTTGCCAGG |
| MYF5 for | CAGTCCTGTCTGGTCCAGAAAG |
| MYF5 rev | GTCCACTATGTTGGATAAGCAATC |
| MYF6 for | CCCTTCAGCTACAGACCCAAAC |
| MYF6 rev | TCCTTAGCCGTTATCACGAGCC |
| MYOD for | CTCCAACTGCTCCGACGGCAT |
| MYOD rev | ACAGGCAGTCTAGGCTCGACAC |
| MYOG for | AGTGCCATCCAGTACATCGAGC |
| MYOG rev | AGGCGCTGTGAGAGCTGCATTC |
| MYMK for | ATGCGTCACGACATCCTGGAGT |
| MYMK rev | CAATGGTCAGGACGCCGAACAT |
| NANOG for | CTCCAACATCCTGAACCTCAGC |
| NANOG rev | CGTCACACCATTGCTATTCTTCG |
| POU5F1 for | CCTGAAGCAGAAGAGGATCACC |
| POU5F1 rev | AAAGCGGCAGATGGTCGTTTGG |
| PROM1 for | CACTACCAAGGACAAGGCGTTC |
| PROM1 rev | CAACGCCTCTTTGGTCTCCTTG |
| SOX2 for | GCTACAGCATGATGCAGGACCA |
| SOX2 rev | TCTGCGAGCTGGTCATGGAGTT |
| TANC1 for | GTGTGTCTGCTGACCAAGAAGG |
| TANC1 rev | GACCACTCACAAGTCAGCAGGT |
| Original Tumor | SRH Cell Line | SRH Xenograft | |
|---|---|---|---|
| AMEL | XX | XX | XX |
| CSF1P0 | 11,12 | 11,12 | 11,12 |
| D13S317 | 11,11 | 11,11 | 11,11 |
| D16S539 | 11,12 | 11,12 | 11,12 |
| D21S11 | 29,31.2 | 29,31.2 | 29,31.2 |
| D5S818 | 12,12 | 12,12 | 12,12 |
| D7S820 | 8,11 | 8,11 | 8,11 |
| TH01 | 9.3,9.3 | 9.3,9.3 | 9.3,9.3 |
| TPOX | 8,9 | 8,9 | 8,9 |
| vWA | 14,17 | 14,17 | 14,17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schleicher, S.; Grote, S.; Malenke, E.; Chan, K.C.-H.; Schaller, M.; Fehrenbacher, B.; Riester, R.; Kluba, T.; Frauenfeld, L.; Boesmueller, H.; et al. Establishment and Characterization of a Sclerosing Spindle Cell Rhabdomyosarcoma Cell Line with a Complex Genomic Profile. Cells 2020, 9, 2668. https://doi.org/10.3390/cells9122668
Schleicher S, Grote S, Malenke E, Chan KC-H, Schaller M, Fehrenbacher B, Riester R, Kluba T, Frauenfeld L, Boesmueller H, et al. Establishment and Characterization of a Sclerosing Spindle Cell Rhabdomyosarcoma Cell Line with a Complex Genomic Profile. Cells. 2020; 9(12):2668. https://doi.org/10.3390/cells9122668
Chicago/Turabian StyleSchleicher, Sabine, Stefan Grote, Elke Malenke, Kenneth Chun-Ho Chan, Martin Schaller, Birgit Fehrenbacher, Rosa Riester, Torsten Kluba, Leonie Frauenfeld, Hans Boesmueller, and et al. 2020. "Establishment and Characterization of a Sclerosing Spindle Cell Rhabdomyosarcoma Cell Line with a Complex Genomic Profile" Cells 9, no. 12: 2668. https://doi.org/10.3390/cells9122668
APA StyleSchleicher, S., Grote, S., Malenke, E., Chan, K. C.-H., Schaller, M., Fehrenbacher, B., Riester, R., Kluba, T., Frauenfeld, L., Boesmueller, H., Göhring, G., Schlegelberger, B., Handgretinger, R., Kopp, H.-G., Traub, F., & Boehme, K. A. (2020). Establishment and Characterization of a Sclerosing Spindle Cell Rhabdomyosarcoma Cell Line with a Complex Genomic Profile. Cells, 9(12), 2668. https://doi.org/10.3390/cells9122668






