Effect of a Lens Protein in Low-Temperature Culture of Novel Immortalized Human Lens Epithelial Cells (iHLEC-NY2)
Abstract
:1. Introduction
2. Materials and Methods
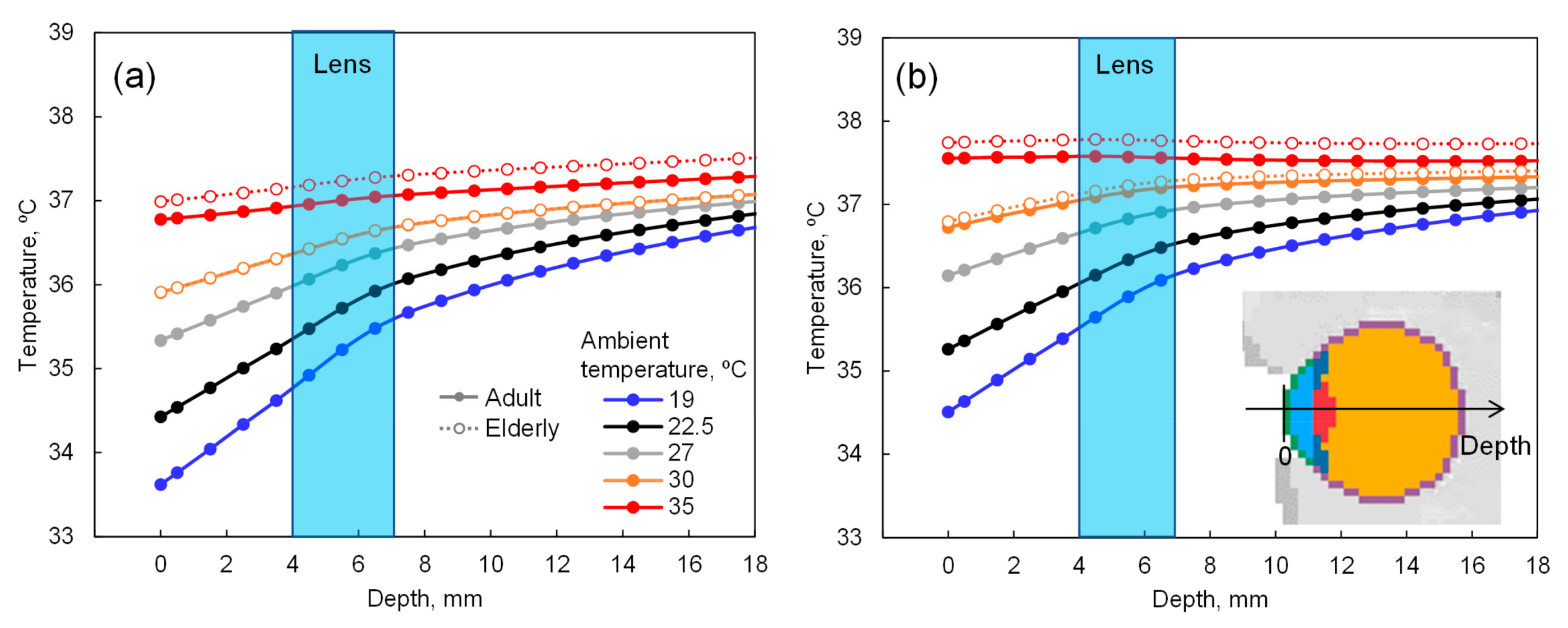
2.1. Computational Methods for Estimation of Eye Temperature
2.2. Primary Culture of Human Lens Epithelial Cells
2.3. Immortalization Gene Transfer to Pseudo-Attachment (att) P Site
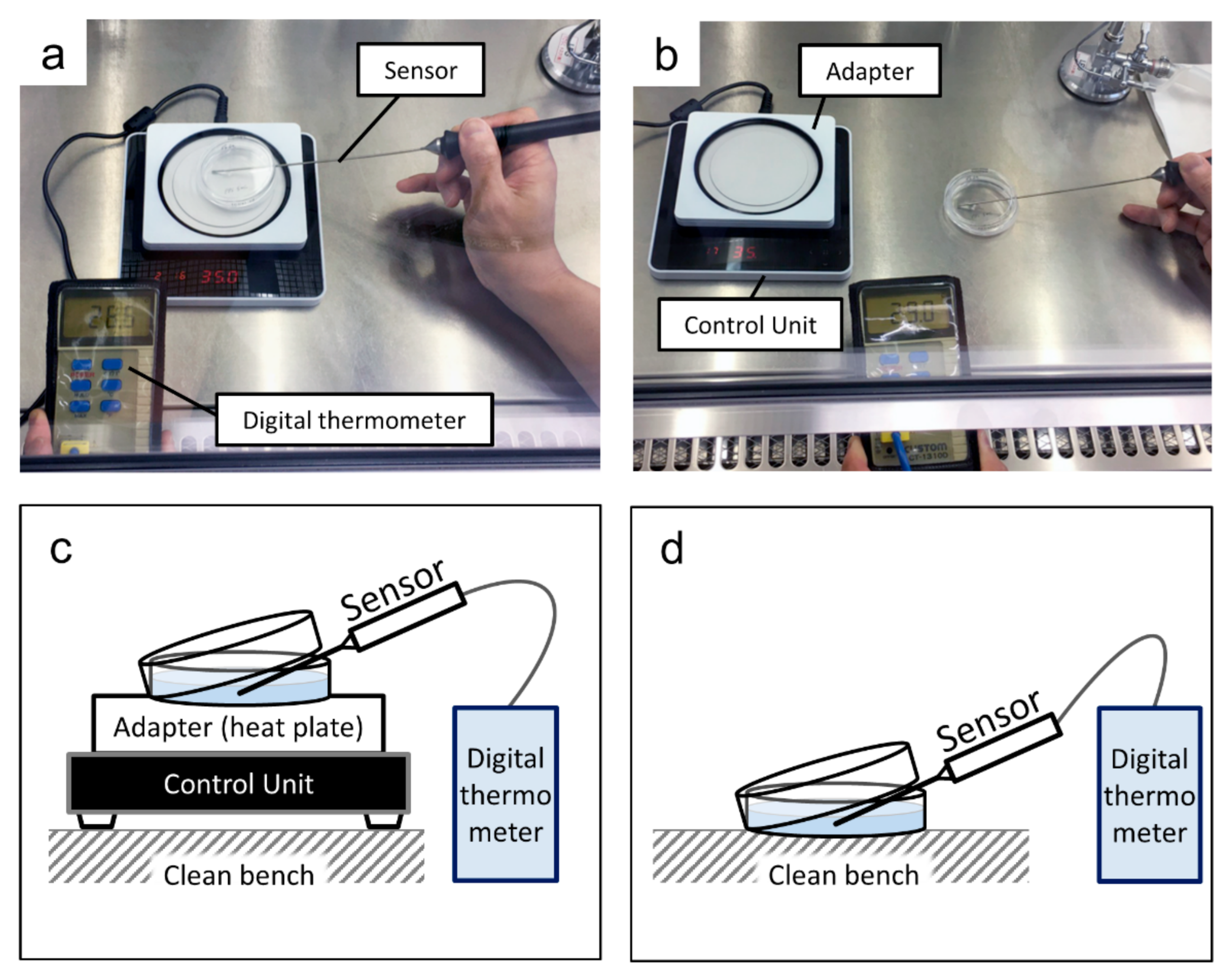
2.4. Temperature Maintenance during Medium Replacement and Cell Growth Curve
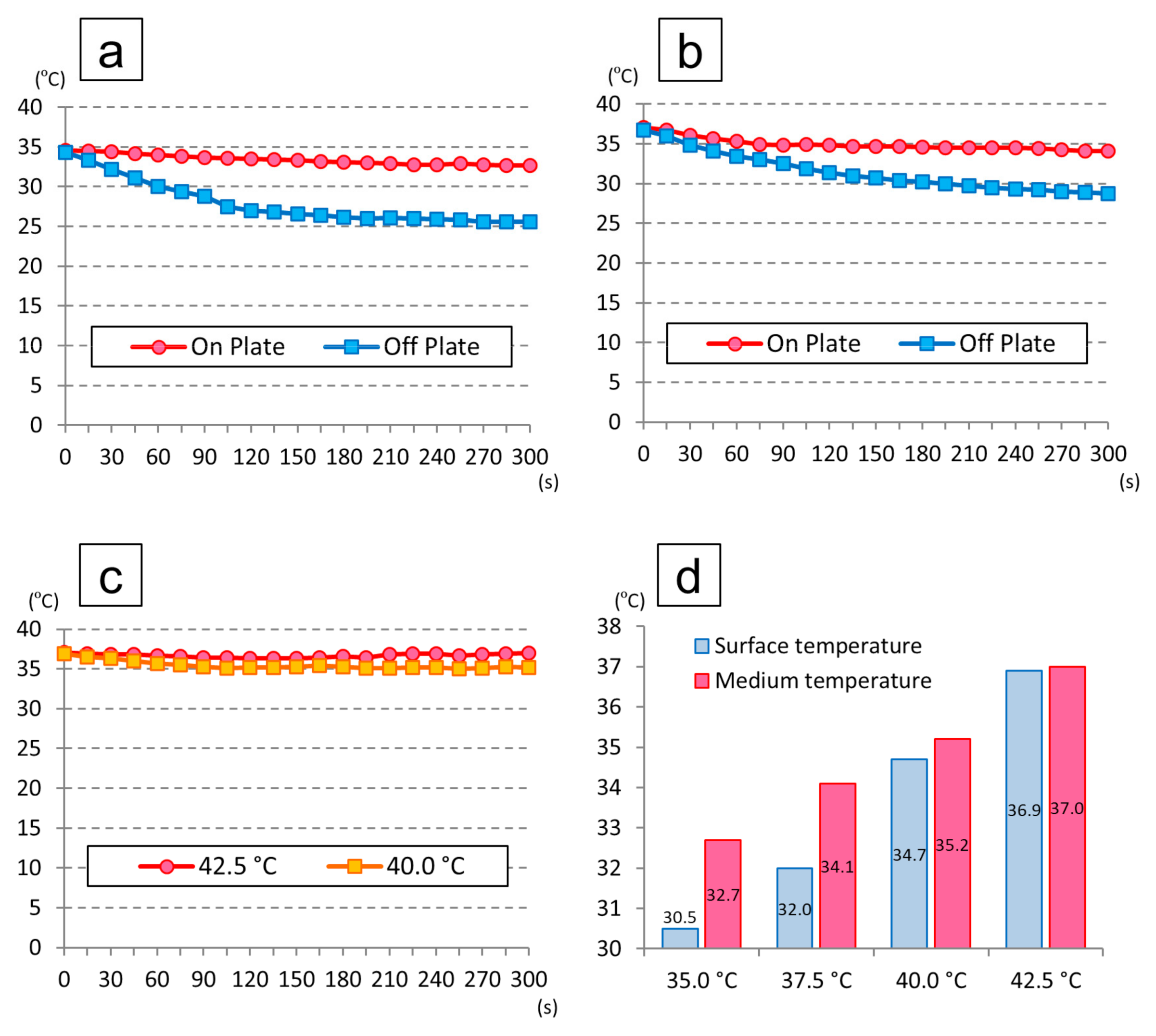
2.4.1. Temperature Maintenance during Medium Replacement
2.4.2. Cell Growth Curve
2.5. Detection of Mycoplasma Infection by Polymerase Chain Reaction (PCR)
2.6. Quantitative Reverse Transcription-PCR
2.7. Western Blotting
2.8. Measurement of Human Aβ1-40 and Aβ1-42 by Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analyses
3. Results
3.1. Computational Results for the Estimation of Eye Temperature
3.2. Temperature Change in the Medium
3.3. The Cell Morphology and Proliferation of the iHLEC-NY2 Cells
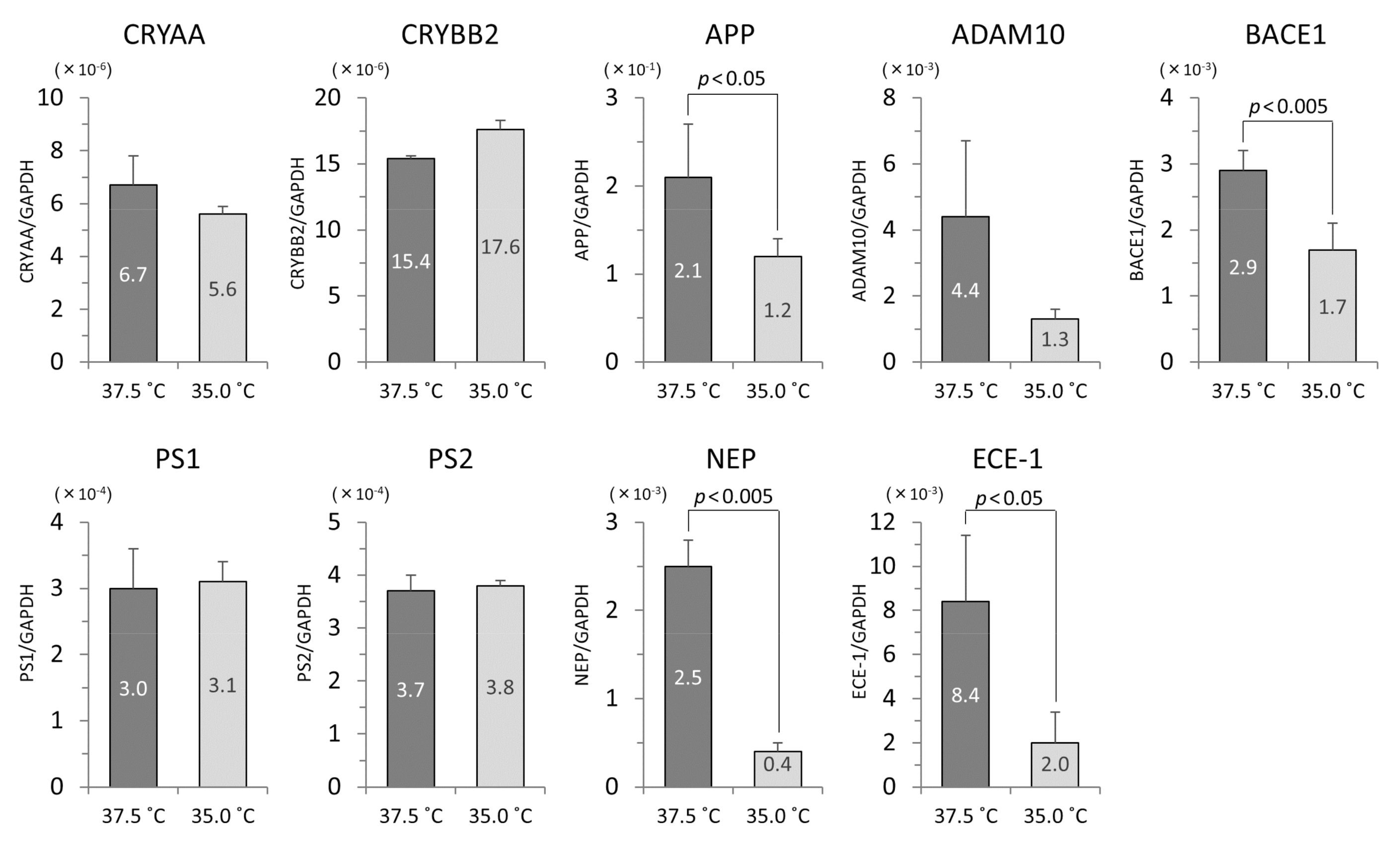
3.4. The mRNA Levels of iHLEC-NY2 and SRA01/04
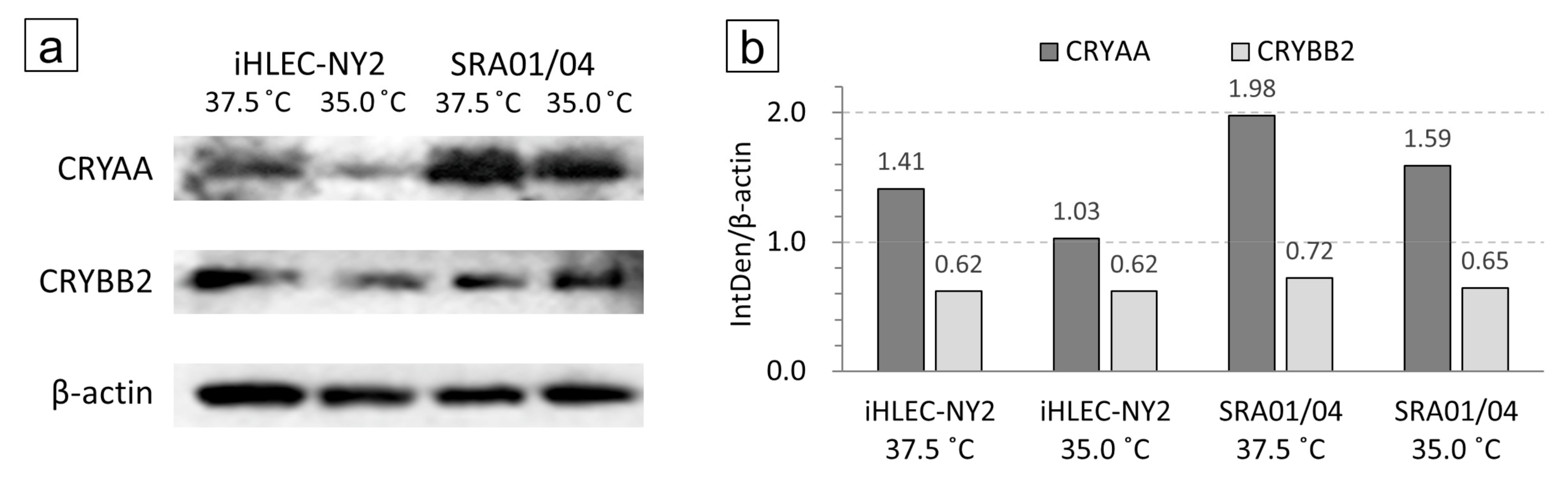
3.5. Analysis of Western Blotting at CRYAA and CRYBB2
3.6. Protein Concentrations of Human Aβ1-40 and Aβ1-42 in Culture Medium
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM10 | A disintegrin and metalloproteinase domain 10 |
| AICD | APP intracellular domain |
| APP | Amyloid precursor protein |
| AQPs | Aquaporins |
| Aβ | Amyloid β |
| BACE1 | β-site APP-cleaving enzyme 1 |
| bFGF | Basic fibroblast growth factor |
| cDNA | Complementary DNA |
| CMV | Cytomegalovirus |
| CRAYY | αA crystalline |
| CRYBB2 | βB2 crystalline |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ECE-1 | Endothelin converting enzyme 1 |
| ECL | Enhanced ChemiLuminescence |
| EF-1α | Elongation Factor-1α |
| ELISA | Enzyme-Linked Immuno Sorbent Assay |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HSPs | Heat shock proteins |
| iHLEC-NY2 | Immortalized human lens epithelial cell line clone NY2 |
| LECs | Lens epithelial cells |
| MEM-NEAA | Minimum essential medium non-essential amino acids |
| mSV40-GFP | Modified SV40 Large T antigen-GFP |
| NEP | Neprilysin |
| PCR | Polymerase chain reaction |
| PMSF | Phenylmethylsulfonyl fluoride |
| PS1 | Presenilin 1 |
| PS2 | Presenilin 2 |
| PVDF | Polyvinylidene difluoride |
| qPCR | Quantitative real-time PCR |
| RIPA | Radioimmune precipitation |
| SD | Standard deviation |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SPSS | Statistical Package for Social Science |
| TBS | Tris-buffered saline |
| TRPV1 | Transient receptor potential cation channel subfamily V member 1 |
| TRPV4 | Transient receptor potential cation channel subfamily V member 4 |
| UV | Ultra Violet |
| WHO | World Health Organization |
References
- Yamamoto, N.; Majima, K.; Marunouchi, T. A study of the proliferating activity in lens epithelium and the identification of tissue-type stem cells. Med. Mol. Morphol. 2008, 41, 83–91. [Google Scholar] [CrossRef]
- Miranda, M.N. The geographic factor in the onset of presbyopia. Trans. Am. Ophthalmol. Soc. 1979, 77, 603–621. [Google Scholar] [PubMed]
- Giblin, F.J.; Leverenz, V.R.; Padgaonkar, V.A.; Unakar, N.J.; Dang, L.; Lin, L.R.; Lou, M.F.; Reddy, V.N.; Borchman, D.; Dillon, J.P. UVA light in vivo reaches the nucleus of the guinea pig lens and produces deleterious, oxidative effects. Exp. Eye Res. 2002, 75, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Jonasson, F.; Shui, Y.B.; Kojima, M.; Ono, M.; Katoh, N.; Cheng, H.M.; Takahashi, N.; Sasaki, K. High prevalence of nuclear cataract in the population of tropical and subtropical areas. Dev. Ophthalmol. 2002, 35, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, L.C.; Hayashi, S.; Yamaoka, K.; Tamiya, N.; Chikuda, M.; Yano, E. Ultraviolet B exposure and type of lens opacity in ophthalmic patients in Japan. Sci. Total Environ. 2003, 302, 53–62. [Google Scholar] [CrossRef]
- Neale, R.E.; Purdie, J.L.; Hirst, L.W.; Green, A.C. Sun exposure as a risk factor for nuclear cataract. Epidemiology 2003, 14, 707–712. [Google Scholar] [CrossRef]
- Pastor-Valero, M.; Fletcher, A.E.; de Stavola, B.L.; Chaques-Alepuz, V. Years of sunlight exposure and cataract: A case-control study in a Mediterranean population. BMC Ophthalmol. 2007, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Sasaki, H.; Jonasson, F.; Kojima, M.; Cheng, H.M. Racial differences of lens transparency properties with aging and prevalence of age-related cataract applying a WHO classification system. Ophthalmic Res. 2004, 36, 332–340. [Google Scholar] [CrossRef]
- Miyashita, H.; Hatsusaka, N.; Shibuya, E.; Mita, N.; Yamazaki, M.; Shibata, T.; Ishida, H.; Ukai, Y.; Kubo, E.; Sasaki, H. Association between ultraviolet radiation exposure dose and cataract in Han people living in China and Taiwan: A cross-sectional study. PLoS ONE 2019, 14, e0215338. [Google Scholar] [CrossRef] [Green Version]
- Ibaraki, N.; Chen, S.C.; Lin, L.R.; Okamoto, H.; Pipas, J.M.; Reddy, V.N. Human lens epithelial cell line. Exp. Eye Res. 1998, 67, 577–585. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Shibata, T.; Kubo, E.; Sasaki, H. A positive feedback loop between nitric oxide and amyloid β (1-42) accelerates mitochondrial damage in human lens epithelial cells. Toxicology 2017, 381, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Mano, Y.; Otake, H.; Shibata, T.; Kubo, E.; Sasaki, H. Amyloid β1-43 accumulates in the lens epithelium of cortical opacification in Japanese patients. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3294–3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodera, S.; Hirata, A.; Miura, F.; Rashed, E.A.; Hatsusaka, N.; Yamamoto, N.; Kubo, E.; Sasaki, H. Model-based approach for analyzing prevalence of nuclear cataracts in elderly residents. Comput. Biol. Med. 2020, 126, 104009. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Hirata, A.; Hasegawa, K.; Kodera, S.; Laakso, I.; Sasaki, D.; Yamashita, T.; Egawa, R.; Horie, Y.; Yazaki, N.; et al. Risk Management of Heatstroke Based on Fast Computation of Temperature and Water Loss Using Weather Data for Exposure to Ambient Heat and Solar Radiation. IEEE Access 2018, 6, 3774–3785. [Google Scholar] [CrossRef]
- Inoue, Y. Longitudinal effects of age on heat-activated sweat gland density and output in healthy active older men. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 72–77. [Google Scholar] [CrossRef]
- Dufour, A.; Candas, V. Ageing and thermal responses during passive heat exposure: Sweating and sensory aspects. Eur. J. Appl. Physiol. 2007, 100, 19–26. [Google Scholar] [CrossRef]
- Hirata, A.; Nomura, T.; Laakso, I. Computational estimation of decline in sweating in the elderly from measured body temperatures and sweating for passive heat exposure. Physiol. Meas. 2012, 33, N51. [Google Scholar] [CrossRef]
- Nagaoka, T.; Watanabe, S.; Sakurai, K.; Kunieda, E.; Watanabe, S.; Taki, M.; Yamanaka, Y. Development of realistic high-resolution whole-body voxel models of Japanese adult males and females of average height and weight, and application of models to radio-frequency electromagnetic-field dosimetry. Phys. Med. Biol. 2004, 49, 1–15. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kato, Y.; Sato, A.; Hiramatsu, N.; Yamashita, H.; Ohkuma, M.; Miyachi, E.; Horiguchi, M.; Hirano, K.; Kojima, H. Establishment of a new immortalized human corneal epithelial cell line (iHCE-NY1) for use in evaluating eye irritancy by in vitro test methods. Vitr. Cell. Dev. Biol. Anim. 2016, 52, 742–748. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Yamamoto, N.; Isogai, S.; Onouchi, T.; Hirayama, M.; Maeda, S.; Ina, T.; Kondo, M.; Imaizumi, K. An analysis of monocytes and dendritic cells differentiated from human peripheral blood monocyte-derived induced pluripotent stem cells. Med. Mol. Morphol. 2020, 53, 63–72. [Google Scholar] [CrossRef]
- Isogai, S.; Yamamoto, N.; Hiramatsu, N.; Goto, Y.; Hayashi, M.; Kondo, M.; Imaizumi, K. Preparation of Induced Pluripotent Stem Cells Using Human Peripheral Blood Monocytes. Cell. Reprogram. 2018, 20, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Kakio, S.; Nakazawa, Y.; Kobata, K.; Funakoshi-Tago, M.; Suzuki, T.; Tamura, H. Roasted Coffee Reduces beta-Amyloid Production by Increasing Proteasomal beta-Secretase Degradation in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2018, 62, e1800238. [Google Scholar] [CrossRef] [PubMed]
- Kodera, S.; Kamiya, T.; Miyazawa, T.; Hirata, A. Correlation between Estimated Thermoregulatory Responses and Pacing in Athletes during Marathon. IEEE Access 2020, 8, 173079–173091. [Google Scholar] [CrossRef]
- Ishida, H.; Shibata, T.; Shibata, S.; Tanaka, Y.; Sasaki, H.; Kubo, E. Lutein plus Water Chestnut (Trapa bispinosa Roxb.) Extract Inhibits the Development of Cataracts and Induces Antioxidant Gene Expression in Lens Epithelial Cells. Biomed. Res. Int. 2020, 2020, 9204620. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Oceanic and Atmospheric Administration (NOAA). Climate Data Online. Available online: https://www.ncdc.noaa.gov/cdo-web/ (accessed on 2 October 2020).
- Vos, J.J.; van Norren, D. Thermal cataract, from furnaces to lasers. Clin. Exp. Optom. 2004, 87, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Rauf, A.; Aihie Sayer, A.; Wormald, R.P.; Cooper, C. Age-related nuclear lens opacities are associated with reduced growth before 1 year of age. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1740–1744. [Google Scholar]
- Truscott, R.J.W.; Friedrich, M.G. Molecular Processes Implicated in Human Age-Related Nuclear Cataract. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5007–5021. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; He, J.; Wang, C.; Wu, H.; Shi, X.; Zhang, H.; Xie, J.; Lee, S.Y. Smoking and risk of age-related cataract: A meta-analysis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3885–3895. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghadyan, A.A.; Cotlier, E. Rise in lens temperature on exposure to sunlight or high ambient temperature. Br. J. Ophthalmol. 1986, 70, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, B. Environmental Temperature and the Ocular Temperature Gradient. Arch Ophthalmol. 1965, 74, 237–243. [Google Scholar] [CrossRef]
- Cumming, R.G.; Mitchell, P.; Smith, W. Diet and cataract: The Blue Mountains Eye Study. Ophthalmology 2000, 107, 450–456. [Google Scholar] [CrossRef]
- Beebe, D.C. Nuclear cataracts and nutrition: Hope for intervention early and late in life. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1531–1534. [Google Scholar]
- West, S.K.; Munoz, B.; Schein, O.D.; Duncan, D.D.; Rubin, G.S. Racial differences in lens opacities: The Salisbury Eye Evaluation (SEE) project. Am. J. Epidemiol. 1998, 148, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Chylack, L.T., Jr.; He, Q.; Wu, S.Y.; Schoenfeld, E.; Friend, J.; Wolfe, J. Risk factors for nuclear opalescence in a longitudinal study. LSC Group. Longitudinal Study of Cataract. Am. J. Epidemiol. 1998, 147, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Storey, P.; Munoz, B.; Friedman, D.; West, S. Racial differences in lens opacity incidence and progression: The Salisbury Eye Evaluation (SEE) study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3010–3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santini, S.A.; Mordente, A.; Meucci, E.; Miggiano, G.A.; Martorana, G.E. Conformational stability of bovine alpha-crystallin. Evidence for a destabilizing effect of ascorbate. Biochem. J. 1992, 287, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luthra, M.; Balasubramanian, D. Nonenzymatic glycation alters protein structure and stability. A study of two eye lens crystallins. J. Biol. Chem. 1993, 268, 18119–18127. [Google Scholar]
- Miesbauer, L.R.; Zhou, X.; Yang, Z.; Yang, Z.; Sun, Y.; Smith, D.L.; Smith, J.B. Post-translational modifications of water-soluble human lens crystallins from young adults. J. Biol. Chem. 1994, 269, 12494–12502. [Google Scholar]
- Garland, D.L.; Duglas-Tabor, Y.; Jimenez-Asensio, J.; Datiles, M.B.; Magno, B. The nucleus of the human lens: Demonstration of a highly characteristic protein pattern by two-dimensional electrophoresis and introduction of a new method of lens dissection. Exp. Eye Res. 1996, 62, 285–291. [Google Scholar] [CrossRef]
- Takata, T.; Matsubara, T.; Nakamura-Hirota, T.; Fujii, N. Negative charge at aspartate 151 is important for human lens alphaA-crystallin stability and chaperone function. Exp. Eye Res. 2019, 182, 10–18. [Google Scholar] [CrossRef]
- Haslbeck, M.; Weinkauf, S.; Buchner, J. Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 2019, 294, 2121–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedziniak, J.A.; Kinoshita, J.H.; Yates, E.M.; Hocker, L.O.; Benedek, G.B. Calcium-induced aggregation of bovine lens alpha crystallins. Investig. Ophthalmol. 1972, 11, 905–915. [Google Scholar]
- Guptasarma, P.; Balasubramanian, D.; Matsugo, S.; Saito, I. Hydroxyl radical mediated damage to proteins, with special reference to the crystallins. Biochemistry 1992, 31, 4296–4303. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.T.; Sen, A.C.; Chakrabarti, B. Micellar subunit assembly in a three-layer model of oligomeric alpha-crystallin. J. Biol. Chem. 1991, 266, 20079–20084. [Google Scholar]
- Raman, B.; Rao, C.M. Chaperone-like activity and temperature-induced structural changes of alpha-crystallin. J. Biol. Chem. 1997, 272, 23559–23564. [Google Scholar] [CrossRef] [Green Version]
- Raman, B.; Ramakrishna, T.; Rao, C.M. Temperature dependent chaperone-like activity of alpha-crystallin. FEBS Lett. 1995, 365, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Maulucci, G.; Papi, M.; Arcovito, G.; De Spirito, M. The thermal structural transition of alpha-crystallin inhibits the heat induced self-aggregation. PLoS ONE 2011, 6, e18906. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, L.E.; Muffat, J.A.; Cherny, R.A.; Moir, R.D.; Ericsson, M.H.; Huang, X.; Mavros, C.; Coccia, J.A.; Faget, K.Y.; Fitch, K.A.; et al. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet 2003, 361, 1258–1265. [Google Scholar] [CrossRef]
- Moncaster, J.A.; Pineda, R.; Moir, R.D.; Lu, S.; Burton, M.A.; Ghosh, J.G.; Ericsson, M.; Soscia, S.J.; Mocofanescu, A.; Folkerth, R.D.; et al. Alzheimer’s disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS ONE 2010, 5, e10659. [Google Scholar] [CrossRef] [Green Version]
- Jun, G.; Moncaster, J.A.; Koutras, C.; Seshadri, S.; Buros, J.; McKee, A.C.; Levesque, G.; Wolf, P.A.; St George-Hyslop, P.; Goldstein, L.E.; et al. δ-Catenin is genetically and biologically associated with cortical cataract and future Alzheimer-related structural and functional brain changes. PLoS ONE 2012, 7, e43728. [Google Scholar] [CrossRef]
- Zheng, H.; Koo, E.H. The amyloid precursor protein: Beyond amyloid. Mol. Neurodegener. 2006, 1, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kukar, T.L.; Ladd, T.B.; Bann, M.A.; Fraering, P.C.; Narlawar, R.; Maharvi, G.M.; Healy, B.; Chapman, R.; Welzel, A.T.; Price, R.W.; et al. Substrate-targeting gamma-secretase modulators. Nature 2008, 453, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.C. Translational control of BACE1 may go awry in Alzheimer’s disease. Neuron 2008, 60, 941–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Takasugi, N.; Tomita, T.; Hayashi, I.; Tsuruoka, M.; Niimura, M.; Takahashi, Y.; Thinakaran, G.; Iwatsubo, T. The role of presenilin cofactors in the gamma-secretase complex. Nature 2003, 422, 438–441. [Google Scholar] [CrossRef]
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; d Paradis, M.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 1994, 265, 1464–1467. [Google Scholar] [CrossRef]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-beta in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef]
- Saito, T.; Suemoto, T.; Brouwers, N.; Sleegers, K.; Funamoto, S.; Mihira, N.; Matsuba, Y.; Yamada, K.; Nilsson, P.; Takano, J.; et al. Potent amyloidogenicity and pathogenicity of Abeta43. Nat. Neurosci. 2011, 14, 1023–1032. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Molazemhosseini, A.; Liu, C.C. In Vitro Quantified Determination of beta-Amyloid 42 Peptides, a Biomarker of Neuro-Degenerative Disorders, in PBS and Human Serum Using a Simple, Cost-Effective Thin Gold Film Biosensor. Biosensors 2017, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Eckman, E.A.; Reed, D.K.; Eckman, C.B. Degradation of the Alzheimer’s amyloid beta peptide by endothelin-converting enzyme. J. Biol. Chem. 2001, 276, 24540–24548. [Google Scholar] [CrossRef] [Green Version]
- Iwata, N.; Takaki, Y.; Fukami, S.; Tsubuki, S.; Saido, T.C. Region-specific reduction of A beta-degrading endopeptidase, neprilysin, in mouse hippocampus upon aging. J. Neurosci. Res. 2002, 70, 493–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco-Quinto, J.; Eckman, C.B.; Eckman, E.A. Major amyloid-beta-degrading enzymes, endothelin-converting enzyme-2 and neprilysin, are expressed by distinct populations of GABAergic interneurons in hippocampus and neocortex. Neurobiol. Aging 2016, 48, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Li, D.; Zheng, T.; Lu, Y. beta-amyloid expression in age-related cataract lens epithelia and the effect of beta-amyloid on oxidative damage in human lens epithelial cells. Mol. Vis. 2017, 23, 1015–1028. [Google Scholar] [PubMed]
- Nakazawa, Y.; Donaldson, P.J.; Petrova, R.S. Verification and spatial mapping of TRPV1 and TRPV4 expression in the embryonic and adult mouse lens. Exp. Eye Res. 2019, 186, 107707. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Takemoto, L.J.; Momose, Y.; Matsumoto, S.; Hiroki, K.; Akaboshi, M. Formation of four isomers at the asp-151 residue of aged human alphaA-crystallin by natural aging. Biochem. Biophys. Res. Commun. 1999, 265, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Matsumoto, S.; Hiroki, K.; Takemoto, L. Inversion and isomerization of Asp-58 residue in human alphaA-crystallin from normal aged lenses and cataractous lenses. Biochim. Biophys. Acta 2001, 1549, 179–187. [Google Scholar] [CrossRef]
- Truscott, R.J. Age-related nuclear cataract: A lens transport problem. Ophthalmic Res. 2000, 32, 185–194. [Google Scholar] [CrossRef]




| 37.5 °C | 35.0 °C | |||||
|---|---|---|---|---|---|---|
| iHLEC-NY2 | SRA01/04 | p Value | iHLEC-NY2 | SRA01/04 | p-Value | |
| CRYAA/GAPDH | 6.7 ± 1.1 (×10−6) | 13.4 ± 2.0 (×10−6) | p < 0.05 | 5.6 ± 0.3 (×10−6) | 7.0 ± 0.9 (×10−6) | n.s |
| CRYBB2/GAPDH | 15.4 ± 0.1 (×10−6) | 6.3 ± 0.1 (×10−6) | p < 0.001 | 17.6 ± 0.7 (×10−6) | 6.2 ± 1.0 (×10−6) | p < 0.01 |
| APP/GAPDH | 2.1 ± 0.6 (×10−1) | 1.5 ± 0.3 (×10−1) | n.s | 1.2 ± 0.2 (×10−1) | 1.4 ± 0.2 (×10−1) | n.s |
| ADAM10/GAPDH | 4.4 ± 2.3 (×10−3) | 2.1 ± 1.0 (×10−3) | n.s | 1.3 ± 0.3 (×10−3) | 1.1 ± 0.2 (×10−3) | n.s |
| BACE1/GAPDH | 2.9 ± 0.3 (×10−3) | 2.1 ± 0.8 (×10−3) | n.s | 1.7 ± 0.4 (×10−3) | 2.2 ± 0.9 (×10−3) | n.s |
| PS1/GAPDH | 3.0 ± 0.6 (×10−4) | 13.4 ± 2.6 (×10−4) | p < 0.05 | 3.1 ± 2.7 (×10−4) | 6.6 ± 5.6 (×10−4) | n.s |
| PS2/GAPDH | 3.7 ± 0.3 (×10−4) | 14.1 ± 4.8 (×10−4) | p < 0.01 | 3.8 ± 0.9 (×10−4) | 13.6 ± 2.6 (×10−4) | p < 0.001 |
| NEP/GAPDH | 2.5 ± 0.3 (×10−3) | 6.2 ± 4.0 (×10−3) | n.s | 0.4 ± 0.1 (×10−3) | 3.5 ± 2.2 (×10−3) | p < 0.05 |
| ECE-1/GAPDH | 8.4 ± 3.0 (×10−3) | 2.8 ± 1.0 (×10−3) | p < 0.05 | 2.0 ± 1.4 (×10−3) | 1.2 ± 0.3 (×10−3) | n.s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, N.; Takeda, S.; Hatsusaka, N.; Hiramatsu, N.; Nagai, N.; Deguchi, S.; Nakazawa, Y.; Takata, T.; Kodera, S.; Hirata, A.; et al. Effect of a Lens Protein in Low-Temperature Culture of Novel Immortalized Human Lens Epithelial Cells (iHLEC-NY2). Cells 2020, 9, 2670. https://doi.org/10.3390/cells9122670
Yamamoto N, Takeda S, Hatsusaka N, Hiramatsu N, Nagai N, Deguchi S, Nakazawa Y, Takata T, Kodera S, Hirata A, et al. Effect of a Lens Protein in Low-Temperature Culture of Novel Immortalized Human Lens Epithelial Cells (iHLEC-NY2). Cells. 2020; 9(12):2670. https://doi.org/10.3390/cells9122670
Chicago/Turabian StyleYamamoto, Naoki, Shun Takeda, Natsuko Hatsusaka, Noriko Hiramatsu, Noriaki Nagai, Saori Deguchi, Yosuke Nakazawa, Takumi Takata, Sachiko Kodera, Akimasa Hirata, and et al. 2020. "Effect of a Lens Protein in Low-Temperature Culture of Novel Immortalized Human Lens Epithelial Cells (iHLEC-NY2)" Cells 9, no. 12: 2670. https://doi.org/10.3390/cells9122670
APA StyleYamamoto, N., Takeda, S., Hatsusaka, N., Hiramatsu, N., Nagai, N., Deguchi, S., Nakazawa, Y., Takata, T., Kodera, S., Hirata, A., Kubo, E., & Sasaki, H. (2020). Effect of a Lens Protein in Low-Temperature Culture of Novel Immortalized Human Lens Epithelial Cells (iHLEC-NY2). Cells, 9(12), 2670. https://doi.org/10.3390/cells9122670







