Preemptive Analgesic Effect of Intrathecal Applications of Neuroactive Steroids in a Rodent Model of Post-Surgical Pain: Evidence for the Role of T-Type Calcium Channels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
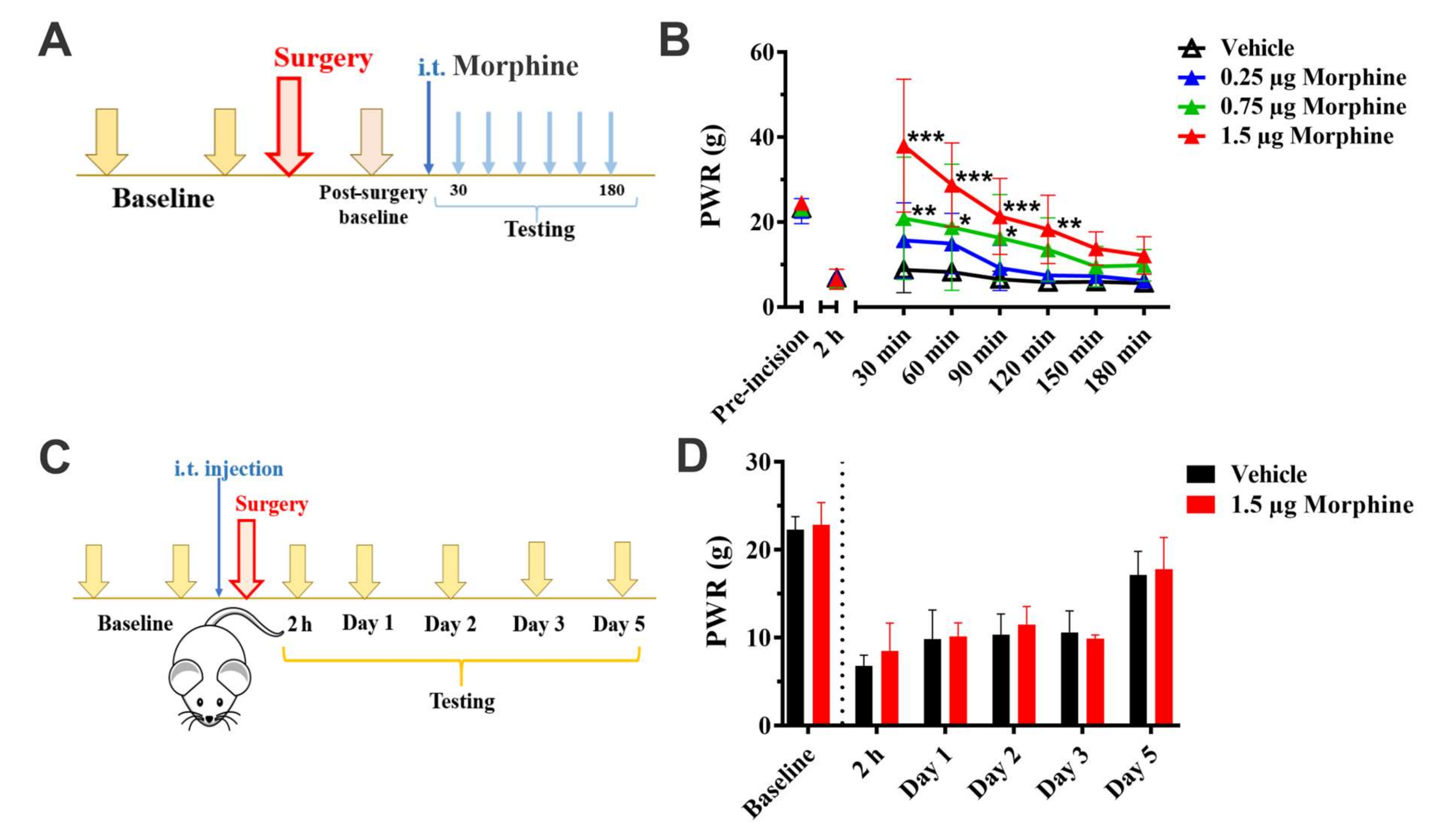
2.3. Incisional Pain Model
2.4. Intrathecal Injections
2.5. Mechanical Sensitivity
2.6. Data Analysis and Statistics
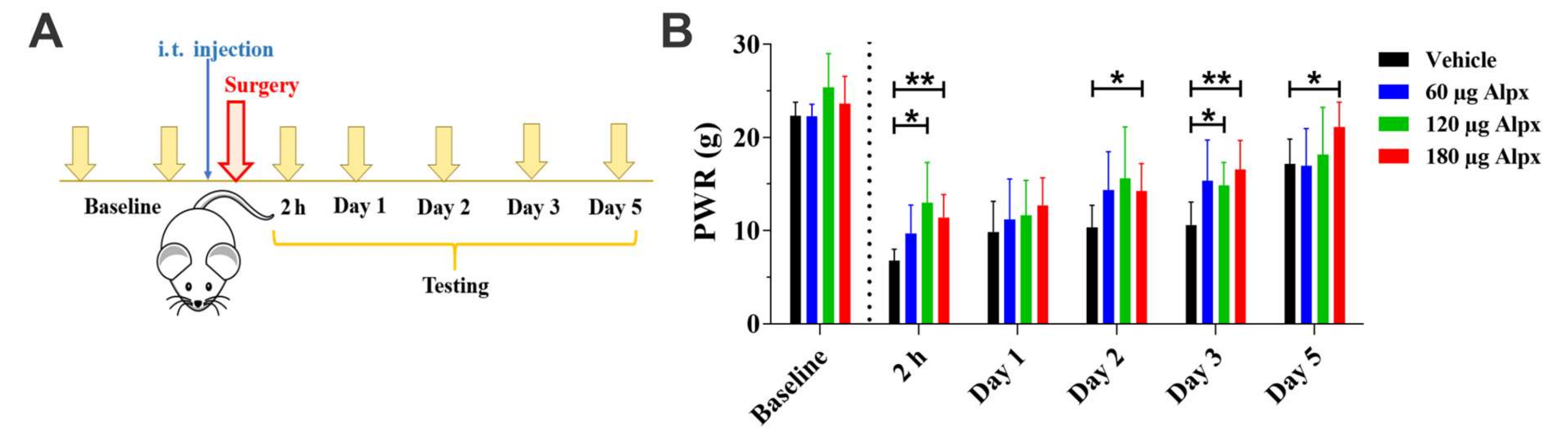
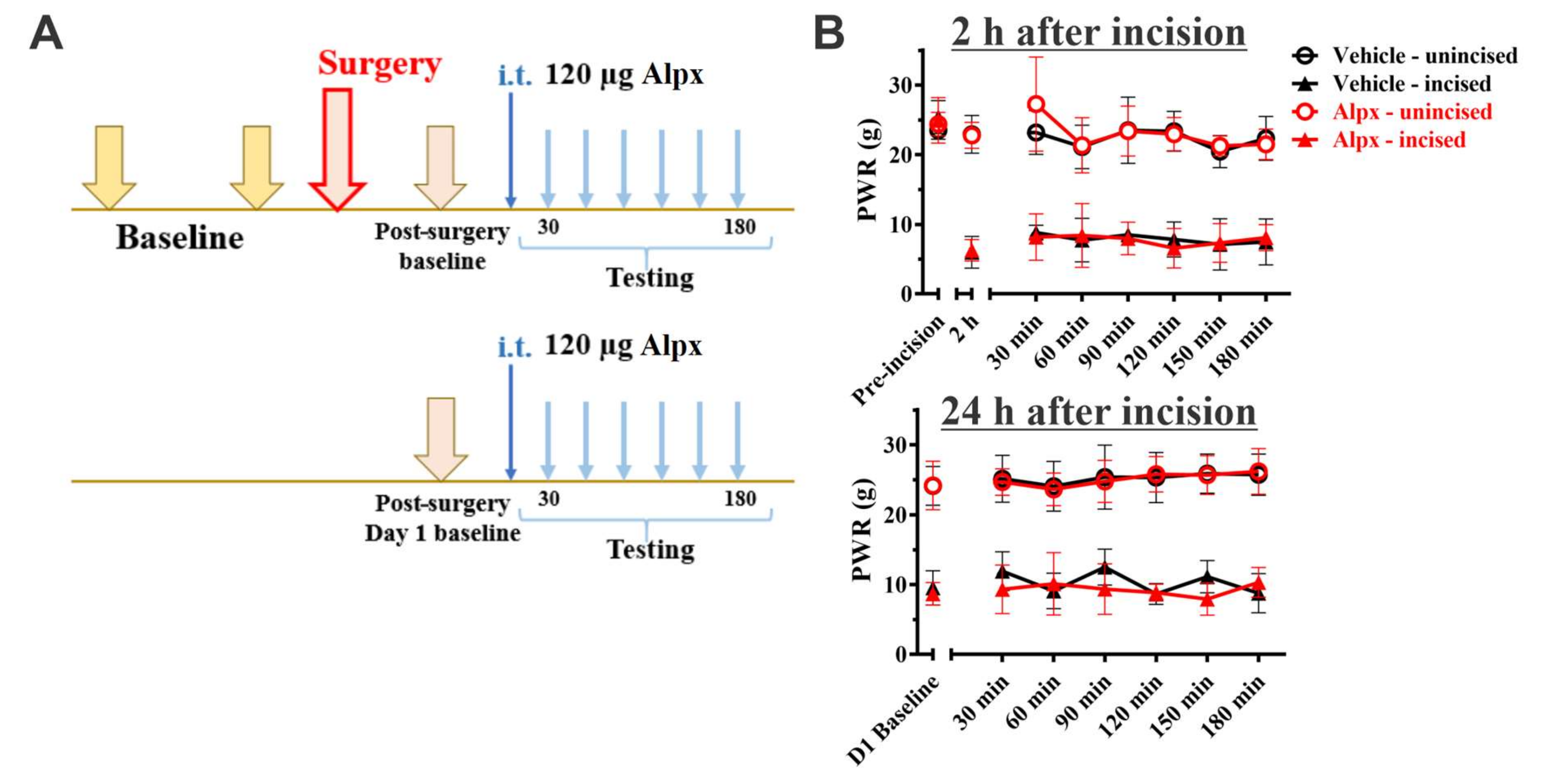
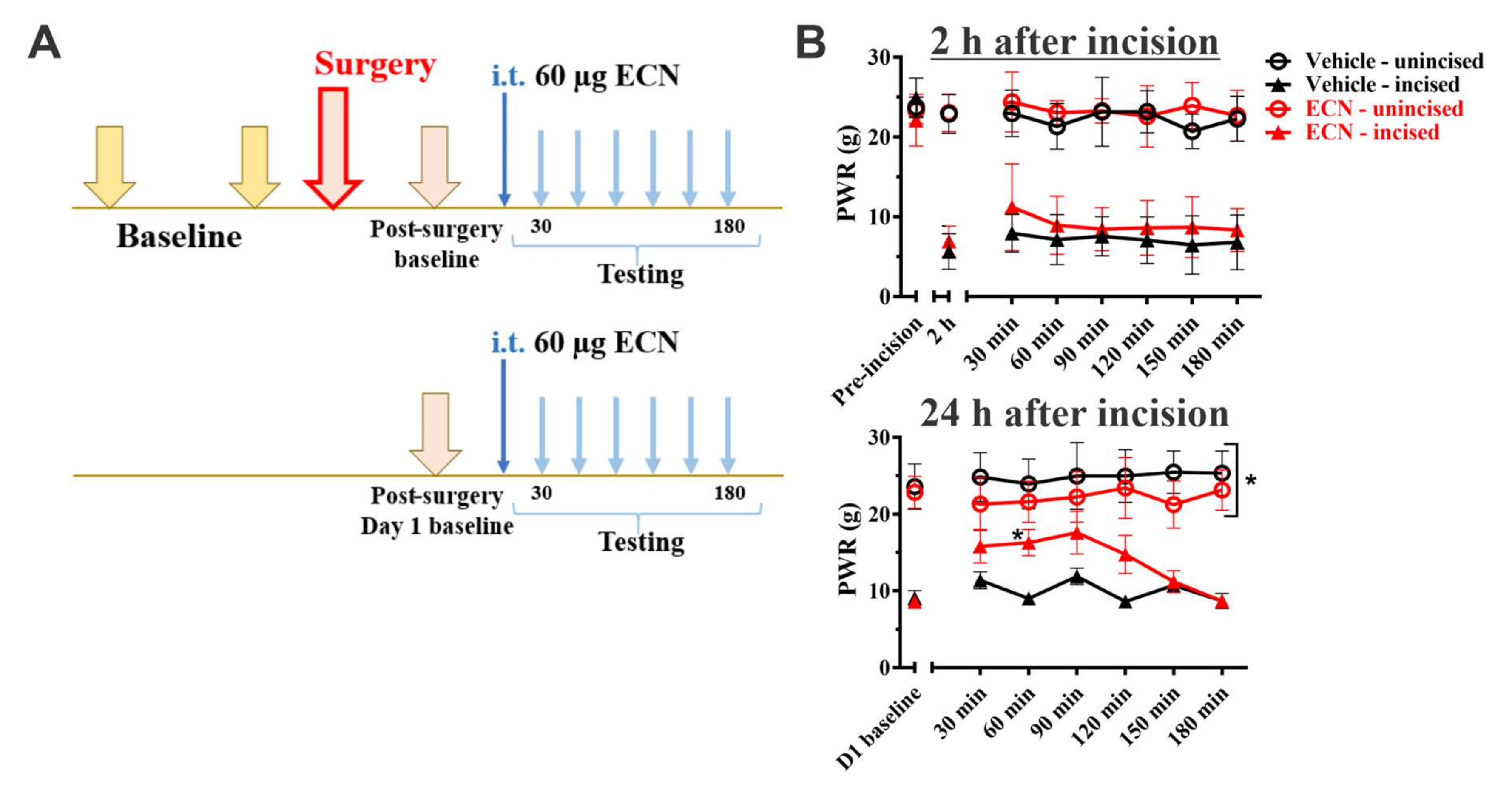
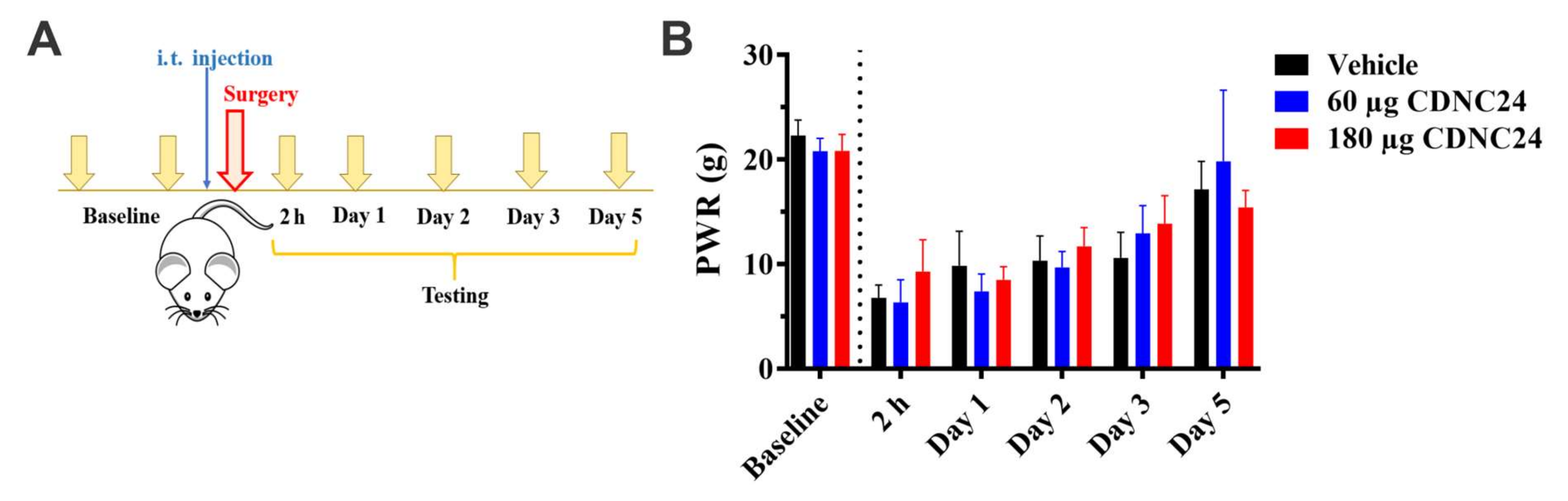
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whiteside, G.T.; Harrison, J.; Boulet, J.; Mark, L.; Pearson, M.; Gottshall, S.; Walker, K. Pharmacological characterization of a rat model of incisional pain. Br. J. Pharmacol. 2003, 141, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.X.; Pettus, M.; Gao, D.; Phillips, C.; Bowersox, S. Effects of intrathecal administration of ziconotide, a selective neuronal N-type calcium channel blocker, on mechanical allodynia and heat hyperalgesia in a rat model of postoperative pain. Pain 2000, 84, 151–158. [Google Scholar] [CrossRef]
- Zahn, P.K.; Brennan, T.J. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology 1999, 90, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.J. Comparison of Pre- versus Post-Incision Administration of Intrathecal Bupivacaine and Intrathecal Morphine in a Rat Model of Postoperative Pain. Anesthesiology 1997, 87, 1517–1528. [Google Scholar] [CrossRef]
- Schug, S.A.; Zech, D.; Grond, S. Adverse effects of systemic opioid analgesics. Drug Saf. 1992, 7, 200–213. [Google Scholar] [CrossRef]
- Meyer, R.; Patel, A.M.; Rattana, S.K.; Quock, T.P.; Mody, S.H. Prescription opiod abuse: A literature review of the clinical and economic burden in the United States. Popul. Health Manag. 2014, 17, 372–387. [Google Scholar] [CrossRef] [Green Version]
- The National Center for Health Statistics. NCHS Data on Drug Poisoning Deaths. December 2012. Available online: http://www.cdc.gov/nchs/data/factsheets/factsheet_drug_poisoning.pdf (accessed on 21 September 2020).
- Jacus, M.O.; Uebele, V.N.; Renger, J.J.; Todorovic, S.M. Presynaptic Cav3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J. Neurosci. 2012, 32, 9374–9382. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.T.; Joksovic, P.M.; Perez-Reyes, E.; Todorovic, S.M. The endogenous redox agent L-cysteine induces T-type Ca2+ channel-dependent sensitization of a novel subpopulation of rat peripheral nociceptors. J. Neurosci. 2005, 25, 8766–8775. [Google Scholar] [CrossRef] [Green Version]
- Todorovic, S.M.; Jevtovic-Todorovic, V.; Meyenburg, A.; Mennerick, S.; Perez-Reyes, E.; Romano, C.; Olney, J.W.; Zorumski, C.F. Redox modulation of T-type calcium channels in rat peripheral nociceptors. Neuron 2001, 31, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Todorovic, S.M.; Pathirathna, S.; Brimelow, B.C.; Jagodic, M.M.; Ko, S.H.; Jiang, X.; Nilsson, K.R.; Zorumski, C.F.; Covey, D.F.; Jevtovic-Todorovic, V. 5β -Reduced Neuroactive Steroids Are Novel Voltage-Dependent Blockers of T-Type Ca2+ Channels in Rat Sensory Neurons in Vitro and Potent Peripheral Analgesics in Vivo. Mol. Pharm. 2004, 66, 1223–1235. [Google Scholar] [CrossRef]
- Bourinet, E.; Alloui, A.; Monteiol, A.; Barrere, C.; Couette, B.; Poirot, O.; Pages, A.; McRory, J.; Snutch, T.P.; Eschalier, A.; et al. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 2005, 24, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoola, C.; Hwang, S.M.; Hong, S.J.; Rose, K.E.; Boyd, C.; Bozic, N.; Park, J.Y.; Osuru, H.P.; DiGruccio, M.R.; Covey, D.F.; et al. Inhibition of CaV3.2 T-Type Calcium Channels in Peripheral Sensory Neurons Contributes to Analgesic Properties of Epipregnanolone. Psychopharmacology 2014, 231, 3503–3515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messinger, R.B.; Naik, A.K.; Jagodic, M.M.; Nelson, M.T.; Lee, W.Y.; Choe, W.J.; Orestes, P.; Latham, J.R.; Todorovic, S.M.; Jevtovic-Todorovic, V. In-vivo silencing of the Cav3.2 T-type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin-induced diabetic neuropathy. Pain 2009, 145, 184–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joksimovic, S.L.; Covey, D.F.; Jevtovic-Todorovic, V.; Todorovic, S.M. Neurosteroids in Pain Management: A New Perspective on an Old Player. Front. Pharmacol. 2018, 9, 1127, eCollection 2018. [Google Scholar] [CrossRef] [Green Version]
- Joksimovic, S.L.; Joksimovic, S.M.; Manzella, F.M.; Asnake, B.; Orestes, P.; Raol, Y.H.; Krishnan, K.; Covey, D.F.; Jevtovic-Todorovic, V.; Todorovic, S.M. Novel neuroactive steroid with hypnotic and T-type calcium channel blocking properties exerts effective analgesia in a rodent model of post-surgical pain. Br. J. Pharm. 2020, 177, 1735–1753. [Google Scholar] [CrossRef]
- Todorovic, S.M.; Prakriya, M.; Nakashima, Y.M.; Nillson, K.R.; Han, M.; Zorumski, C.F.; Covey, D.F.; Lingle, C.J. Enantioselective blockade of T-type Ca2+ current in adult rat sensory neurons by a steroid that lacks GABA-modulatory activity. Mol. Pharmacol. 1998, 54, 918–927. [Google Scholar] [CrossRef] [Green Version]
- Pathirathna, S.; Brimelow, B.C.; Jagodic, M.M.; Krishnan, K.; Jiang, X.; Zorumski, C.F.; Mennerick, S.; Covey, D.F.; Todorovic, S.M.; Jevtovic-Todorovic, V. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5alpha-reduced neuroactive steroids. Pain 2005, 114, 429–443. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Ralvenius, W.T.; Acuña, M.A. Restoring the spinal pain gate: GABA(A) receptors as targets for novel analgesics. Adv. Pharmacol. 2015, 73, 71–96. [Google Scholar]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 11, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Mogil, J.S.; Chanda, M.L. The case for the inclusion of female subjects in basic science studies of pain. Pain 2005, 117, 1–5. [Google Scholar] [CrossRef]
- Han, M.; Zorumski, C.F.; Covey, D.F. Neurosteroid Analogues. 4. The Effect of Methyl Substitution at the C-5 and C-10 Positions of Neurosteroids on Electrophysiological Activity at GABA(A) Receptors. J. Med. Chem. 1996, 39, 4218–4232. [Google Scholar] [CrossRef] [PubMed]
- Atluri, N.; Joksimovic, S.M.; Oklopcic, A.; Milanovic, D.; Klawitter, J.; Eggan, P.; Krishnan, K.; Covey, D.F.; Todorovic, S.M.; Jevtovic-Todorovic, V. A Neurosteroid Analogue with T-Type Calcium Channel Blocking Properties Is an Effective Hypnotic, but Is Not Harmful to Neonatal Rat Brain. Br. J. Anaesth. 2018, 120, 768–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesic, V.; Joksimovic, S.M.; Quillinan, N.; Krishnan, K.; Covey, D.F.; Todorovic, S.M.; Jevtovic-Todorovic, V. Neuroactive steroids alphaxalone and CDNC24 are effective hypnotics and potentiators of GABAA currents, but are not neurotoxic to the developing rat brain. Br. J. Anaesth. 2020, 124, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.J.; Vandermeulen, E.P.; Gebhart, G.F. Characterization of a Rat Model of Incisional Pain. Pain 1996, 64, 493–501. [Google Scholar] [CrossRef]
- Brennan, T.J. Pathophysiology of postoperative pain. Pain 2011, 152, S33–S40. [Google Scholar] [CrossRef] [Green Version]
- Zorumski, C.F.; Mennerick, S.; Isenberg, K.E.; Covey, D.F. Potential clinical uses of neuroactive steroids. Curr. Opin. Investig. Drugs 2000, 1, 360–369. [Google Scholar]
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef]
- Meyer, L.; Taleb, O.; Patte-Mensah, C.; Mensah-Nyagan, A.G. Neurosteroids and neuropathic pain management: Basic evidence and therapeutic perspectives. Front. Neuroendocr. 2019, 55, 100795. [Google Scholar] [CrossRef]
- Choi, S.; Na, H.S.; Kim, J.; Lee, J.; Lee, S.; Kim, D.; Park, J.; Chen, C.C.; Campbell, K.P.; Shin, H.S. Attenuated Pain Responses in Mice Lacking CaV3.2 T-Type Channels. Genes Brain Behav. 2007, 6, 425–431. [Google Scholar] [CrossRef]
- Pathirathna, S.; Todorovic, S.M.; Covey, D.F.; Jevtovic-Todorovic, V. 5α-Reduced Neuroactive Steroids Alleviate Thermal and Mechanical Hyperalgesia in Rats With Neuropathic Pain. Pain 2005, 117, 326–339. [Google Scholar] [CrossRef]
- Choe, W.; Messinger, R.B.; Leach, E.; Eckle, V.-S.; Obradovic, A.; Salajegheh, R.; Jevtovic-Todorovic, V.; Todorovic, S.M. TTA-P2 is a potent and selective blocker of T-type calcium channels in rat sensory neurons and a novel antinociceptive agent. Mol. Pharm. 2011, 80, 900–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensah-Nyagan, A.G.; Kibaly, C.; Schaeffer, V.; Venard, C.; Meyer, L.; Patte-Mensah, C. Endogenous Steroid Production in the Spinal Cord and Potential Involvement in Neuropathic Pain Modulation. J. Steroid Biochem. Mol. Biol. 2008, 109, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.M.; Pereverzev, A.; Schneider, T.; Covey, D.F.; Lingle, C.J. Blockade of Ba2+ current through human alpha1E channels by two steroid analogs, (+)-ACN and (+)-ECN. Neuropharmacology 1999, 38, 843–855. [Google Scholar] [CrossRef]
- Jiang, X.; Manion, B.D.; Benz, A.; Rath, N.P.; Evers, A.S.; Zorumski, C.F.; Mennerick, S.; Covey, D.F. Neurosteroid analogues. 9. Conformationally constrained pregnanes: Structure-activity studies of 13,24-cyclo-18,21-dinorcholane analogues of the GABA modulatory and anesthetic steroids (3alpha,5alpha)- and (3alpha,5beta)-3-hydroxypregnan-20-one. J. Med. Chem. 2003, 46, 5334–5348. [Google Scholar] [CrossRef]
- Baulieu, E.E.; Robel, P. Neurosteroids: A New Brain Function? J. Steroid Biochem. Mol. Biol. 1990, 37, 395–403. [Google Scholar] [CrossRef]
- Byrne, K.; Smith, C. Preemptive Analgesia: An Unobtainable Goal? J. Cardiothorac. Vasc. Anesth. 2019, 33, 460–461. [Google Scholar] [CrossRef] [Green Version]
- Chapman, C.R.; Duncan, A.S.; Lipman, A.G. Quality of Postoperative Pain Management in American versus European Institutions. J. Pain Palliat. Care Pharm. 2013, 27, 350–358. [Google Scholar] [CrossRef]
- Chang, W.K.; Tao, Y.X.; Chuang, C.C.; Chen, P.T.; Chan, K.H.; Chu, Y.C. Lack of beneficial effect for preemptive analgesia in postoperative pain control: Verifying the efficacy of preemptive analgesia with N-methyl-D-aspartate receptor antagonists in a modified animal model of postoperative pain. Anesth. Analg. 2011, 112, 710–718. [Google Scholar] [CrossRef]
- Ong, C.K.; Lirk, P.; Seymour, R.A.; Jenkins, B.J. The efficacy of preemptive analgesia for acute postoperative pain management: A meta-analysis. Anesth. Analg. 2005, 100, 757–773. [Google Scholar] [CrossRef]
- Møiniche, S.; Kehlet, H.; Dahl, J.B. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: The role of timing of analgesia. Anesthesiology 2002, 96, 725–741. [Google Scholar] [CrossRef]
- Kissin, I. Preemptive analgesia: Terminology and clinical relevance. Anesth. Analg. 1994, 79, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, O.; Dahl, B.; Thomsen, B.A.; Kitter, B.; Sonne, N.; Dahl, J.B.; Kehlet, H. A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Euro. Spine J. 2013, 22, 2089–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluka, K.A.; Radhakrishnan, R.; Benson, C.J.; Eshcol, J.O.; Price, M.P.; Babinski, K.; Audette, K.M.; Yeomans, D.C.; Wilson, S.P. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain 2007, 129, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Deval, E.; Noël, J.; Lay, N.; Alloui, A.; Diochot, S.; Friend, V.; Jodar, M.; Lazdunski, M.; Lingueglia, E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008, 27, 3047–3055. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tat, Q.L.; Joksimovic, S.M.; Krishnan, K.; Covey, D.F.; Todorovic, S.M.; Jevtovic-Todorovic, V. Preemptive Analgesic Effect of Intrathecal Applications of Neuroactive Steroids in a Rodent Model of Post-Surgical Pain: Evidence for the Role of T-Type Calcium Channels. Cells 2020, 9, 2674. https://doi.org/10.3390/cells9122674
Tat QL, Joksimovic SM, Krishnan K, Covey DF, Todorovic SM, Jevtovic-Todorovic V. Preemptive Analgesic Effect of Intrathecal Applications of Neuroactive Steroids in a Rodent Model of Post-Surgical Pain: Evidence for the Role of T-Type Calcium Channels. Cells. 2020; 9(12):2674. https://doi.org/10.3390/cells9122674
Chicago/Turabian StyleTat, Quy L., Srdjan M. Joksimovic, Kathiresan Krishnan, Douglas F. Covey, Slobodan M. Todorovic, and Vesna Jevtovic-Todorovic. 2020. "Preemptive Analgesic Effect of Intrathecal Applications of Neuroactive Steroids in a Rodent Model of Post-Surgical Pain: Evidence for the Role of T-Type Calcium Channels" Cells 9, no. 12: 2674. https://doi.org/10.3390/cells9122674



