Role of Aquaporins in the Physiological Functions of Mesenchymal Stem Cells
Abstract
1. Introduction
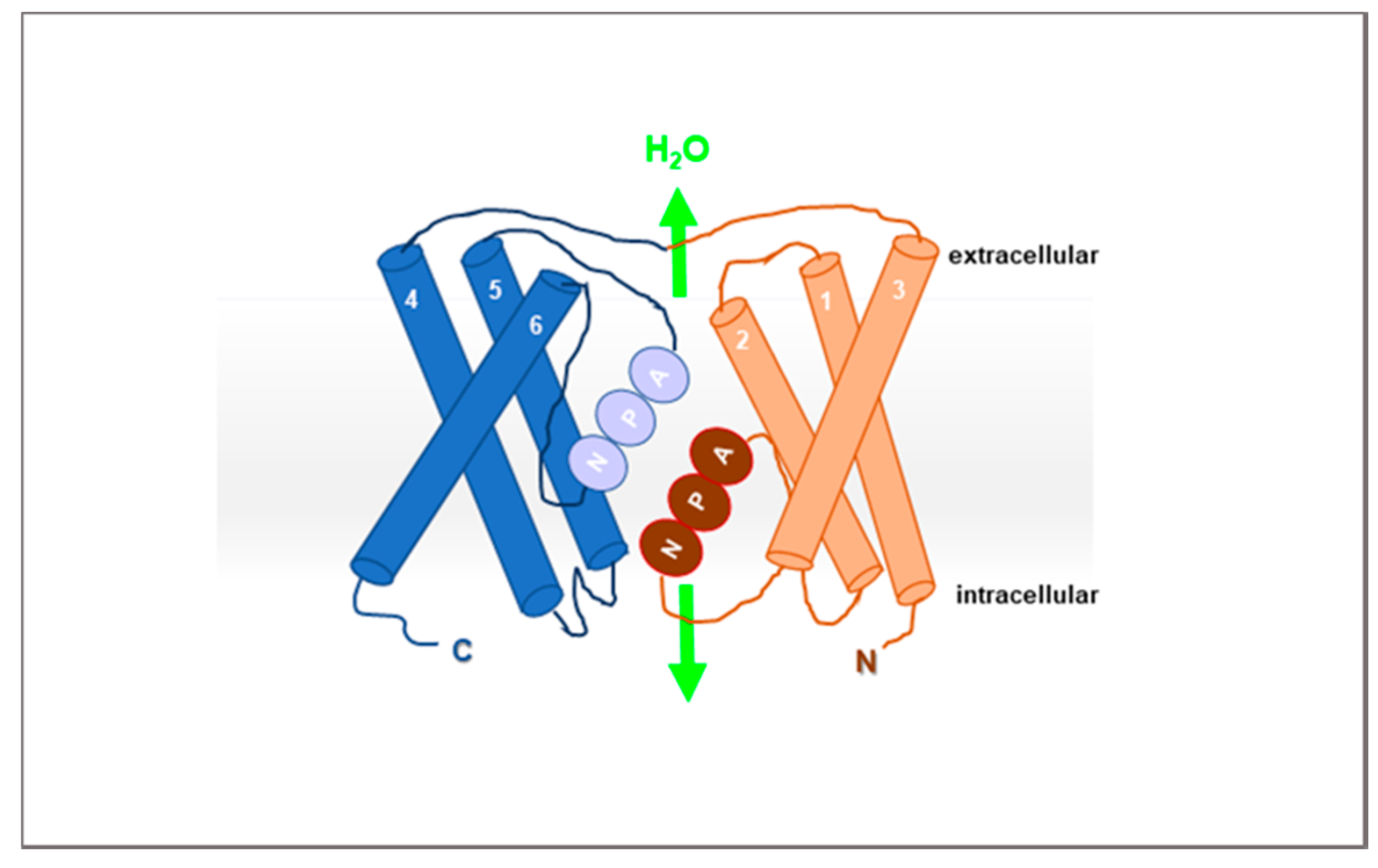
2. AQPs
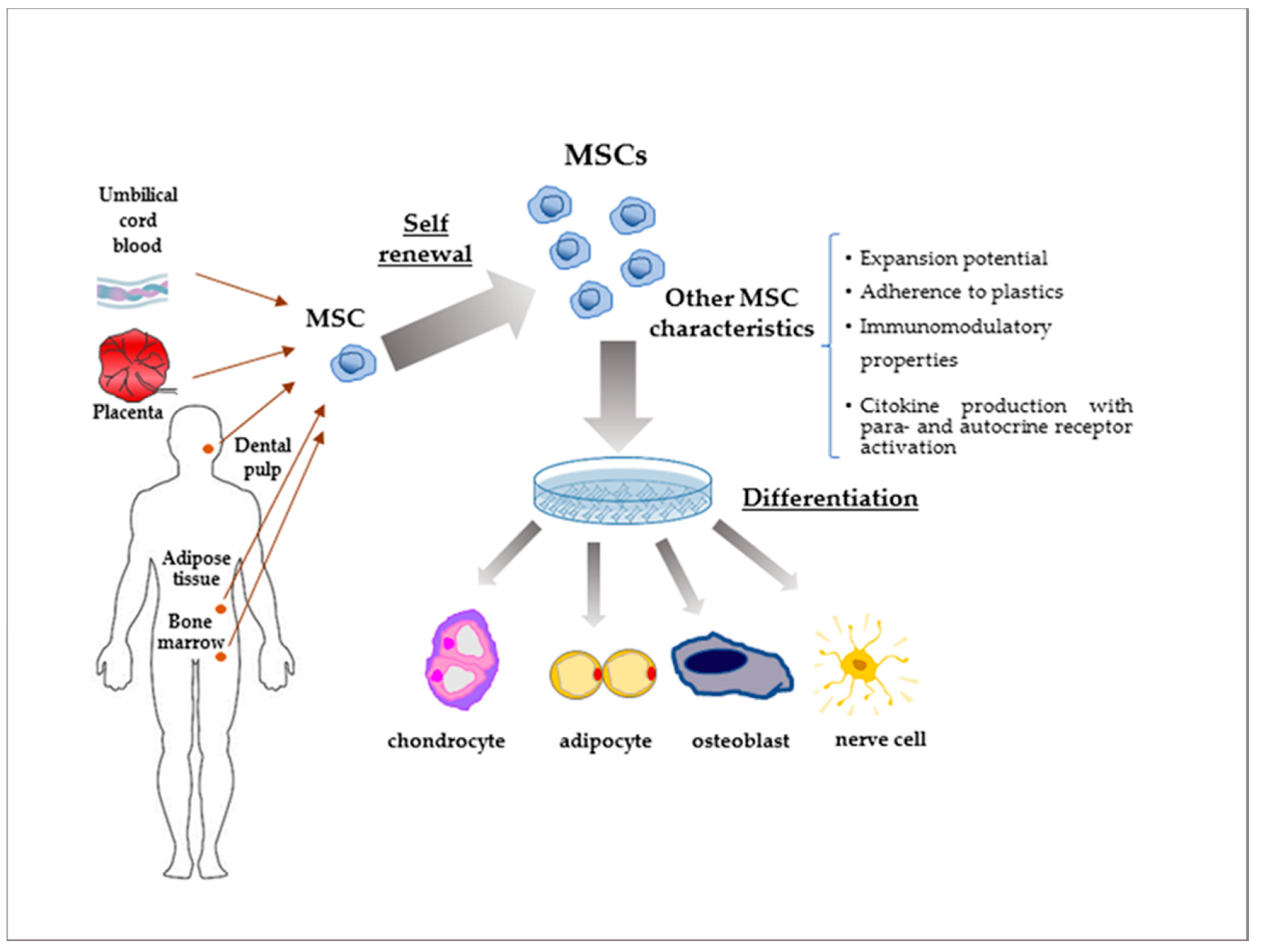
3. MSCs
4. Physiological Roles of AQPs in Driving MSC Function
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benga, G. Water channel proteins (later called aquaporins) and relatives: Past, present, and future. IUBMB Life 2009, 61, 112–133. [Google Scholar] [CrossRef] [PubMed]
- Benga, G. On the definition, nomenclature and classification of water channel proteins (aquaporins and relatives). Mol. Aspects Med. 2012, 33, 514–517. [Google Scholar] [CrossRef]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta 2014, 1840, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Day, R.E.; Salman, M.M.; Conner, M.T.; Bill, R.M.; Conner, A.C. Beyond water homeostasis: Diverse functional roles of mammalian aquaporins. Biochim. Biophys. Acta 2015, 1850, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Squillacioti, C.; Mirabella, N.; Meli, R. Aquaporins in health and disease: An overview focusing on the gut of different species. Int. J. Mol. Sci. 2016, 17, 1213. [Google Scholar] [CrossRef]
- Verkman, A. Aquaporins at a glance. J. Cell. Sci. 2011, 124, 2107–2112. [Google Scholar] [CrossRef]
- Login, F.H.; Jensen, H.H.; Pedersen, G.A.; Koffman, J.S.; Kwon, T.H.; Parsons, M.; Nejsum, L.N. Aquaporins differentially regulate cell-cell adhesion in MDCK cells. FASEB J. 2019, 33, 6980–6994. [Google Scholar] [CrossRef]
- Elkhider, A.; Wang, B.; Ouyang, X.; Al-Azab, M.; Walana, W.; Sun, X.; Li, H.; Tang, Y.; Wei, J.; Li, X. Aquaporin 5 promotes tumor migration and angiogenesis in non-small cell lung cancer cell line H1299. Oncol. Lett. 2020, 19, 1665–1672. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazik, H.; Giordano, E.; Barbanti Brodano, G.; Griffoni, C.; De Falco, E.; Pelagalli, A. Substantial overview on Mesenchymal Stem Cell biological and physical properties as an opportunity in translational medicine. Int. J. Mol. Sci. 2019, 20, 5386. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal stem cell migration and tissue repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Te lindert, M.; Krause, M.; Alexander, S.; Te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Donders, R.; Bogie, J.F.J.; Ravanidis, S.; Gervois, P.; Vanheusden, M.; Marée, R.; Schrynemackers, M.; Smeets, H.J.M.; Pinxteren, J.; Gijbels, K.; et al. Human Wharton’s jelly-derived stem cells display a distinct immunomodulatory and proregenerative transcriptional signature compared to bone marrow-derived stem cells. Stem Cells Develop. 2018, 27, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Beeravolu, N.; McKee, C.; Alamri, A.; Mikhael, S.; Brown, C.; Perez-Cruet, M.; Rasul Chaudhry, G. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J. Vis. Exp. 2017, 122, 55224. [Google Scholar] [CrossRef]
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The analysis of in vivo aging in human bone marrow mesenchymal stromal cells using colony-forming unit-fibroblast assay and the cd45(low)cd271(+) phenotype. Stem Cells Int. 2019, 5197983. [Google Scholar] [CrossRef]
- Zhu, C.; Yu, J.; Pan, Q.; Yang, J.; Hao, G.; Wang, Y.; Li, L.; Cao, H. Hypoxia-inducible factor-2 αpromotes the proliferation of human placenta-derived mesenchymal stem cells through the MAPK/ERK signaling pathway. Sci. Rep. 2016, 6, 35489. [Google Scholar] [CrossRef]
- Fontaine, M.J.; Shih, H.; Schäfer, R.; Pittenger, M.F. Unraveling the mesenchymal stromal cells’ paracrine immunomodulatory effects. Transfus. Med. Rev. 2016, 30, 37–43. [Google Scholar] [CrossRef]
- Konala, V.B.R.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol. Renal Physiol. 2000, 278, F13–F28. [Google Scholar] [CrossRef]
- Rojek, A.; Praetorius, J.; Frokiaer, J.; Nielsen, S.; Fenton, R.A. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef]
- Ishibashi, K.; Hara, S.; Kondo, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009, 13, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Han, B.-G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature 2001, 414, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Hub, J.S.; de Groot, B.L. Mechanism of selectivity in aquaporins and aquaglyceroporins. Proc. Natl. Acad. Sci. USA 2008, 105, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Kreida, S.; Törnroth-Horsefield, S. Structural insights into aquaporin selectivity and regulation. Curr. Opin. Struct. Biol. 2015, 33, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Preston, G.M.; Smith, B.L.; Guggino, W.B.; Agre, P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. 1994, 269, 14648–14654. [Google Scholar]
- Jensen, M.Ø.; Tajkhorshid, E.; Schulten, K. The mechanism of glycerol conduction in aquaglyceroporins. Structure 2001, 9, 1083–1093. [Google Scholar] [CrossRef]
- Conner, A.C.; Bill, R.M.; Conner, M.T. An emerging consensus on aquaporin translocation as a regulatory mechanism. Mol. Membr. Biol. 2013, 30, 1–12. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins in Clinical Medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef]
- Dasdelen, D.; Mogulkoc, R.; Baltaci, A.K. Aquaporins and roles in brain health and brain injury. Mini Rev. Med. Chem. 2020, 20, 498–512. [Google Scholar] [CrossRef]
- Badaut, J.; Lasbennes, F.; Magistretti, P.J.; Regli, L. Aquaporins in brain: Distribution, physiology, and pathophysiology. J. Cereb. Blood Flow Metab. 2002, 22, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Schey, K.L.; Wang, Z.; Wenke, J.L.; Qi, Y. Aquaporins in the eye: Expression, function, and roles in ocular disease. Biochim. Biophys. Acta 2014, 1840, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Lamagna, B.; Ciaramella, P.; Lamagna, F.; Di Loria, A.; Brunetti, A.; Pelagalli, A. Aquaporin 1 (AQP1) expression in healthy dog tears. Animals 2020, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; He, R.H.; Sun, C.C.; Zhang, Y.; Meng, Q.X.; Ma, Y.Y. Function of aquaporins in female and male reproductive systems. Hum Reprod. Update 2006, 12, 785–795. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, Z.; Bazer, F.W.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Aquaporins in the female reproductive system of mammals. Front. Biosci. 2015, 20, 838–871. [Google Scholar]
- Morató, M.Y. R.; Rodríguez-Gil, J.E.; Bonet, S.; Prieto-Martínez, N. Aquaporins in the male reproductive tract and sperm: Functional implications and cryobiology. Reprod. Dom. Anim. 2017, 52, 12–27. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Bernardino, R.L.; Soveral, G.; Calamita, G.; Alves, M.G.; Oliveira, P.F. Aquaporins and male (in)fertility: Expression and role throughout the male reproductive tract. Arch. Biochem. Biophys. 2020, 679, 108222. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Valen, G.; Vaage, J. Cardiac aquaporins. Basic Res. Cardiol. 2013, 108, 393. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Lodder, E.M.; Wilders, R. Aquaporin Channels in the Heart—Physiology and Pathophysiology. Int. J. Mol. Sci. 2019, 20, 2039. [Google Scholar] [CrossRef]
- Noda, Y.; Sohara, E.; Ohta, E.; Sasaki, S. Aquaporins in kidney pathophysiology. Nat. Rev. Nephrol. 2010, 6, 168–178. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Jiang, T.; Yang, B. Aquaporins in Urinary System. Adv. Exp. Med. Biol. 2017, 969, 131–148. [Google Scholar] [PubMed]
- Laforenza, U. Water channel proteins in the gastrointestinal tract. Mol. Aspects Med. 2012, 33, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Huebert, R.C.; Splinter, P.L.; García, F.; Marinelli, R.A.; LaRusso, N.F. Expression and localization of aquaporin water channels in rat hepatocytes. Evidence for a role in canalicular bile secretion. J. Biol. Chem. 2002, 277, 22710–22717. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, R.A.; Gradilone, S.A.; Carreras, F.I.; Calamita, G.; Lehmann, G.L. Liver aquaporins: Significance in canalicular and ductal bile formation. Ann. Hepatol. 2004, 3, 130–136. [Google Scholar] [CrossRef]
- Portincasa, P.; Calamita, G. Water channel proteins in bile formation and flow in health and disease: When immiscible becomes miscible. Mol. Aspects Med. 2012, 33, 651–664. [Google Scholar] [CrossRef]
- Wittekindt, O.H.; Dietl, P. Aquaporins in the lung. Pflügers Archiv Eur. J. Physiol. 2019, 471, 519–532. [Google Scholar] [CrossRef]
- Delporte, C.; Steinfeld, S. Distribution and roles of aquaporins in salivary glands. Biochim. Biophys. Acta 2006, 1758, 1061–1070. [Google Scholar] [CrossRef]
- D’Agostino, C.; Elkashty, O.A.; Chivasso, C.; Perret, J.; Tran, S.D.; Delporte, C. Insight into salivary gland aquaporins. Cells 2020, 9, 1547. [Google Scholar] [CrossRef]
- Boury-Jamot, M.; Daraspe, J.; Bonté, F.; Perrier, E.; Schnebert, S.; Dumas, M.; Verbavatz, J.M. Skin aquaporins: Function in hydration, wound healing, and skin epidermis homeostasis. Handb. Exp. Pharmacol. 2009, 190, 205–217. [Google Scholar]
- Zhu, C.; Chen, Z.; Jiang, Z. Expression, distribution and role of aquaporin water channels in human and animal stomach and intestines. Int. J. Mol. Sci. 2016, 17, 1399. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Maly, K.; Uberall, F.; Hoflacher, J.; Grunicke, H. Stimulation of K+ transport systems by Ha-ras. J. Biol. Chem. 1991, 266, 8230–8235. [Google Scholar] [PubMed]
- Lang, F.; Ritter, M.; Gamper, N.; Huber, S.; Fillon, S.; Tanneur, V.; Lepple-Wienhues, A.; Szabo, I.; Gulbins, E. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell Physiol. Biochem. 2000, 10, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Föller, M.; Lang, K.S.; Lang, P.A.; Ritter, M.; Gulbins, E.; Vereninov, A.; Huber, S.M. Ion channels in cell proliferation and apoptotic cell death. J. Membr. Biol. 2005, 205, 147–157. [Google Scholar] [CrossRef]
- Rouzaire-Dubois, B.; Dubois, J.M. K+ channel block-induced mammalian neuroblastoma cell swelling: A possible mechanism to influence proliferation. J. Physiol. 1998, 510, 93–102. [Google Scholar] [CrossRef]
- Kunzelmann, K. Ion channels and cancer. J. Membrane Biol. 2005, 205, 159–173. [Google Scholar] [CrossRef]
- Delporte, C.; Chen, Z.J.; Baum, B.J. Aquaporin 1 Expression during the Cell Cycle in A5 Cells. Biochem. Biophys. Res. Commun. 1996, 228, 223–228. [Google Scholar] [CrossRef]
- Yoneda, K.; Yamamoto, N.; Asai, K.; Sobue, K.; Fujita, Y.; Fujita, M.; Mase, M.; Yamada, K.; Nakanishi, M.; Tada, T.; et al. Regulation of aquaporin in astrocytes. Mol. Brain Res. 2001, 89, 94–102. [Google Scholar] [CrossRef]
- Rivarola, V.; Flamenco, P.; Melamud, L.; Galizia, L.; Ford, P.; Capurro, C. Adaptation to alkalosis induces cell cycle delay and apoptosis in cortical collecting duct cells: Role of aquaporin-2. J. Cell Physiol. 2010, 224, 405–413. [Google Scholar] [CrossRef]
- Di Giusto, G.; Flamenco, P.; Rivarola, V.; Fernández, J.; Melamud, L.; Ford, P.; Capurro, C. Aquaporin 2-increased renal cell proliferation is associated with cell volume regulation. J. Cell Biochem. 2012, 113, 3721–3729. [Google Scholar] [CrossRef]
- Shimizu, H.; Shiozaki, A.; Ichikawa, D.; Fujiwara, H.; Konishi, H.; Ishii, H.; Komatsu, S.; Kubota, T.; Okamoto, K.; Kishimoto, M.; et al. The expression and role of Aquaporin 5 in esophageal squamous cell carcinoma. J. Gastroenterol. 2014, 49, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Galán-Cobo, A.; Ramírez-Lorca, R.; Serna, A.; Echevarría, M. Overexpression of AQP3 modifies the cell cycle and the proliferation rate of mammalian cells in culture. PLoS ONE 2015, 10, e0137692. [Google Scholar] [CrossRef] [PubMed]
- Galán-Cobo, A.; Ramírez-Lorca, R.; Toledo-Aral, J.J.; Echevarría, M. Aquaporin-1 plays important role in proliferation by affecting cell cycle progression. J. Cell Physiol. 2016, 231, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Bichet, D.G. Genetics and diagnosis of central diabetes insipidus. Ann. Endocrinol. 2012, 73, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Varadaraj, K.; Kumari, S.S.; Patil, R.; Wax, M.B.; Mathias, R.T. Functional characterization of a human aquaporin 0 mutation that leads to a congenital dominant lens cataract. Exp Eye Res. 2008, 87, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012, 11, 535–544. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Murchison, C.F.; Westaway, S.K.; Simon, M.J.; Erten-Lyons, D.; Kaye, J.A.; Quinn, J.F.; Iliff, J.J. The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease. Alzheimers Dement. 2017, 3, 348–359. [Google Scholar] [CrossRef]
- Senk, B.; Goricar, K.; Kovac, V.; Dolzan, V.; Franko, A. Genetic polymorphisms in aquaporin 1 as risk factors for malignant mesothelioma and biomarkers of response to cisplatin treatment. Radiol. Oncol. 2019, 53, 96–104. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Scuteri, A.; Miloso, M.; Foudah, D.; Orciani, M.; Cavaletti, G.; Tredici, G. Mesenchymal stem cells neuronal differentiation ability: A real perspective for nervous system repair? Curr. Stem Cell Res. Ther. 2011, 6, 82–92. [Google Scholar] [CrossRef]
- Haynesworth, S.E.; Goshima, J.; Goldberg, V.M.; Caplan, A.I. Characterization of cells with osteogenic potential from human marrow. Bone 1992, 13, 81–88. [Google Scholar] [CrossRef]
- Halvorsen, Y.; Wilkison, W.; Gimble, J. Adipose-derived stromal cells—their utility and potential in bone formation. Int. J. Obes. 2000, 24, S41–S44. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Potential therapeutic applications of muscle-derived mesenchymal stem and progenitor cells. Expert Opin Biol Ther. 2010, 10, 505–517. [Google Scholar] [CrossRef]
- Sun, T.; Yu, C.; Gao, Y.; Zhao, C.; Hua, J.; Cai, L.; Guan, W.; Ma, Y. Establishment and biological characterization of a dermal mesenchymal stem cells line from bovine. Biosci. Rep. 2014, 34, e00101. [Google Scholar] [CrossRef]
- Huang, Y.S.; Li, I.H.; Chueh, S.H.; Hueng, D.Y.; Tai, M.C.; Liang, C.M.; Lien, S.B.; Sytwu, H.K.; Ma, K.H. Mesenchymal stem cells from rat olfactory bulbs can differentiate into cells with cardiomyocyte characteristics. J. Tissue Eng. Regen. Med. 2015, 9, E191–201. [Google Scholar] [CrossRef]
- Yoshida, S.; Tomokiyo, A.; Hasegawa, D.; Hamano, S.; Sugii, H.; Maeda, H. Insight into the role of dental pulp stem cells in regenerative therapy. Biology 2020, 9, E160. [Google Scholar] [CrossRef]
- Fontanella, R.; Pelagalli, A.; Nardelli, A.; D'Alterio, C.; Ieranò, C.; Cerchia, L.; Lucarelli, E.; Scala, S.; Zannetti, A. A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett. 2016, 370, 100–107. [Google Scholar] [CrossRef]
- Hill, B.S.; Pelagalli, A.; Passaro, N.; Zannetti, A. Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget 2017, 8, 73296–73311. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Hill, B.S.; Fontanella, R.; Greco, A.; Gramanzini, M.; Auletta, L.; Gargiulo, S.; Albanese, S.; Lucarelli, E.; Cerchia, L.; et al. Inhibition of bone marrow-derived mesenchymal stem cells homing towards triple-negative breast cancer microenvironment using an anti-PDGFRβ aptamer. Theranostics 2017, 7, 3595–3607. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.S.; Sarnella, A.; D'Avino, G.; Zannetti, A. Recruitment of stromal cells into tumour microenvironment promote the metastatic spread of breast cancer. Semin. Cancer Biol. 2020, 60, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Denu, R.A.; Dollar, B.A.; Escalante, L.E.; Kuether, J.P.; Callander, N.S.; Asimakopoulos, F.; Hematti, P. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br. J. Haematol. 2012, 158, 336–346. [Google Scholar] [CrossRef]
- Castellone, M.D.; Laatikainen, L.E.; Laurila, J.P.; Langella, A.; Hematti, P.; Soricelli, A.; Salvatore, M.; Laukkanen, M.O. Brief report: Mesenchymal stromal cell atrophy in coculture increases aggressiveness of transformed cells. Stem Cells 2013, 31, 1218–1223. [Google Scholar] [CrossRef]
- Cammarota, F.; Laukkanen, M.O. Mesenchymal stem/stromal cells in stromal evolution and cancer progression. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.S. Usage of human mesenchymal stem cells in cell-based therapy: Advantages and disadvantages. Dev. Reprod. 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Viganò, M.; Sansone, V.; d’Agostino, M.C.; Romeo, P.; Perucca Orfei, C.; de Girolamo, L. Mesenchymal stem cells as therapeutic target of biophysical stimulation for the treatment of musculoskeletal disorders. J. Orthop. Surg. Res. 2016, 11, 163. [Google Scholar] [CrossRef]
- Eder, C.; Schmidt-Bleek, K.; Geissler, S.; Sass, F.A.; Maleitzke, T.; Pumberger, M.; Perka, C.; Duda, G.N.; Winkler, T. Mesenchymal stromal cell and bone marrow concentrate therapies for musculoskeletal indications: A concise review of current literature. Mol. Biol. Rep. 2020, 47, 4789–4814. [Google Scholar] [CrossRef]
- Blundell, R.; Shah, M. Neurodegenerative Diseases and Stem Cell Transplantation. J. Stem Cell Res. Ther. 2015, 5, 277. [Google Scholar]
- Crisostomo, P.R.; Wang, Y.; Markel, T.A.; Wang, M.; Lahm, T.; Meldrum, D.R. Human mesenchymal stem cells stimulated by TNF-α, LPS, or hypoxia produce growth factors by an NFκB- but not JNK-dependent mechanism. Am. J. Physiol. Physiol. 2008, 294, C675–C682. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.Y.; Cho, H.J.; Kang, H.J.; Kim, T.S.; Kim, M.H.; Chung, J.H.; Bae, J.W.; Oh, B.H.; Park, Y.B.; Kim, H.S. Pre-Treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J. Am. Coll. Cardiol. 2008, 51, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Haynesworth, S.E.; Baber, M.A.; Caplan, A.I. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and IL-1α. J. Cell Physiol. 1996, 166, 585–592. [Google Scholar] [CrossRef]
- Marakhova, I.; Domnina, A.; Shatrova, A.; Borodkina, A.; Burova, E.; Pugovkina, N.; Zemelko, V.; Nikolsky, N. Proliferation-related changes in K+content in human mesenchymal stem cells. Sci. Rep. 2019, 9, 346. [Google Scholar] [CrossRef]
- Lang, F.; Shumilina, E.; Ritter, M.; Gulbins, E.; Vereninov, A.; Huber, S.M. Ion channels and cell volume in regulation of cell proliferation and apoptotic cell death. Contrib. Nephrol. 2006, 152, 142–160. [Google Scholar]
- Stutzin, A.; Hoffmann, E.K. Swelling-activated ion channels: Functional regulation in cell-swelling, proliferation and apoptosis. Acta Physiol. 2006, 187, 27–42. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Pedersen, S.F. Cell volume homeostatic mechanisms: Effectors and signalling pathways. Acta Physiol. 2011, 202, 465–485. [Google Scholar] [CrossRef]
- Urrego, D.; Tomczak, A.P.; Zahed, F.; Stühmer, W.; Pardo, L.A. Potassium channels in cell cycle and cell proliferation. Phil. Trans. R. Soc. B. 2014, 369, 20130094. [Google Scholar] [CrossRef]
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 2006, 24, 462–471. [Google Scholar] [CrossRef]
- Sethe, S.; Scutt, A.; Stolzing, A. Aging of mesenchymal stem cells. Ageing Res. Rev. 2006, 5, 91–116. [Google Scholar] [CrossRef]
- Zaim, M.; Karaman, S.; Cetin, G.; Isik, S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann. Hematol. 2012, 91, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; De Bari, C. The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 2010, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Azuma, Y.; Fukuyama, R.; Hattori, Y.; Yoshida, C.; Koida, M.; Ogita, K.; Komori, T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J. Cell Biol. 2004, 166, 85–95. [Google Scholar] [CrossRef]
- Akune, T.; Ohba, S.; Kamekura, S.; Yamaguchi, M.; Chung, U.I.; Kubota, N.; Terauchi, Y.; Harada, Y.; Azuma, Y.; Nakamura, K.; et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Investig. 2004, 113, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Oreffo, R.O.; Kusec, V.; Romberg, S.; Triffitt, J.T. Human bone marrow osteoprogenitors express estrogen receptor-alpha and bone morphogenetic proteins 2 and 4 mRNA during osteoblastic differentiation. J. Cell Biochem. 1999, 75, 382–392. [Google Scholar] [CrossRef]
- Peng, Y.; Kang, Q.; Cheng, H.; Li, X.; Sun, M.H.; Jiang, W.; Luu, H.H.; Park, J.Y.; Haydon, R.C.; He, T.C. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J. Cell Biochem. 2003, 90, 1149–1165. [Google Scholar] [CrossRef]
- Luo, Q.; Kang, Q.; Si, W.; Jiang, W.; Park, J.K.; Peng, Y.; Li, X.; Luu, H.H.; Luo, J.; Montag, A.G.; et al. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J. Biol. Chem. 2004, 279, 55958–55968. [Google Scholar] [CrossRef]
- Singh, P.; Yash Roy, R.C.; Hoque, M. Augmented bone-matrix formation and osteogenesis under magnetic field stimulation in vivo XRD, TEM and SEM investigations. Indian J. Biochem. Biophys. 2006, 43, 167–172. [Google Scholar]
- Luo, J.; Chen, J.; Deng, Z.L.; Luo, X.; Song, W.X.; Sharff, K.A.; Tang, N.; Haydon, R.C.; Luu, H.H.; He, T.C. Wnt signaling and human diseases: What are the therapeutic implications? Lab. Invest. 2007, 87, 97–103. [Google Scholar] [CrossRef]
- Luu, H.H.; Song, W.X.; Luo, X.; Manning, D.; Luo, J.; Deng, Z.L.; Sharff, K.A.; Montag, A.G.; Haydon, R.C.; He, T.C. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J. Orthop. Res. 2007, 25, 665–677. [Google Scholar] [CrossRef]
- Choi, Y.H.; Burdick, M.D.; Strieter, R.M. Human circulating fibrocytes have the capacity to differentiate osteoblasts and chondrocytes. Int. J. Biochem. Cell Biol. 2010, 42, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Ashjian, P.H.; Elbarbary, A.S.; Edmonds, B.; DeUgarte, D.; Zhu, M.; Zuk, P.A.; Lorenz, H.P.; Benhaim, P.; Hedrick, M.H. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast. Reconstr. Surg. 2003, 111, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Lin, G.; Fandel, T.; Banie, L.; Lue, T.F.; Lin, C.S. Insulin growth factor signaling mediates neuron-like differentiation of adipose-tissue-derived stem cells. Differentiation 2008, 76, 488–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavlova, G.; Lopatina, T.; Kalinina, N.; Rybalkina, E.; Parfyonova, Y.; Tkachuk, V.; Revishchin, A. In vitro neuronal induction of adipose-derived stem cells and their fate after transplantation into injured mouse brain. Curr. Med. Chem. 2012, 19, 5170–5177. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Keely, P.J. The role of focal adhesion kinase in tumor initiation and progression. Cell Adh. Migr. 2009, 3, 347–350. [Google Scholar] [CrossRef]
- Shi, C. Recent progress toward understanding the physiological function of bone marrow mesenchymal stem cells. Immunology 2012, 136, 133–138. [Google Scholar] [CrossRef]
- Juuti-Uusitalo, K.; Delporte, C.; Gr´egoire, F.; Perret, J.; Huhtala, H.; Savolainen, V.; Nymark, S.; Hyttinen, J.; Uusitalo, H.; Willermain, F.; et al. Aquaporin expression and function in human pluripotent stem cell–derived retinal pigmented epithelial cells. Ophthalmol. Vis. Sci. 2013, 54, 3510–3519. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, Y.; Liu, J.; Ren, J.; Wu, J.; Zhu, N. AQP5 regulates the proliferation and differentiation of epidermal stem cells in skin aging. Braz. J. Med. Biol. Res. 2020, 53, e10009. [Google Scholar] [CrossRef]
- Gao, P.; Yang, J.; Gao, X.; Xu, D.; Niu, D.; Li, J.; Wen, Q. Salvianolic acid B improves bone marrow-derived mesenchymal stem cell differentiation into alveolar epithelial cells type I via Wnt signaling. Mol. Med. Rep. 2015, 12, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Nardelli, A.; Fontanella, R.; Zannetti, A. Inhibition of AQP1 hampers osteosarcoma and hepatocellular carcinoma progression mediated by bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2016, 17, 1102. [Google Scholar] [CrossRef] [PubMed]
- Gouda, Z.A.; Ali Khalifa, M.E.; Shalaby, S.M.; Hussein, S. Mechanistic effect of human umbilical cord blood derived mesenchymal stem cells on the submandibular salivary gland in ovariectomized rats. Biochem. Cell Biol. 2018, 96, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Beijer, N.R.M.; Nauryzgaliyeva, Z.M.; Arteaga, E.M.; Pieuchot, L.; Anselme, K.; van de Peppel, J.; Vasilevich, A.S.; Groen, N.; Roumans, N.; Hebels, D.G.A.J.; et al. Dynamic adaptation of mesenchymal stem cell physiology upon exposure to surface micropatterns. Sci. Rep. 2019, 9, 9099. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Rui, Y.; Xu, L.; Wan, C.; Jiang, X.; Li, G. Aqp1 enhances migration of bone marrow mesenchymal stem cells through regulation of FAK and β-catenin. Stem Cells Dev. 2014, 23, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Nardelli, A.; Lucarelli, E.; Zannetti, A.; Brunetti, A. Autocrine signals increase ovine mesenchymal stem cells migration through Aquaporin-1 and CXCR4 overexpression. J. Cell Physiol. 2018, 233, 6241–6249. [Google Scholar] [CrossRef]
- Graziano, A.C.E.; Avola, R.; Pannuzzo, G. Cardile, Aquaporin 1 and 3 modification as a result of chondrogenic differentiation of human mesenchymal stem cell. J. Cell Physiol. 2018, 233, 2279–2291. [Google Scholar] [CrossRef]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Cardile, V. Human mesenchymal stem cells from adipose tissue differentiated into neuronal or glial phenotype express different aquaporins. Mol. Neurobiol. 2017, 54, 8308–8320. [Google Scholar] [CrossRef]
- Avril-Delplanque, A.; Casal, I.; Castillon, N.; Hinnrasky, J.; Puchelle, E.; Péault, B. Aquaporin-3 expression in human fetal airway epithelial progenitor cells. Stem Cells 2005, 23, 992–1001. [Google Scholar] [CrossRef]
- Fossdal, G.; Vik-Mo, E.O.; Sandberg, C.; Varghese, M.; Kaarbø, M.; Telmo, E.; Langmoen, I.A.; Murrell, W. Aqp 9 and brain tumour stem cells. Sci. World J. 2012, 915176. [Google Scholar] [CrossRef]
- Cavazzin, C.; Ferrari, D.; Facchetti, F.; Russignan, A.; Vescovi, A.L.; La Porta, C.A.M.; Gritti, A. Unique expression and localization of aquaporin- 4 and aquaporin-9 in murine and human neural stem cells and in their glial progeny. Glia 2006, 53, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Fan, Y.; Xie, J.; Ding, J.; Sha, L.; Shi, X.; Sun, X.; Hu, G. AQP4 Knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J. Cell Sci. 2008, 121, 4029–4036. [Google Scholar] [CrossRef] [PubMed]
- La Porta, C.A.M.; Gena, P.; Gritti, A.; Fascio, U.; Svelto, M.; Calamita, G. Adult murine CNS stem cells express aquaporin channels. Biol. Cell 2006, 98, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, G.; Fu, X.; Xu, S.; Wang, T.; Zhang, Q.; Yang, Y. Aquaporin 3 maintains the stemness of CD133+ hepatocellular carcinoma cells by activating STAT3. Cell Death Dis. 2019, 10, 465. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Zhang, Y.; Jing, H. Molecular mechanism of aquaporin (AQP3) in regulating differentiation and apoptosis of lung cancer stem cells through Wnt/GSK-3β/β-Catenin pathway. J BUON 2020, 25, 828–834. [Google Scholar]
- Zheng, X.; Li, C.; Yu, K.; Shi, S.; Chen, H.; Qian, Y.; Mei, Z. Aquaporin-9, mediated by IGF2, suppresses liver cancer stem cell properties via augmenting ros/β-catenin/foxo3a signaling. Mol. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Hui, T.H.; Cho, W.C.; Fong, H.W.; Yu, M.; Kwan, K.W.; Ngan, K.C.; Wong, K.H.; Tan, Y.; Yao, S.; Jiang, H.; et al. An electro-osmotic microfluidic system to characterize cancer cell migration under confinement. J. R. Soc. Interface 2019, 16, 20190062. [Google Scholar] [CrossRef]
- Pasantes-Morales, H.; Cruz-Rangel, S. Brain volume regulation: Osmolytes and aquaporin perspectives. Neuroscience 2010, 168, 871–884. [Google Scholar] [CrossRef]
- Nielsen, S.; Smith, B.L.; Christensen, E.I.; Agre, P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA. 1993, 90, 7275–7279. [Google Scholar] [CrossRef]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef]
- Nagelhus, E.A.; Veruki, M.L.; Torp, R.; Haug, F.M.; Laake, J.H.; Nielsen, S.; Agre, P.; Ottersen, O.P. Aquaporin-4 water channel protein in the rat retina and optic nerve: Polarized expression in Muller cells and fibrous astrocytes. J. Neurosci. 1998, 18, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Frigeri, A.; Nicchia, G.P.; Nico, B.; Quondamatteo, F.; Herken, R.; Roncali, L.; Svelto, M. Aquaporin-4 deficiency in skeletal muscle and brain of dystrophic mdx mice. FASEB J. 2001, 15, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Elkjaer, M.L.; Vajda, Z.; Nejsum, L.N.; Kwon, T.; Jensen, U.B.; Amiry-Moghaddam, M.; Frokiaer, J.; Nielsen, S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem. Biophys. Res. Commun. 2000, 276, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Frigeri, A.; Nico, B.; Ribatti, D.; Svelto, M. Tissue distribution and membrane localization of aquaporin-9 water channel: Evidence for sex-linked differences in liver. J. Histochem. Cytochem. 2001, 49, 1547–1556. [Google Scholar] [CrossRef]
- Nicchia, G.P.; Frigeri, A.; Liuzzi, G.M.; Svelto, M. Inhibition of aquaporin-4 expression in astrocytes by RNAi determines alteration in cell morphology, growth, and water transport and induces changes in ischemia-related genes. FASEB J. 2003, 17, 1508–1510. [Google Scholar] [CrossRef]
- Binder, D.K.; Papadopoulos, M.C.; Haggie, P.M.; Verkman, A.S. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J. Neurosci. 2004, 24, 8049–8056. [Google Scholar] [CrossRef]
- Fujii, F.; Nodasaka, Y.; Nishimura, G.; Tamura, M. Anoxia induces matrix shrinkage accompanied by an increase in light scattering in isolated brain mitochondria. Brain Res. 2004, 999, 29–39. [Google Scholar] [CrossRef]
- Griesdale, D.E.; Honey, C.R. Aquaporins and brain edema. Surg. Neurol. 2004, 61, 418–421. [Google Scholar] [CrossRef]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef]
- Calamita, G.; Ferri, D.; Gena, P.; Liquori, G.E.; Cavalier, A.; Thomas, D.; Svelto, M. The inner mitochondrial membrane has Aquaporin-8 water channels and is highly permeable to water. J. Biol. Chem. 2005, 280, 17149–17153. [Google Scholar] [CrossRef]
- Li, Y.; Schmidt-Edelkraut, U.; Poetz, F.; Oliva, I.; Mandl, C.; Hölzl-Wenig, G.; Schönig, K.; Bartsch, D.; Ciccolini, F. γ-aminobutyric A receptor (GABA(A)R) regulates Aquaporin 4 expression in the subependymal zone: Relevance to neural precursors and water exchange. J. Biol. Chem. 2015, 290, 4343–4355. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Khan, M.; Gao, H.; Hao, F.; Sun, M.; Zhong, L.; Lu, C.; Feng, X.; Ma, T. Increased differentiation capacity of bone marrow-derived mesenchymal stem cells in aquaporin-5 deficiency. Stem Cells Dev. 2012, 21, 2495–2507. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Xiao, L.; Dai, G.; Lu, P.; Wang, Y.; Rui, Y. AQP1 modulates tendon stem/progenitor cells senescence during tendon aging. Cell Death Dis. 2020, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- De Ieso, M.L.; Yool, A.J. Mechanisms of aquaporin-facilitated cancer invasion and metastasis. Front. Chem. 2018, 6, 135. [Google Scholar] [CrossRef]
- Hui, T.H.; Kwan, K.W.; Yip, T.T.C.; Fong, H.W.; Ngan, K.C.; Yu, M.; Yao, S.; Ngan, A.H.W.; Lin, Y. Regulating the membrane transport activity and death of cells via electroosmotic manipulation. Biophys. J. 2016, 110, 2769–2778. [Google Scholar] [CrossRef]
- Günther, H.S.; Schmidt, N.O.; Phillips, H.S.; Kemming, D.; Kharbanda, S.; Soriano, R.; Modrusan, Z.; Meissner, H.; Westphal, M.; Lamszus, K. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene 2007, 27, 2897–2909. [Google Scholar] [CrossRef]
- Schulte, A.; Günther, H.S.; Phillips, H.S.; Kemming, D.; Martens, T.; Kharbanda, S.; Soriano, R.H.; Modrusan, Z.; Zapf, S.; Westphal, M.; et al. A distinct subset of glioma cell lines with stem cell – like properties reflects the transcriptional phenotype of glioblastomas and overexpresses CXCR4 as therapeutic target. Glia 2011, 59, 590–602. [Google Scholar] [CrossRef]
| AQP Classification | Isoform | Permeability | ||||||
|---|---|---|---|---|---|---|---|---|
| H2O | Glycerol | NO | H2O2 | NH3 and/or Ammonia | Urea | Uncertain | ||
| AQPs | AQP0 | + | / | / | / | + | / | / |
| AQP1 | + | / | + | + | + | / | / | |
| AQP2 | + | / | / | / | / | / | / | |
| AQP4 | + | / | + | / | / | / | / | |
| AQP5 | + | / | / | + | / | / | / | |
| AQP6 | + | + | / | / | + | + | / | |
| AQP8 | + | + | / | + | + | + | / | |
| Aquaglyceroporins | AQP3 | + | + | / | + | + | + | / |
| AQP7 | + | + | / | / | + | + | / | |
| AQP9 | + | + | / | + | + | + | / | |
| AQP10 | + | + | / | / | + | + | / | |
| Unorthodox-AQPs | AQP11 | / | / | / | / | / | / | + |
| AQP12 | / | / | / | / | / | / | + | |
| Tissue Localisation | Aqp Isoform | Main AQP Roles | Main References |
|---|---|---|---|
| Brain | AQP1, AQP3, AQP4, AQP6 |
| [30,31] |
| Eye | AQP0, AQP1, AQP3, AQP4, AQP5, |
| [32,33] |
| Reproductive Tract | |||
| Female | AQP1, AQP3, AQP4, AQP5, AQP7, AQP8, AQP9, AQP10, AQP11 |
| [34,35] |
| Male | AQP1, AQP2, AQP3, AQP6, AQP7, AQP9, AQP11, AQP12 |
| [34,36,37] |
| Heart | AQP1, AQP3, AQP4, AQP6, AQP7, AQP9, AQP11 |
| [38,39] |
| Kidney | AQP1, AQP2, AQP3, AQP4, AQP5, AQP6, AQP7, AQP11 |
| [40,41] |
| Intestine | |||
| Small | AQP1, AQP3, AQP4, AQP5, AQP8, AQP9, AQP10 |
| [4,42] |
| Large | AQP1, AQP3, AQP4, AQP5, AQP6, AQP7, AQP8, AQP9, AQP11 |
| [4,42] |
| Liver | AQP1, AQP3, AQP7, AQP8, AQP9, AQP11 |
| [43,44,45] |
| Lung | AQP1, AQP3, AQP4, AQP5 |
| [46] |
| Salivary glands | AQP1, AQP3, AQP4, AQP5, AQP8 |
| [47,48] |
| Skin | AQP1, AQP3, AQP5, AQP9, AQP10 |
| [49] |
| Stomach | AQP1, AQP3, AQP4, AQP5, AQP7, AQP11 |
| [42,50] |
| Stem Cell Type | Aqp Isoform | AQP Role | Reference |
|---|---|---|---|
| MSCs | AQP1 |
| [125,126,127] |
| AQP1, AQP3, AQP4, AQP7 |
| [127,128] | |
| progenitor MSC | AQP3 |
| [127,129] |
| glioblastoma stem-cell | AQP4, AQP9 |
| [130] |
| NSCs | AQP4 |
| [131,132] |
| AQP8 |
| [133] | |
| AQP9 |
| [131] | |
| EPSCs | AQP5 |
| [120] |
| LCSCs | AQP3 |
| [134,135] |
| AQP9 |
| [136] | |
| Lung CSCs | AQP4 |
| [137] |
| CSCs | AQP4 |
| [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannetti, A.; Benga, G.; Brunetti, A.; Napolitano, F.; Avallone, L.; Pelagalli, A. Role of Aquaporins in the Physiological Functions of Mesenchymal Stem Cells. Cells 2020, 9, 2678. https://doi.org/10.3390/cells9122678
Zannetti A, Benga G, Brunetti A, Napolitano F, Avallone L, Pelagalli A. Role of Aquaporins in the Physiological Functions of Mesenchymal Stem Cells. Cells. 2020; 9(12):2678. https://doi.org/10.3390/cells9122678
Chicago/Turabian StyleZannetti, Antonella, Gheorghe Benga, Arturo Brunetti, Francesco Napolitano, Luigi Avallone, and Alessandra Pelagalli. 2020. "Role of Aquaporins in the Physiological Functions of Mesenchymal Stem Cells" Cells 9, no. 12: 2678. https://doi.org/10.3390/cells9122678
APA StyleZannetti, A., Benga, G., Brunetti, A., Napolitano, F., Avallone, L., & Pelagalli, A. (2020). Role of Aquaporins in the Physiological Functions of Mesenchymal Stem Cells. Cells, 9(12), 2678. https://doi.org/10.3390/cells9122678







