Glycochenodeoxycholate Promotes Liver Fibrosis in Mice with Hepatocellular Cholestasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
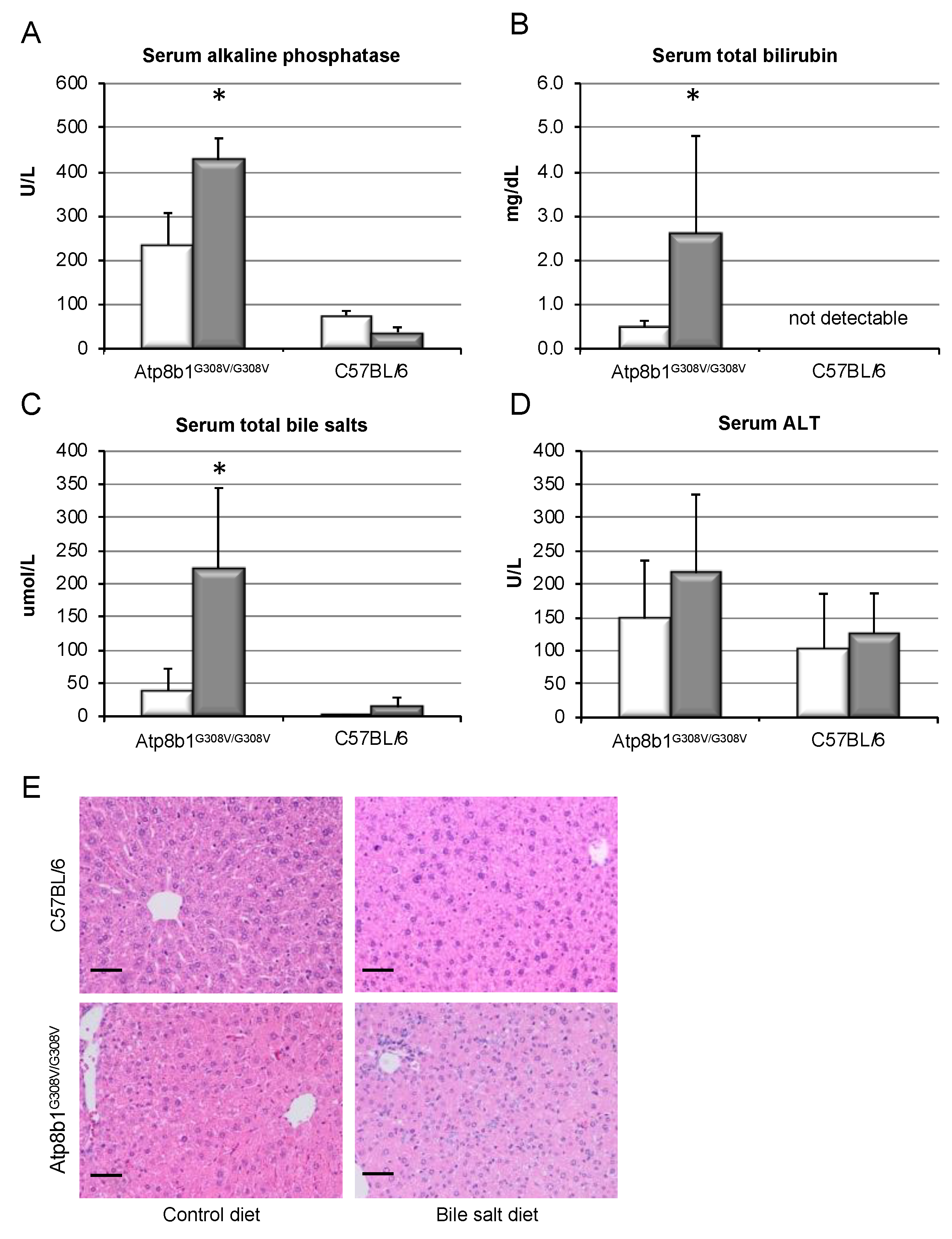
2.2. Serum Biochemistry and Serum Bile Salt Measurements
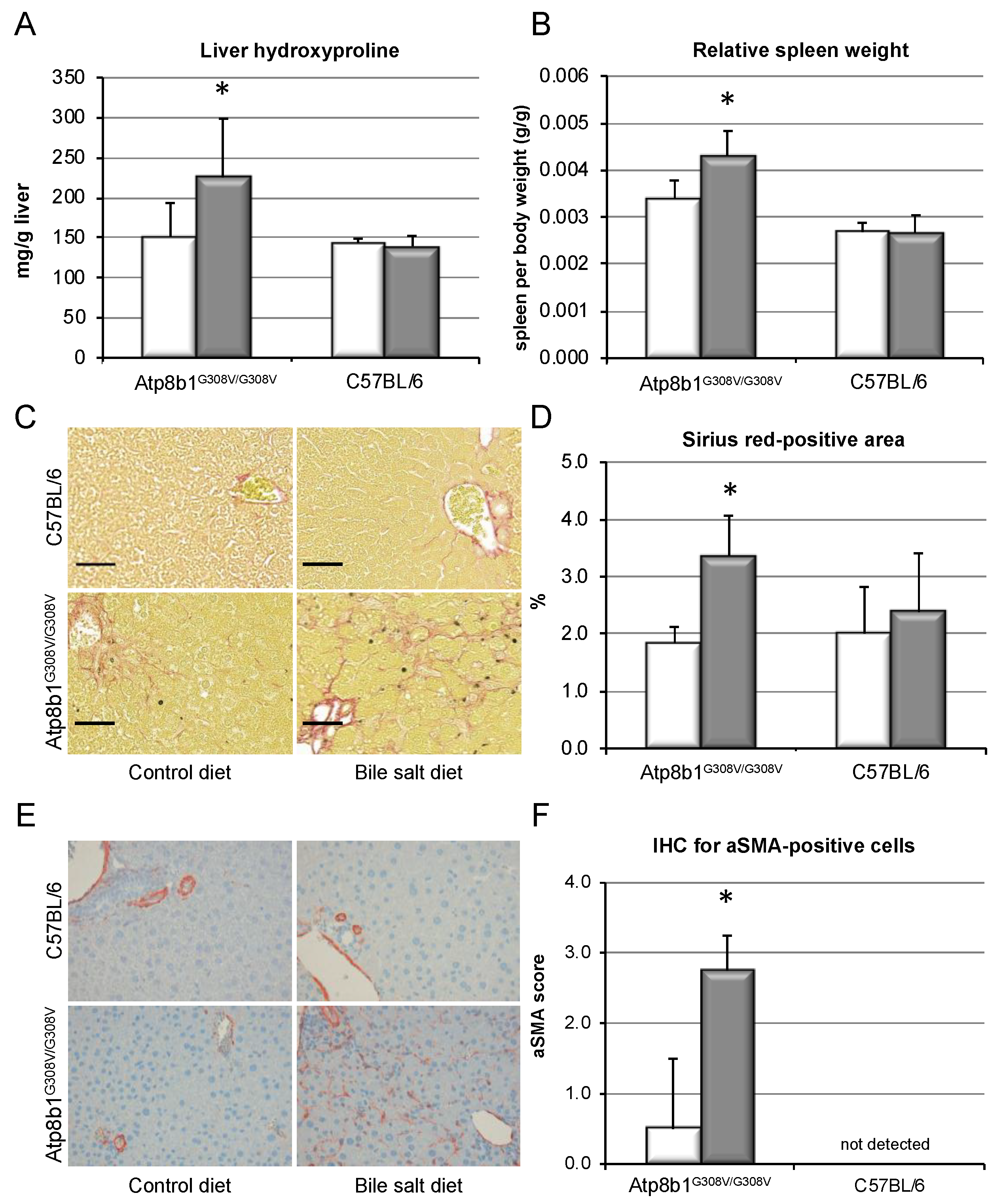
2.3. Liver Histology, Immunohistochemistry, and Hydroxyproline Quantification
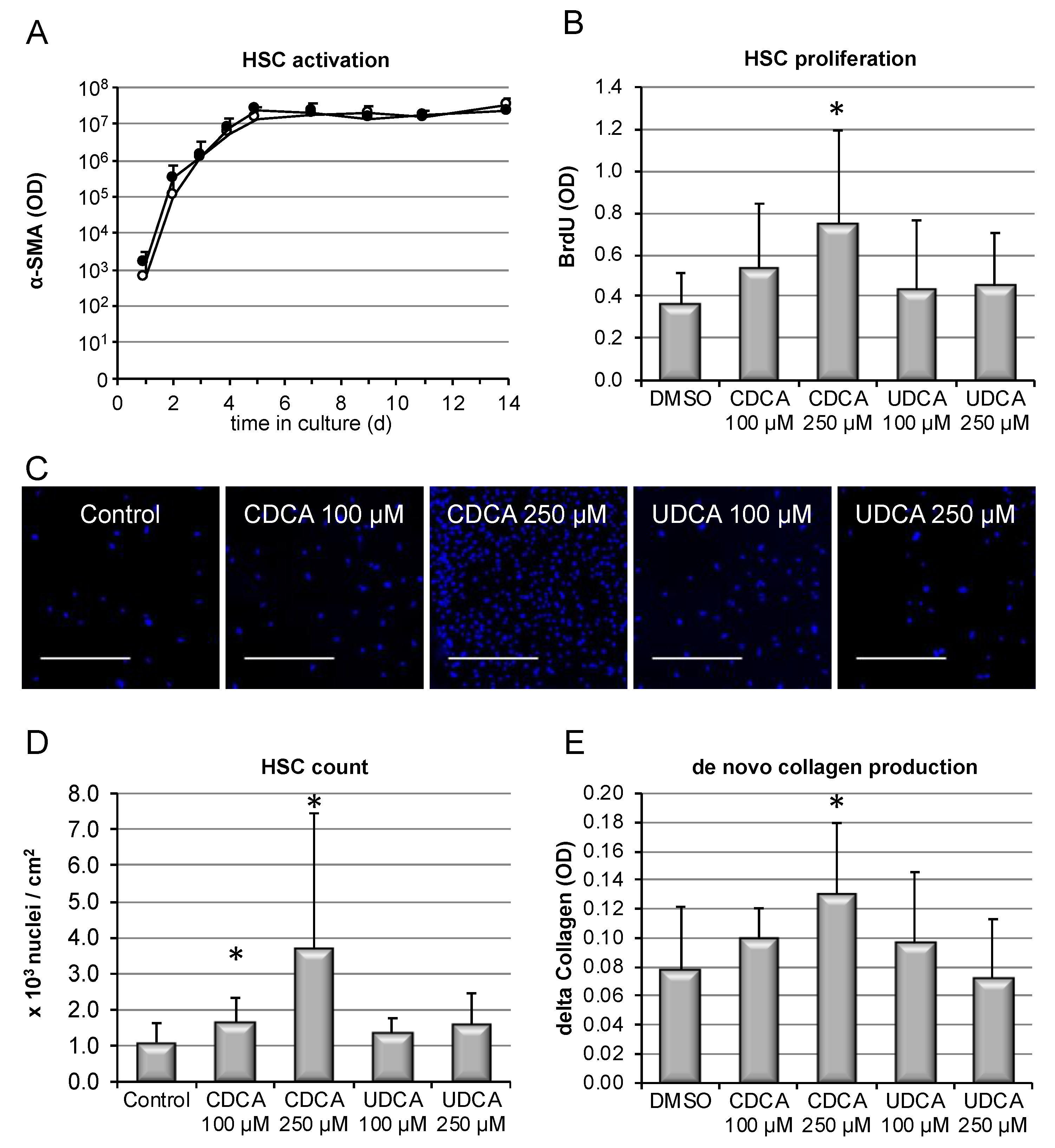
2.4. Hepatic Stellate Cell Isolation and Culture
2.5. Collagen Quantification In Vitro
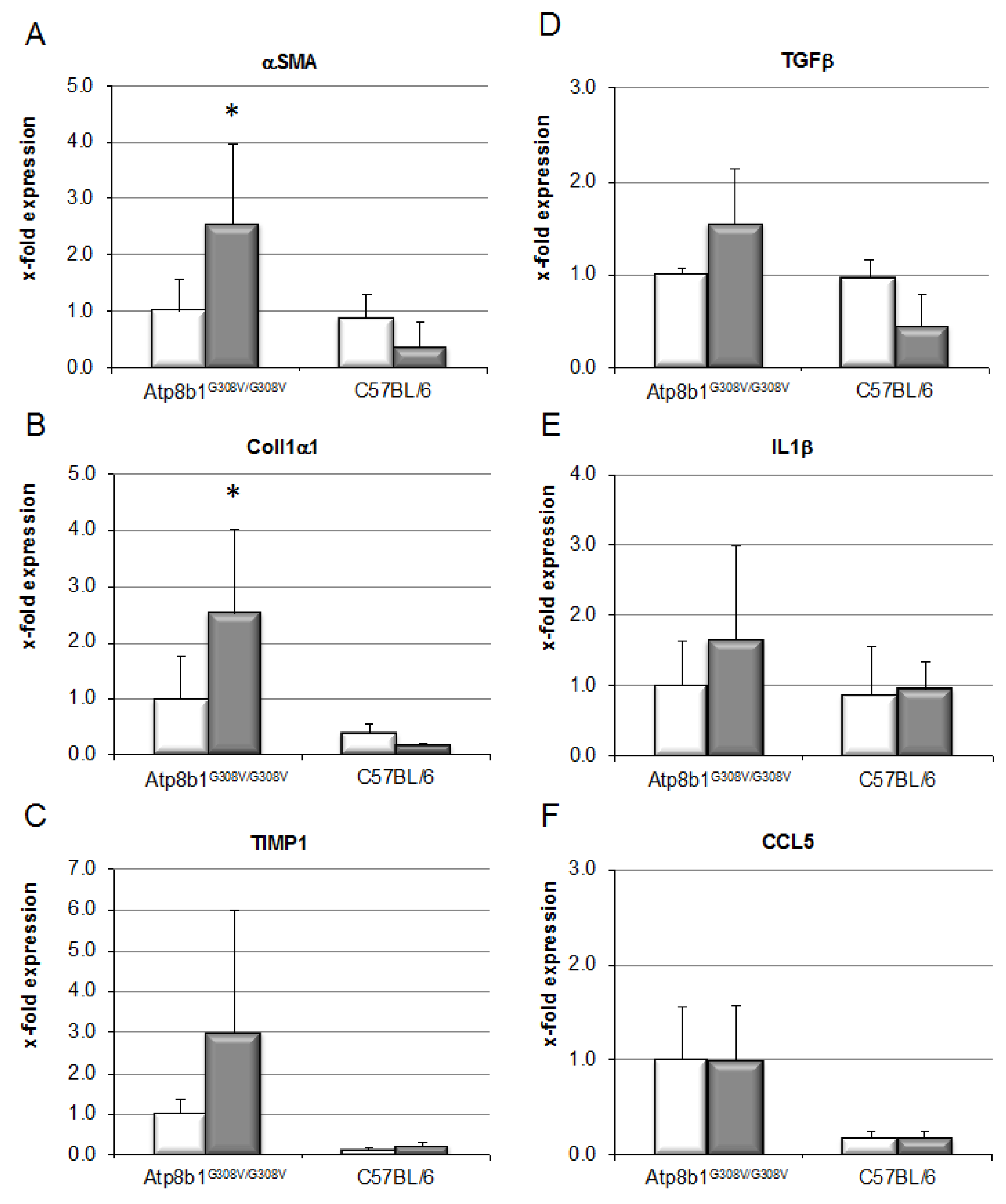
2.6. Quantitative Real-Time PCR
2.7. Western Blotting
2.8. Statistics
3. Results
3.1. Bile Salt Feeding Induces Chronic Cholestasis in Atp8b1G308V/G308V Mice without Relevant Liver Damage
3.2. GCDCA Feeding Induces a “Humanized” Bile Salt Pool
3.3. Chronic Cholestasis, upon Supplementation of GCDCA, Induces Liver Fibrosis in Atp8b1G308V/G308V Mice
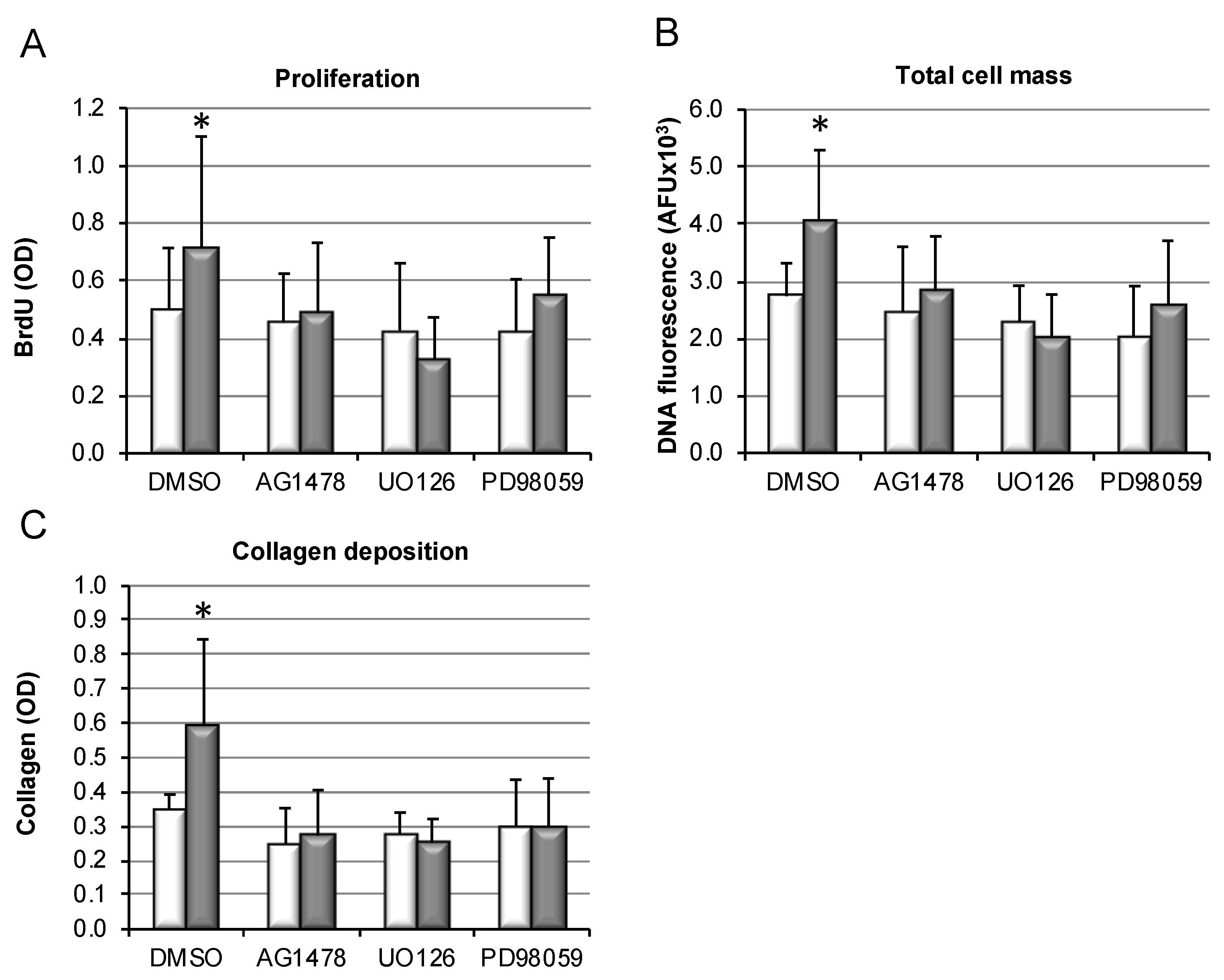
3.4. Hydrophobic, but Not Hydrophilic, Bile Salts Promote Proliferation and Collagen Deposition by Primary HSCs
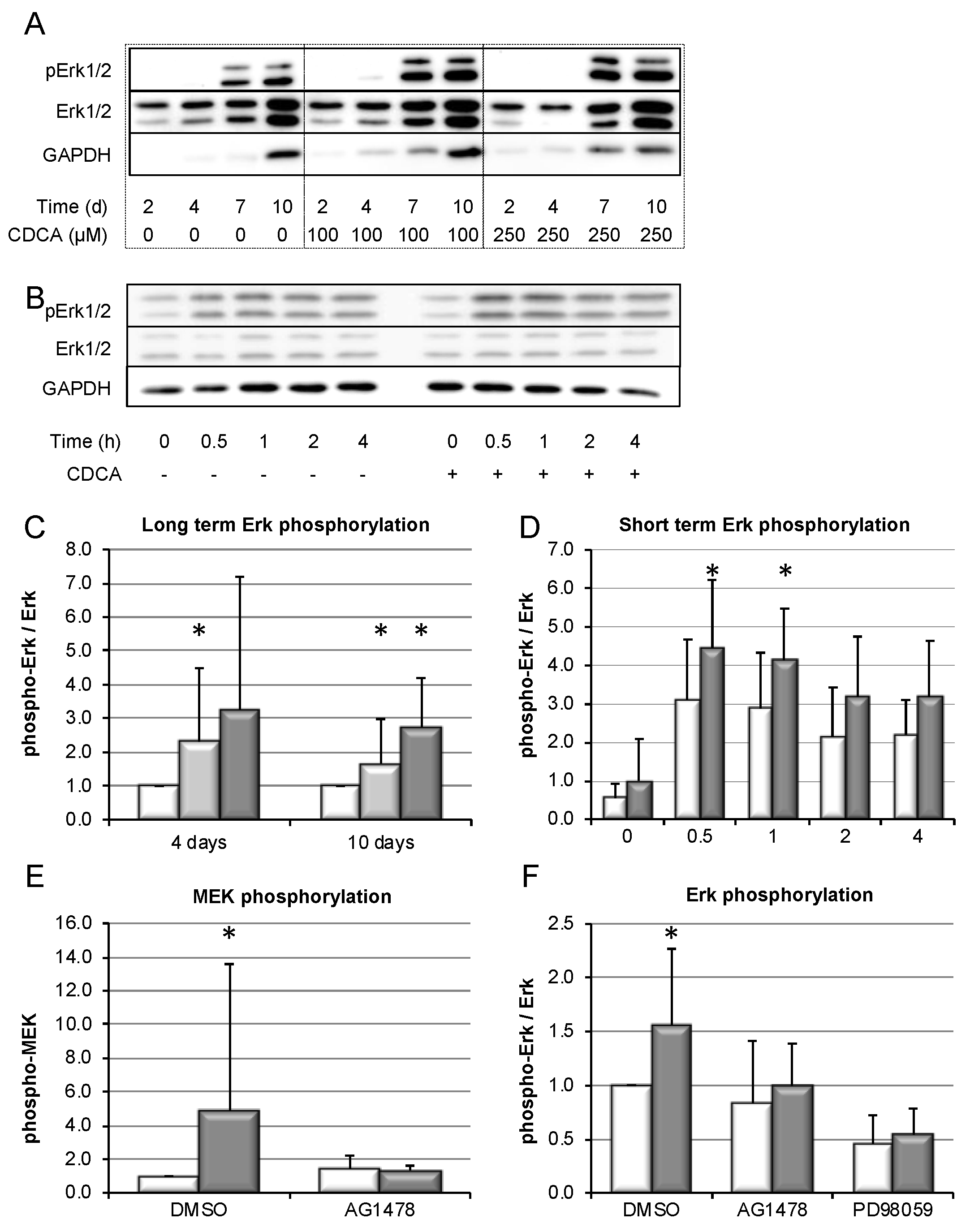
3.5. Bile-Salt-Induced HSC Proliferation is Associated with Activation of the MEK/Erk Signaling Cascade
3.6. Bile-Salt-Induced HSC Proliferation and Collagen Deposition Is Diminished by MEK/Erk Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Strautnieks, S.S.; Bull, L.N.; Knisely, A.S.; Kocoshis, S.A.; Dahl, N.; Arnell, H.; Sokal, E.; Dahan, K.; Childs, S.; Ling, V.; et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat. Genet. 1998, 20, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Greim, H.; Trülzsch, D.; Czygan, P.; Rudick, J.; Hutterer, F.; Schaffner, F.; Popper, H. Mechanism of cholestasis. 6. Bile acids in human livers with or without biliary obstruction. Gastroenterology 1972, 63, 846–850. [Google Scholar] [CrossRef]
- Greim, H.; Trülzsch, D.; Roboz, J.; Dressler, K.; Czygan, P.; Hutterer, F.; Schaffner, F.; Popper, H. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology 1972, 63, 837–845. [Google Scholar] [CrossRef]
- Murphy, G.M.; Ross, A.; Billing, B.H. Serum bile acids in primary biliary cirrhosis. Gut 1972, 13, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Dyrszka, H.; Salen, G.; Zaki, F.G.; Chen, T.; Mosbach, E. Hepatic Toxicity in the Rhesus Monkey Treated with Chenodeoxycholic Acid for 6 Months: Biochemical and Ultrastructural Studies. Gastroenterology 1976, 70, 93–104. [Google Scholar] [CrossRef]
- Malhi, H.; Gores, G.J. Cellular and molecular mechanisms of liver injury. Gastroenterology 2008, 134, 1641–1654. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef]
- Guicciardi, M.; Gores, G. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig. Liver Dis. 2002, 34, 387–392. [Google Scholar] [CrossRef]
- Fickert, P.; Wagner, M. Biliary bile acids in hepatobiliary injury—What is the link? J. Hepatol. 2017, 67, 619–631. [Google Scholar] [CrossRef]
- Patel, T.; Bronk, S.F.; Gores, G.J. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J. Clin. Investig. 1994, 94, 2183–2192. [Google Scholar] [CrossRef]
- Faubion, W.A.; Guicciardi, M.E.; Miyoshi, H.; Bronk, S.F.; Roberts, P.J.; Svingen, P.A.; Kaufmann, S.H.; Gores, G.J. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J. Clin. Investig. 1999, 103, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, R.; Graf, D.; Häussinger, D. Bile salt-induced hepatocyte apoptosis involves epidermal growth factor receptor-dependent CD95 tyrosine phosphorylation. Gastroenterology 2003, 125, 839–853. [Google Scholar] [CrossRef]
- Reinehr, R.; Becker, S.; Keitel, V.; Eberle, A.; Grether–Beck, S.; Häussinger, D. Bile salt-induced apoptosis involves NADPH oxidase isoform activation. Gastroenterology 2005, 129, 2009–2031. [Google Scholar] [CrossRef] [PubMed]
- Rust, C.; Wild, N.; Bernt, C.; Vennegeerts, T.; Wimmer, R.; Beuers, U. Bile acid-induced apoptosis in hepatocytes is caspase-6-dependent. J. Biol. Chem. 2009, 284, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, S.; Gates, A.; Wimmer, R.; Beuers, U.; Anwer, M.S.; Rust, C.; Webster, C.R. Phosphatidylinositol-3-kinase p110gamma contributes to bile salt-induced apoptosis in primary rat hepatocytes and human hepatoma cells. J. Hepatol. 2010, 53, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Denk, G.; Omary, A.-J.; Reiter, F.P.; Hohenester, S.; Wimmer, R.; Holdenrieder, S.; Rust, C. Soluble intracellular adhesion molecule, M30 and M65 as serum markers of disease activity and prognosis in cholestatic liver diseases. Hepatol. Res. 2014, 44, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Canbay, A.; Taimr, P.; Torok, N.; Higuchi, H.; Friedman, S.; Gores, G.J. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab. Investig. 2003, 83, 655–663. [Google Scholar] [CrossRef]
- Zhan, S.S.; Jiang, J.X.; Wu, J.; Halsted, C.; Friedman, S.L.; Zern, M.A.; Torok, N.J. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology 2006, 43, 435–443. [Google Scholar] [CrossRef]
- Watanabe, A.; Hashmi, A.; Gomes, D.A.; Town, T.; Badou, A.; Flavell, R.A.; Mehal, W.Z. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology 2007, 46, 1509–1518. [Google Scholar] [CrossRef]
- Myung, S.J.; Yoon, J.H.; Gwak, G.Y.; Kim, W.; Yang, J.I.; Lee, S.H.; Jang, J.J.; Lee, H.-S. Bile acid-mediated thrombospondin-1 induction in hepatocytes leads to transforming growth factor-beta-dependent hepatic stellate cell activation. Biochem. Biophys. Res. Commun. 2007, 353, 1091–1096. [Google Scholar] [CrossRef]
- Fickert, P.; Zollner, G.; Fuchsbichler, A.; Stumptner, C.; Weiglein, A.H.; Lammert, F.; Marschall, H.-U.; Tsybrovskyy, O.; Zatloukal, K.; Denk, H.; et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology 2002, 123, 1238–1251. [Google Scholar] [CrossRef] [PubMed]
- Fickert, P.; Stöger, U.; Fuchsbichler, A.; Moustafa, T.; Marschall, H.-U.; Weiglein, A.H.; Tsybrovskyy, O.; Jaeschke, H.; Zatloukal, K.; Denk, H.; et al. A New Xenobiotic-Induced Mouse Model of Sclerosing Cholangitis and Biliary Fibrosis. Am. J. Pathol. 2007, 171, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.D.; Paumgartner, G.; Wahlström, A.; Schwabl, P.; Reiberger, T.; Leditznig, N.; Stojakovic, T.; Rohr-Udilova, N.; Chiba, P.; Marschall, H.-U.; et al. Metabolic preconditioning protects BSEP/ABCB11−/− mice against cholestatic liver injury. J. Hepatol. 2017, 66, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ghallab, A.; Hofmann, U.; Sezgin, S.; Vartak, N.; Hassan, R.; Zaza, A.; Godoy, P.; Schneider, K.M.; Guenther, G.; Ahmed, Y.A. Bile Microinfarcts in Cholestasis Are Initiated by Rupture of the Apical Hepatocyte Membrane and Cause Shunting of Bile to Sinusoidal Blood. Hepatology 2019, 69, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Salem, M.; Yousef, I.M.; Tuchweber, B.; Lam, P.; Childs, S.J.; Helgason, C.D.; Ackerley, C.; Phillips, M.J.; Ling, V. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc. Natl. Acad. Sci. USA 2001, 98, 2011–2016. [Google Scholar] [CrossRef]
- Pinzani, M.; Luong, T.V. Pathogenesis of biliary fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1279–1283. [Google Scholar] [CrossRef]
- Paulusma, C.C.; Groen, A.; Kunne, C.; Ho-Mok, K.S.; Spijkerboer, A.L.; De Waart, D.R.; Hoek, F.J.; Vreeling, H.; Hoeben, K.A.; Van Marle, J.; et al. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology 2006, 44, 195–204. [Google Scholar] [CrossRef]
- Paulusma, C.C.; De Waart, D.R.; Kunne, C.; Mok, K.S.; Elferink, R.P.J.O. Activity of the Bile Salt Export Pump (ABCB11) Is Critically Dependent on Canalicular Membrane Cholesterol Content. J. Boil. Chem. 2009, 284, 9947–9954. [Google Scholar] [CrossRef]
- Pawlikowska, L.; Groen, A.; Eppens, E.F.; Kunne, C.; Ottenhoff, R.; Looije, N.; Killeen, N.P.; Elferink, R.P.O.; Knisely, A.; Bull, L.N.; et al. A mouse genetic model for familial cholestasis caused by ATP8B1 mutations reveals perturbed bile salt homeostasis but no impairment in bile secretion. Hum. Mol. Genet. 2004, 13, 881–892. [Google Scholar] [CrossRef]
- Kunne, C.; Acco, A.; Hohenester, S.; Duijst, S.; De Waart, D.R.; Zamanbin, A.; Elferink, R.P.O. Defective bile salt biosynthesis and hydroxylation in mice with reduced cytochrome P450 activity. Hepatology 2013, 57, 1509–1517. [Google Scholar] [CrossRef]
- Dilger, K.; Hohenester, S.; Winkler-Budenhofer, U.; Bastiaansen, B.A.; Schaap, F.G.; Rust, C.; Beuers, U. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J. Hepatol. 2012, 57, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; O’Brien, W. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin. Chim. Acta 1980, 104, 161–167. [Google Scholar] [CrossRef]
- Friedman, S.L.; Roll, F. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal. Biochem. 1987, 161, 207–218. [Google Scholar] [CrossRef]
- Kunne, C.; De Graaff, M.; Duijst, S.; De Waart, D.R.; Elferink, R.P.J.O.; Paulusma, C.C. Hepatic cytochrome P450 deficiency in mouse models for intrahepatic cholestasis predispose to bile salt-induced cholestasis. Lab. Investig. 2014, 94, 1103–1113. [Google Scholar] [CrossRef][Green Version]
- Groen, A.; Romero, M.R.; Kunne, C.; Hoosdally, S.J.; Dixon, P.H.; Wooding, C.; Williamson, C.; Seppen, J.; Oever, K.V.D.; Mok, K.S.; et al. Complementary Functions of the Flippase ATP8B1 and the Floppase ABCB4 in Maintaining Canalicular Membrane Integrity. Gastroenterology 2011, 141, 1927–1937.e4. [Google Scholar] [CrossRef]
- Maroni, L.; Hohenester, S.D.; Van De Graaf, S.F.; Tolenaars, D.; Van Lienden, K.; Verheij, J.; Marzioni, M.; Karlsen, T.H.; Elferink, R.P.O.; Beuers, U. Knockout of the primary sclerosing cholangitis-risk gene Fut2 causes liver disease in mice. Hepatology 2017, 66, 542–554. [Google Scholar] [CrossRef]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. New Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef]
- Cai, S.-Y.; Ouyang, X.; Chen, Y.; Soroka, C.J.; Wang, J.; Mennone, A.; Wang, Y.; Mehal, W.Z.; Jain, D.; Boyer, J.L. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2017, 2, e90780. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Trussoni, C.E.; Krishnan, A.; Bronk, S.F.; Pisarello, M.J.L.; O’Hara, S.P.; Splinter, P.L.; Gao, Y.; Vig, P.; Revzin, A.; et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J. Hepatol. 2018, 69, 676–686. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Ridolfi, F.; Hannivoort, R.; Saccomanno, S.; Homan, M.; De Minicis, S.; Jansen, P.L.; Candelaresi, C.; Benedetti, A.; Moshage, H. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology 2005, 128, 1042–1055. [Google Scholar] [CrossRef]
- Sommerfeld, A.; Reinehr, R.; Häussinger, D. Bile Acid-induced Epidermal Growth Factor Receptor Activation in Quiescent Rat Hepatic Stellate Cells Can Trigger Both Proliferation and Apoptosis. J. Boil. Chem. 2009, 284, 22173–22183. [Google Scholar] [CrossRef] [PubMed]
- Saga, K.; Iwashita, Y.; Hidano, S.; Aso, Y.; Isaka, K.; Kido, Y.; Tada, K.; Takayama, H.; Masuda, T.; Hirashita, T.; et al. Secondary Unconjugated Bile Acids Induce Hepatic Stellate Cell Activation. Int. J. Mol. Sci. 2018, 19, 3043. [Google Scholar] [CrossRef] [PubMed]
- Schliess, F.; Kurz, A.K.; Dahl, S.V.; Häussinger, D. Mitogen-activated protein kinases mediate the stimulation of bile acid secretion by tauroursodeoxycholate in rat liver. Gastroenterology 1997, 113, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F. The enterohepatic circulation of bile acids in mammals: Form and functions. Front Biosci 2009, 14, 2584–2598. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, S.; Wenniger, L.M.; Paulusma, C.C.; van Vliet, S.J.; Jefferson, D.M.; Elferink, R.P.; Beuers, U. A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 2012, 55, 173–183. [Google Scholar] [CrossRef]
- Beuers, U.; Hohenester, S.; Wenniger, L.J.M.D.B.; Kremer, A.E.; Jansen, P.L.M.; Elferink, R.P.J.O. The biliary HCO3− umbrella: A unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology 2010, 52, 1489–1496. [Google Scholar] [CrossRef]
- Ichikawa, M.; Kato, Y.; Miyauchi, S.; Sawada, Y.; Iga, T.; Fuwa, T.; Hanano, M.; Sugiyama, Y. Effect of perfusate pH on the influx of 5-5′-dimethyl-oxazolidine-2,4-dione and dissociation of epidermal growth factor from the cell-surface receptor: The existence of the proton diffusion barrier in the Disse space. J. Hepatol. 1994, 20, 190–200. [Google Scholar] [CrossRef]
- Cronholm, T.; Sjövall, J. Bile acids in portal blood of fats fed different diets and cholestyramine. Bile acids and steroids 189. JBIC J. Boil. Inorg. Chem. 1967, 2, 375–383. [Google Scholar]
- Miethke, A.G.; Zhang, W.; Simmons, J.; Taylor, A.E.; Shi, T.; Shanmukhappa, S.K.; Karns, R.; White, S.; Jegga, A.G.; Lages, C.S. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology 2016, 63, 512–523. [Google Scholar] [CrossRef]
- Baghdasaryan, A.; Fuchs, C.D.; Österreicher, C.H.; Lemberger, U.J.; Halilbasic, E.; Påhlman, I.; Graffner, H.; Krones, E.; Fickert, P.; Wahlström, A.; et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J. Hepatol. 2016, 64, 674–681. [Google Scholar] [CrossRef]
- Hegade, V.S.; Jones, D.E.; Hirschfield, G.M. Apical Sodium-Dependent Transporter Inhibitors in Primary Biliary Cholangitis and Primary Sclerosing Cholangitis. Dig. Dis. 2017, 35, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, N.; Liu, S.B.; Sverdlov, D.Y.; Yoshida, S.; Nasser, I.; Ke, Q.; Kang, P.M.; Popov, Y.V. A New Mdr2−/− Mouse Model of Sclerosing Cholangitis with Rapid Fibrosis Progression, Early-Onset Portal Hypertension, and Liver Cancer. Am. J. Pathol. 2015, 185, 325–334. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohenester, S.; Kanitz, V.; Kremer, A.E.; Paulusma, C.C.; Wimmer, R.; Kuehn, H.; Denk, G.; Horst, D.; Oude Elferink, R.; Beuers, U. Glycochenodeoxycholate Promotes Liver Fibrosis in Mice with Hepatocellular Cholestasis. Cells 2020, 9, 281. https://doi.org/10.3390/cells9020281
Hohenester S, Kanitz V, Kremer AE, Paulusma CC, Wimmer R, Kuehn H, Denk G, Horst D, Oude Elferink R, Beuers U. Glycochenodeoxycholate Promotes Liver Fibrosis in Mice with Hepatocellular Cholestasis. Cells. 2020; 9(2):281. https://doi.org/10.3390/cells9020281
Chicago/Turabian StyleHohenester, Simon, Veronika Kanitz, Andreas E. Kremer, Coen C. Paulusma, Ralf Wimmer, Helen Kuehn, Gerald Denk, David Horst, Ronald Oude Elferink, and Ulrich Beuers. 2020. "Glycochenodeoxycholate Promotes Liver Fibrosis in Mice with Hepatocellular Cholestasis" Cells 9, no. 2: 281. https://doi.org/10.3390/cells9020281
APA StyleHohenester, S., Kanitz, V., Kremer, A. E., Paulusma, C. C., Wimmer, R., Kuehn, H., Denk, G., Horst, D., Oude Elferink, R., & Beuers, U. (2020). Glycochenodeoxycholate Promotes Liver Fibrosis in Mice with Hepatocellular Cholestasis. Cells, 9(2), 281. https://doi.org/10.3390/cells9020281





