A New Highlight of Ephedra alata Decne Properties as Potential Adjuvant in Combination with Cisplatin to Induce Cell Death of 4T1 Breast Cancer Cells In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Drugs, Antibodies, and Chemical Reagents
2.3. Preparation of Ephedra alata Extract
2.4. LC–MS/MS Analysis
2.5. Cell Viability Assays
2.6. Combination Index Analysis
2.7. Apoptosis Analysis
2.8. Flow Cytometry Analysis of Apoptosis
2.9. Western Blotting
2.10. Preparation of Mitochondrial and Cytosolic Protein Extracts
2.11. Mouse Strain and Tumor Growth Experiments
2.12. Biochemical Assays and Analysis
2.13. Statistical Analysis
3. Results
3.1. Identification and Quantification of Phenolic Compounds in E.A. Extracts
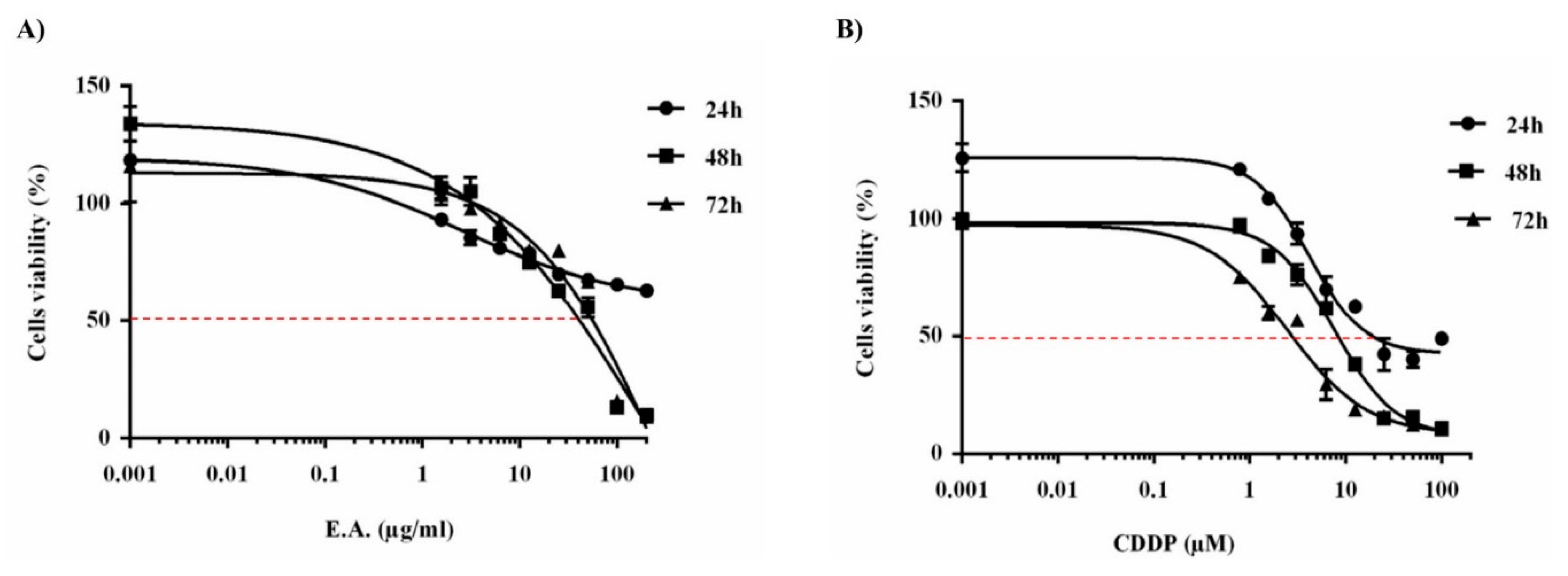
3.2. E.A. Extract Decreased Breast Cancer Cells Viability
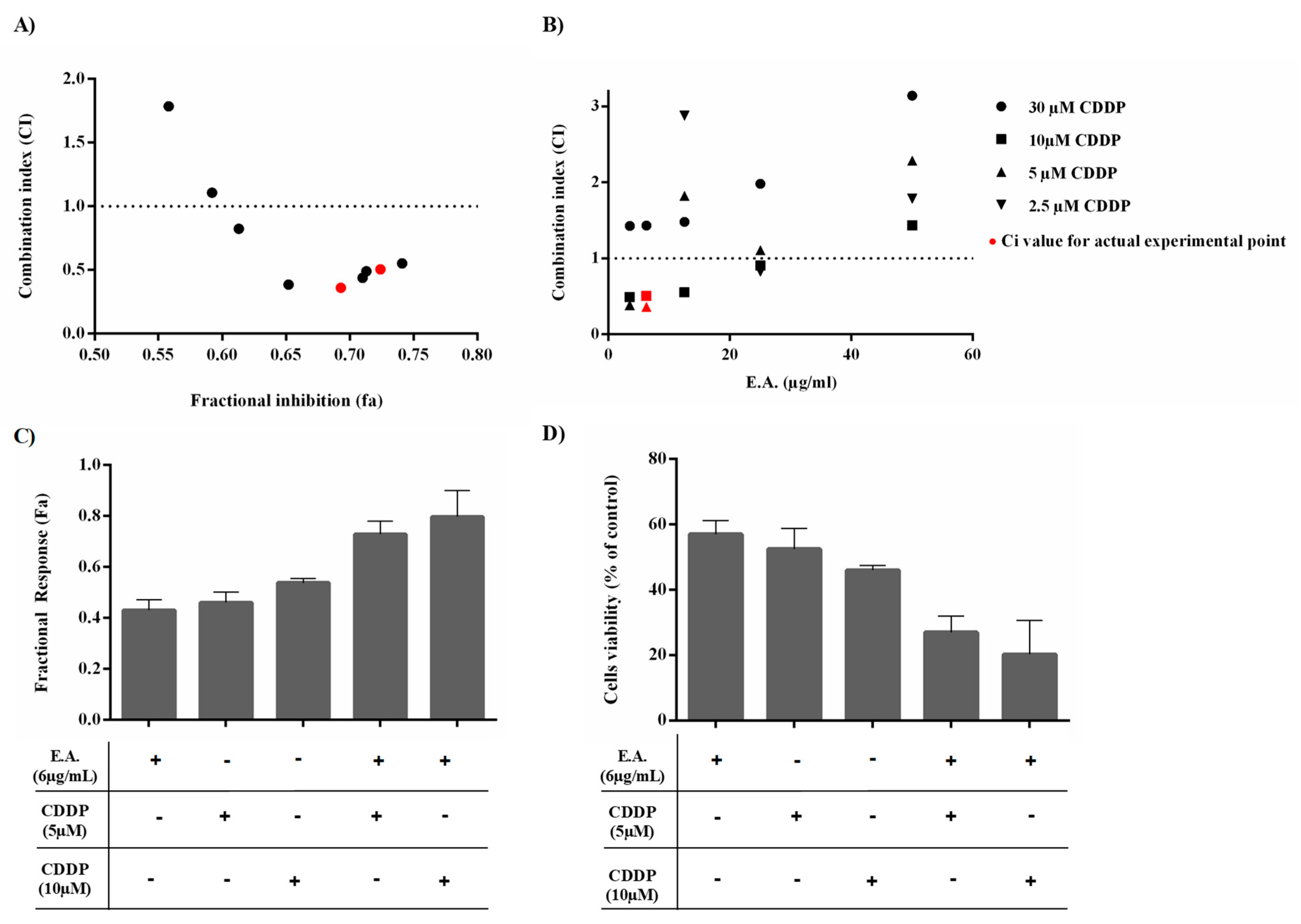
3.3. E.A. Synergized with CDDP to Reduce 4T1 Breast Cancer Cell Viability
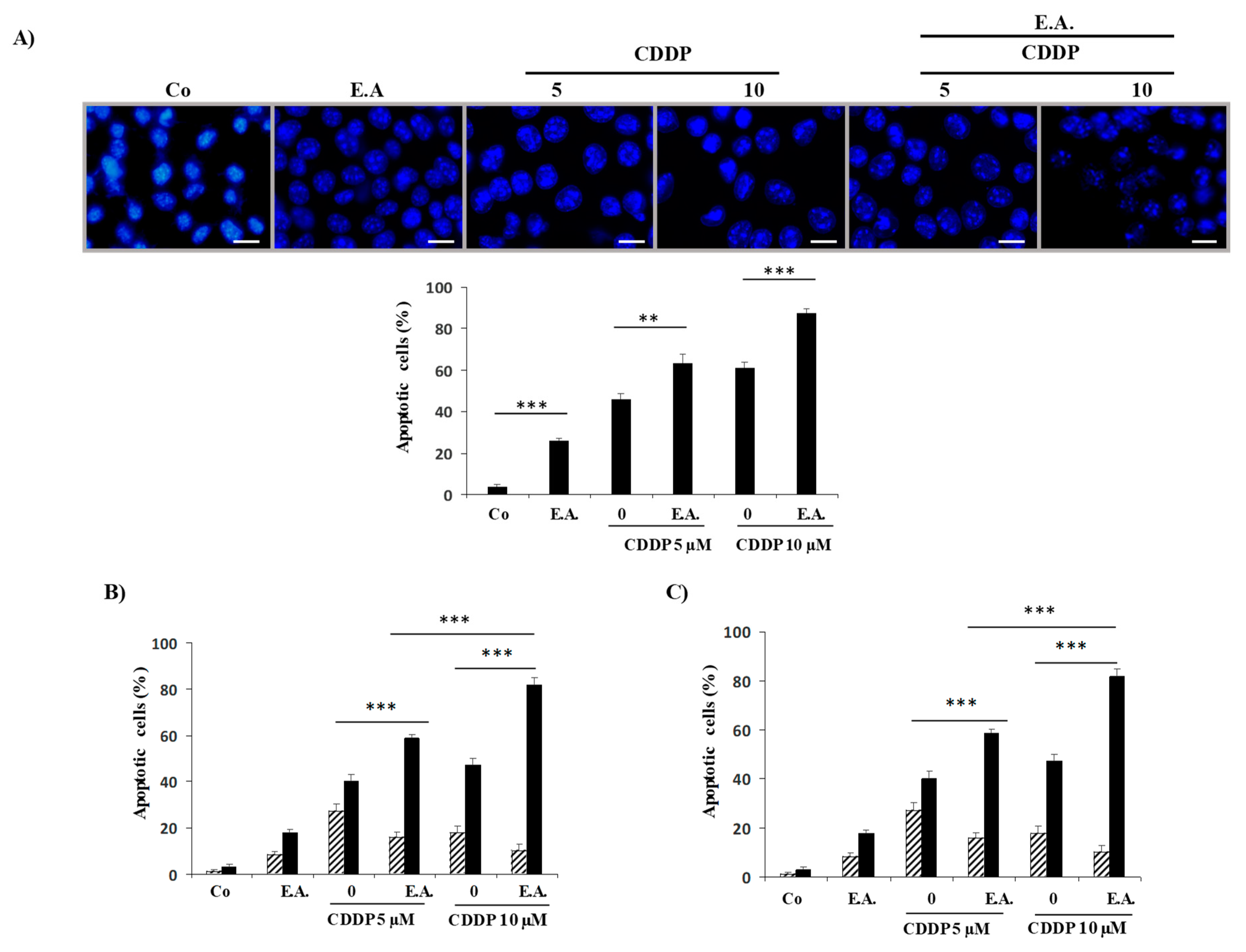
3.4. E.A. Synergized with CDDP to Enhance Apoptosis in 4T1 Breast Cancer Cells
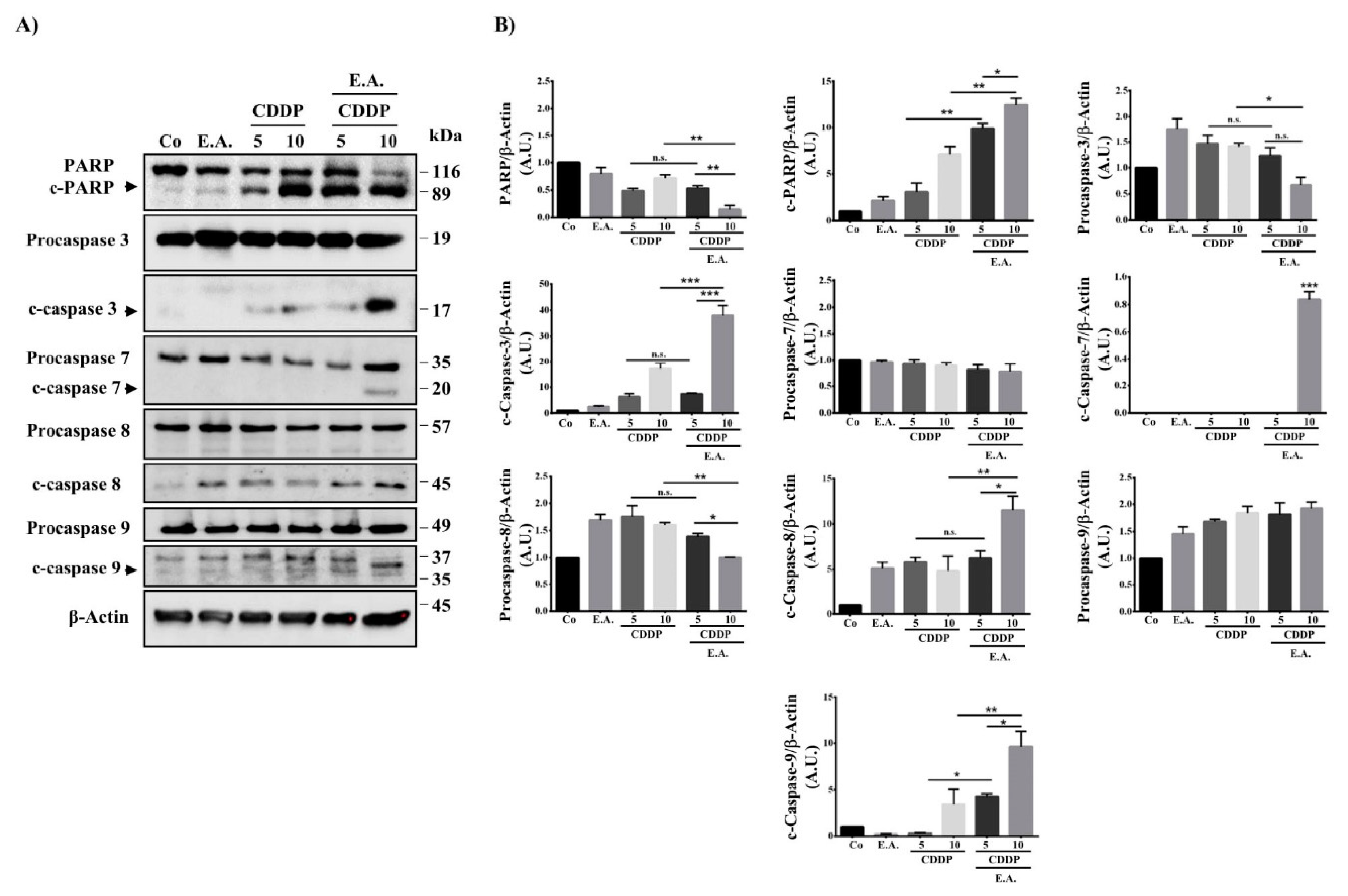
3.5. E.A. Sensitized Breast Cancer Cells to CDDP-Mediated Caspase Activation
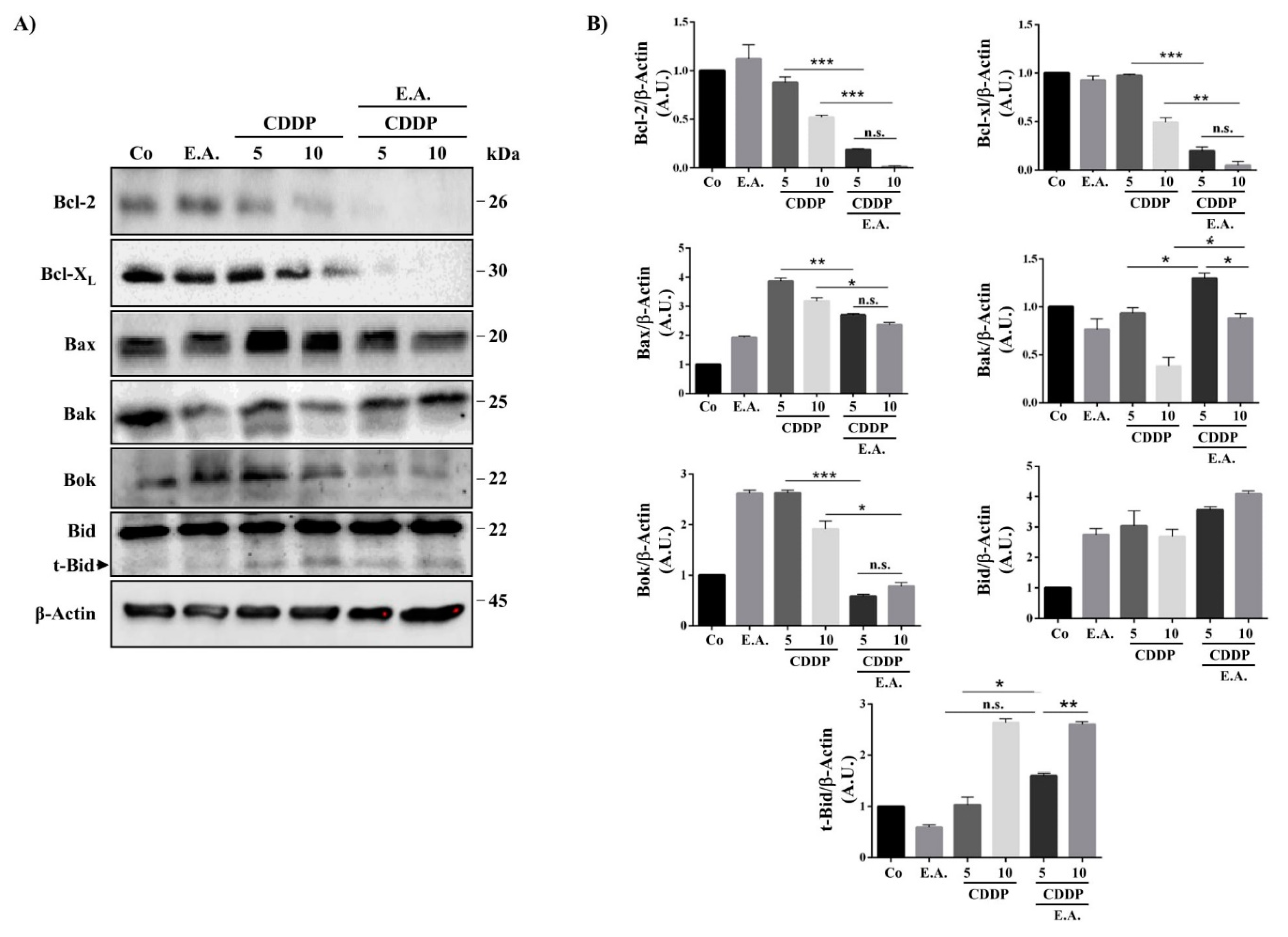
3.6. E.A./CDDP Combination-Induced Apoptosis through a Modulation of Bcl-2 Proteins Family
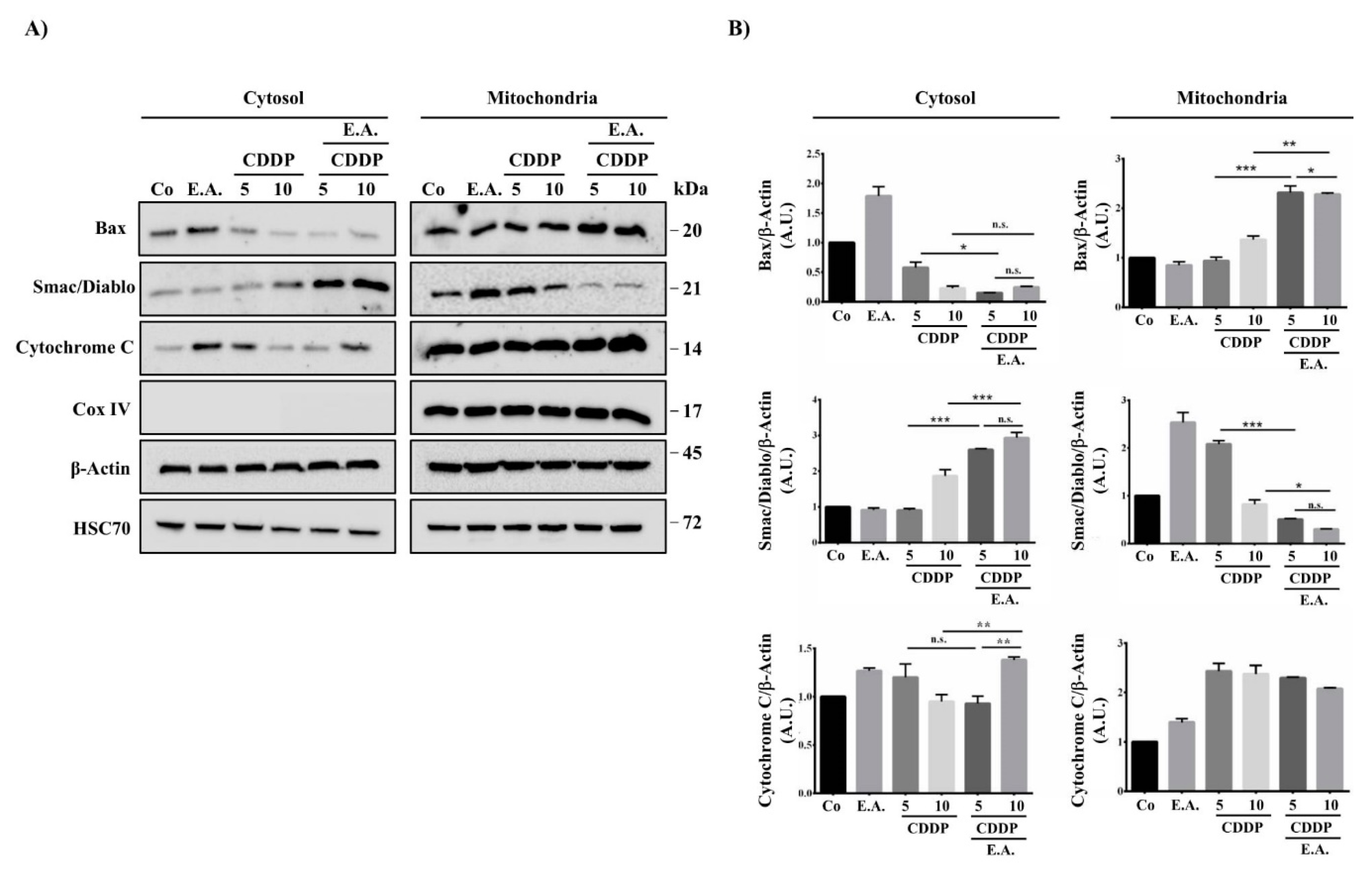
3.7. E.A./CDDP Combination Induced Smac/Diablo and Cytochrome C Release from the Mitochondria
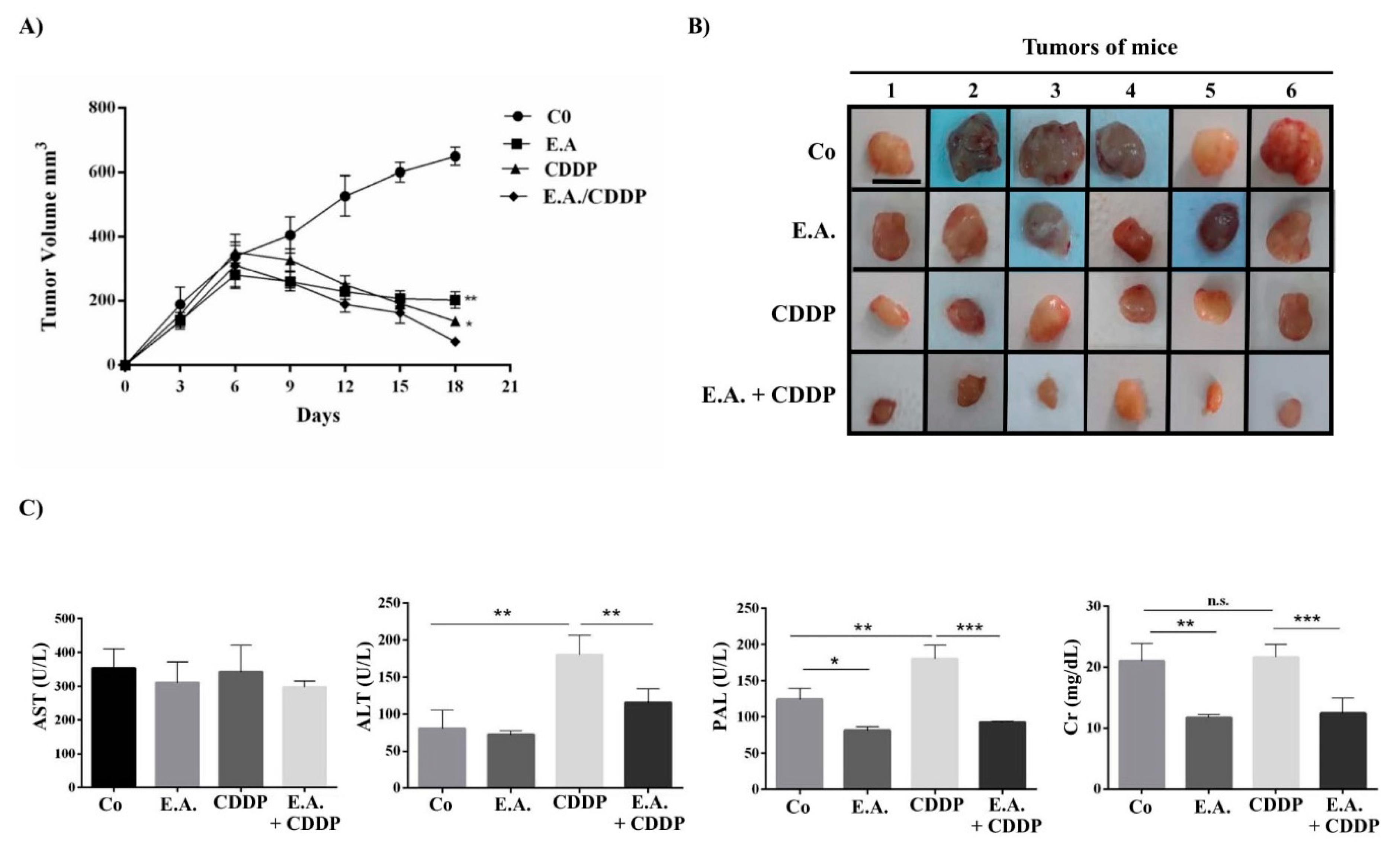
3.8. E.A./CDDP Combination Synergized to Prevent Murine Mammary Carcinoma Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA. Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.M.; Harvey, H.; Lipton, A. Metastatic breast cancer: Overview of treatment. Clin. Orthop. Relat. Res. 2003, 415, S132–S137. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.P.; Boult, J.K.; Vasudev, N.S.; Reynolds, A.R. Monitoring the vascular response and resistance to sunitinib in renal cell carcinoma in vivo with susceptibility contrast MRI. Cancer Res. 2017, 77, 4127–4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef]
- Pegram, M.D.; Lipton, A.; Hayes, D.F.; Weber, B.L.; Baselga, J.M.; Tripathy, D.; Baly, D.; Baughman, S.A.; Twaddell, T.; Glaspy, J.A.; et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol. 1998, 16, 2659–2671. [Google Scholar] [CrossRef]
- Gerratana, L.; Fanotto, V.; Pelizzari, G.; Agostinetto, E.; Puglisi, F. Do platinum salts fit all triple negative breast cancers? Cancer Treat. Rev. 2016, 48, 34–41. [Google Scholar] [CrossRef]
- Pattarawat, P.; Wallace, S.; Pfisterer, B.; Odoi, A.; Wang, H.R. Formulation of a triple combination gemcitabine plus romidepsin + cisplatin regimen to efficaciously and safely control triple-negative breast cancer tumor development. Cancer Chemother. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Al-Eitan, L.N.; Alzoubi, K.H.; Al-Smadi, L.I.; Khabour, O.F. Vitamin E protects against cisplatin-induced genotoxicity in human lymphocytes. Toxicol. In Vitro 2020, 62, 104672. [Google Scholar] [CrossRef]
- Shruthi, S.; Bhasker Shenoy, K. Genoprotective effects of gallic acid against cisplatin induced genotoxicity in bone marrow cells of mice. Toxicol. Res. 2018, 7, 951–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hu, P.C.; Gao, F.F.; Lv, J.W.; Xu, S.; Kuang, C.C.; Wei, L.; Zhang, J.W. The protective effect of curcumin on hepatotoxicity and ultrastructural damage induced by cisplatin. Ultrastruct. Pathol. 2014, 38, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.; Gimazane, A.; Lizard, G.; Izard, J.C.; Solary, E.; Latruffe, N.; Delmas, D. Effects of resveratrol analogs on cell cycle progression, cell cycle associated proteins and 5fluoro-uracil sensitivity in human derived colon cancer cells. Int J. Cancer 2009, 124, 2780–2788. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Gao, W.; Zhang, M.J.; Chan, J.Y.; Wong, T.S. Curcumin enhances cisplatin sensitivity by suppressing NADPH oxidase 5 expression in human epithelial cancer. Oncol. Lett. 2019, 18, 2132–2139. [Google Scholar] [CrossRef] [Green Version]
- Czarnomysy, R.; Surazynski, A.; Poplawska, B.; Rysiak, E.; Pawlowska, N.; Czajkowska, A.; Bielawski, K.; Bielawska, A. Synergistic action of cisplatin and echistatin in MDA-MB-231 breast cancer cells. Mol. Cell. Biochem. 2017, 427, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Jaradat, N.A.; Shawahna, R.; Eid, A.M.; Al-Ramahi, R.; Asma, M.K.; Zaid, A.N. Herbal remedies use by breast cancer patients in the West Bank of Palestine. J. Ethnopharmacol. 2016, 178, 1–8. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Al-Ramahi, R.; Zaid, A.N.; Ayesh, O.I.; Eid, A.M. Ethnopharmacological survey of herbal remedies used for treatment of various types of cancer and their methods of preparations in the West Bank-Palestine. BMC Complement. Altern. Med. 2016, 16, 93. [Google Scholar] [CrossRef] [Green Version]
- Hegazi, G.; El-Lamey, T.M. In vitro production of some phenolic compounds from Ephedra alata Decne. J. Appl. Env. Biol. Sci 2011, 1, 158–163. [Google Scholar]
- Ibragic, S.; Sofic, E. Chemical composition of various Ephedra species. Bosn J. Basic Med. Sci 2015, 15, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.-M.; Zhi-Bin, W.; Ping, X.; Qiu-Hong, W.; He, B.; Kuang, H.-X. Phytochemistry and pharmacology of genus Ephedra. Chin. J. Nat. Med. 2018, 16, 811–828. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Abu-Lafi, S.; Abbadi, J.; Alamarneh, A.A.; Sawahreh, R.A.; Odeh, I. Analysis of phenolic and flavonoids of wild Ephedra alata plant extracts by LC/PDA and LC/MS and their antioxidant activity. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y.; Hikino, H.; Tsurufuji, S.; Watanabe, M.; Ohuchi, K. Antiinflammatory actions of ephedrines in acute inflammations. Planta Med. 1985, 51, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shu, Z.; Xing, N.; Xu, B.; Wang, C.; Sun, G.; Sun, X.; Kuang, H. A pure polysaccharide from Ephedra sinica treating on arthritis and inhibiting cytokines expression. Int. J. Biol. Macromol. 2016, 86, 177–188. [Google Scholar] [CrossRef]
- Ghasemi, M.; Azarnia, M.; Jamali, M.; Mirabolghasemi, G.; Nazarian, S.; Naghizadeh, M.M.; Rajabi, M.; Tahamtani, Y. Protective effects of Ephedra pachyclada extract on mouse models of carbon tetrachloride-induced chronic and acute liver failure. Tissue Cell 2014, 46, 78–85. [Google Scholar] [CrossRef]
- Yamada, I.; Goto, T.; Takeuchi, S.; Ohshima, S.; Yoneyama, K.; Shibuya, T.; Kataoka, E.; Segawa, D.; Sato, W.; Dohmen, T. Mao (Ephedra sinica Stapf) protects against d-galactosamine and lipopolysaccharide-induced hepatic failure. Cytokine 2008, 41, 293–301. [Google Scholar] [CrossRef]
- Khan, A.; Jan, G.; Khan, A.; Gul Jan, F.; Bahadur, A.; Danish, M. In Vitro Antioxidant and Antimicrobial Activities of Ephedra gerardiana (Root and Stem) Crude Extract and Fractions. Evid. -Based Complement. Altern. Med. 2017, 2017, 4040254. [Google Scholar] [CrossRef] [Green Version]
- Parsaeimehr, A.; Sargsyan, E.; Javidnia, K. A comparative study of the antibacterial, antifungal and antioxidant activity and total content of phenolic compounds of cell cultures and wild plants of three endemic species of Ephedra. Molecules 2010, 15, 1668–1678. [Google Scholar] [CrossRef]
- Danciu, C.; Muntean, D.; Alexa, E.; Farcas, C.; Oprean, C.; Zupko, I.; Bor, A.; Minda, D.; Proks, M.; Buda, V.; et al. Phytochemical Characterization and Evaluation of the Antimicrobial, Antiproliferative and Pro-Apoptotic Potential of Ephedra alata Decne. Hydroalcoholic Extract against the MCF-7 Breast Cancer Cell Line. Molecules 2018, 24, 13. [Google Scholar] [CrossRef] [Green Version]
- Nam, N.H.; Lee, C.W.; Hong, D.H.; Kim, H.M.; Bae, K.H.; Ahn, B.Z. Antiinvasive, antiangiogenic and antitumour activity of Ephedra sinica extract. Phytother. Res. 2003, 17, 70–76. [Google Scholar] [CrossRef]
- Ala, A.A.; Olotu, B.B.; Ohia, C.M.D. Assessment of cytotoxicity of leaf extracts of Andrographis paniculata and Aspilia africana on murine cells in vitro. Arch. Basic Appl. Med. 2018, 6, 61–65. [Google Scholar] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Cotte, A.K.; Aires, V.; Fredon, M.; Limagne, E.; Derangere, V.; Thibaudin, M.; Humblin, E.; Scagliarini, A.; de Barros, J.P.; Hillon, P.; et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat. Commun. 2018, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins (Basel) 2019, 11, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baarine, M.; Thandapilly, S.J.; Louis, X.L.; Mazue, F.; Yu, L.; Delmas, D.; Netticadan, T.; Lizard, G.; Latruffe, N. Pro-apoptotic versus anti-apoptotic properties of dietary resveratrol on tumoral and normal cardiac cells. Genes Nutr. 2011, 6, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Nivelle, L.; Hubert, J.; Courot, E.; Jeandet, P.; Aziz, A.; Nuzillard, J.M.; Renault, J.H.; Clement, C.; Martiny, L.; Delmas, D.; et al. Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor. Molecules 2017, 22, 474. [Google Scholar] [CrossRef]
- Petrelli, F.; Barni, S.; Bregni, G.; de Braud, F.; Di Cosimo, S. Platinum salts in advanced breast cancer: A systematic review and meta-analysis of randomized clinical trials. Breast Cancer Res. Treat. 2016, 160, 425–437. [Google Scholar] [CrossRef]
- Thornberry, N.A. Caspases: Key mediators of apoptosis. Chem. Biol. 1998, 5, R97–103. [Google Scholar] [CrossRef] [Green Version]
- Carlini, E.A. Plants and the central nervous system. Pharmacol. Biochem. Behav. 2003, 75, 501–512. [Google Scholar] [CrossRef]
- Mazue, F.; Delmas, D.; Murillo, G.; Saleiro, D.; Limagne, E.; Latruffe, N. Differential protective effects of red wine polyphenol extracts (RWEs) on colon carcinogenesis. Food Funct. 2014, 5, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Chalons, P.; Amor, S.; Courtaut, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; Chabert, P.; Schni-Kerth, V.; Aires, V.; Delmas, D. Study of Potential Anti-Inflammatory Effects of Red Wine Extract and Resveratrol through a Modulation of Interleukin-1-Beta in Macrophages. Nutrients 2018, 10, 1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasiri, N.; Rahmati, M.; Ahmadi, L.; Eskandari, N.; Motedayyen, H. Therapeutic potential of quercetin on human breast cancer in different dimensions. Inflammopharmacology 2019. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different antitumor effects of quercetin, quercetin-3′-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef]
- Mellado, M.; Soto, M.; Madrid, A.; Montenegro, I.; Jara-Gutierrez, C.; Villena, J.; Werner, E.; Godoy, P.; Aguilar, L.F. In vitro antioxidant and antiproliferative effect of the extracts of Ephedra chilensis K Presl aerial parts. BMC Complement. Altern. Med. 2019, 19, 53. [Google Scholar] [CrossRef]
- Al-Awaida, W.; Al-Hourani, B.J.; Akash, M.; Talib, W.H.; Zein, S.; Falah, R.R.; Aburubaiha, Z. In vitro anticancer, anti-inflammatory, and antioxidant potentials of Ephedra aphylla. J. Cancer Res. Ther. 2018, 14, 1350–1354. [Google Scholar] [CrossRef]
- Wei, M.C.; Lindsten, T.; Mootha, V.K.; Weiler, S.; Gross, A.; Ashiya, M.; Thompson, C.B.; Korsmeyer, S.J. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000, 14, 2060–2071. [Google Scholar] [PubMed]
- Lutter, M.; Perkins, G.A.; Wang, X. The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC Cell Biol. 2001, 2, 22. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel) 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [Green Version]
- Tsang, R.Y.; Al-Fayea, T.; Au, H.J. Cisplatin overdose: Toxicities and management. Drug Saf. 2009, 32, 1109–1122. [Google Scholar] [CrossRef]

| Compound | Content (%) |
|---|---|
| Caffeic acid derivative | 0.54 |
| Citric acid | 0.28 |
| Syringic acid hexoside | 0.70 |
| Syringic acid | 0.73 |
| Gallocatechin | 0.40 |
| Ferulic acid | 0.19 |
| Quercetin dihexoside | 1.02 |
| Vicenin-2 | 9.33 |
| Quercetin-3-O-galactoside | 1.95 |
| Isoorientin-4-O-glucoside | 1.74 |
| 6-Hydroxyquercetin-3-O-di-hexose | 14.67 |
| Quercetin 3-O-glucoside | 2.48 |
| Luteolin-8-C-β-d-glucopyranoside | 0.18 |
| Quercetin | 19.85 |
| Myricetin hexoside | 8.59 |
| Quercetin 3-O-[6”-(3-hydroxyl-3-methylglutaryl)-β-d-galactoside] | 24.48 |
| Myricetin-3-O-glucoside | 3.05 |
| Quercetin 3-O-rhamnoside | 0.76 |
| 7-O-rhamnoside | |
| Kaempferol-O-di-deoxyhexoside | 1.08 |
| IC50 | 24 h | 48 h | 72 h |
|---|---|---|---|
| E.A. | - | 65 µg/mL | 30 µg/mL |
| CDDP | 20 µM | 10 µM | 3 µM |
| Ratio Cytosol/Mitochondria Fractions | |||
|---|---|---|---|
| Bax | Smac/Diablo | Cytochrome c | |
| E.A. | 2.1 | 0.38 | 0.91 |
| CCDP 5 µM | 0.62 | 0.43 | 0.45 |
| CCDP 10 µM | 0.25 | 0.90 | 0.55 |
| E.A. + CCDP 5 µM | 0.12 | 5.10 | 0.41 |
| E.A. + CCDP 10 µM | 0.10 | 9.69 | 0.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sioud, F.; Amor, S.; Toumia, I.b.; Lahmar, A.; Aires, V.; Chekir-Ghedira, L.; Delmas, D. A New Highlight of Ephedra alata Decne Properties as Potential Adjuvant in Combination with Cisplatin to Induce Cell Death of 4T1 Breast Cancer Cells In Vitro and In Vivo. Cells 2020, 9, 362. https://doi.org/10.3390/cells9020362
Sioud F, Amor S, Toumia Ib, Lahmar A, Aires V, Chekir-Ghedira L, Delmas D. A New Highlight of Ephedra alata Decne Properties as Potential Adjuvant in Combination with Cisplatin to Induce Cell Death of 4T1 Breast Cancer Cells In Vitro and In Vivo. Cells. 2020; 9(2):362. https://doi.org/10.3390/cells9020362
Chicago/Turabian StyleSioud, Fairouz, Souheila Amor, Imène ben Toumia, Aida Lahmar, Virginie Aires, Leila Chekir-Ghedira, and Dominique Delmas. 2020. "A New Highlight of Ephedra alata Decne Properties as Potential Adjuvant in Combination with Cisplatin to Induce Cell Death of 4T1 Breast Cancer Cells In Vitro and In Vivo" Cells 9, no. 2: 362. https://doi.org/10.3390/cells9020362
APA StyleSioud, F., Amor, S., Toumia, I. b., Lahmar, A., Aires, V., Chekir-Ghedira, L., & Delmas, D. (2020). A New Highlight of Ephedra alata Decne Properties as Potential Adjuvant in Combination with Cisplatin to Induce Cell Death of 4T1 Breast Cancer Cells In Vitro and In Vivo. Cells, 9(2), 362. https://doi.org/10.3390/cells9020362






