Uncoupling of the Astrocyte Syncytium Differentially Affects AQP4 Isoforms
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Perfusion and Tissue Preparation for Electron Microscopy
2.3. Postembedding Immunogold Electron Microscopy
2.4. Immunogold Quantitation and Data Analysis
2.5. Preparation of Total Protein Lysates from Brain Regions
2.6. SDS-PAGE and Western Blotting
2.7. RNA Isolation and Reverse Transcriptase Quantitative PCR (RT-qPCR)
3. Results
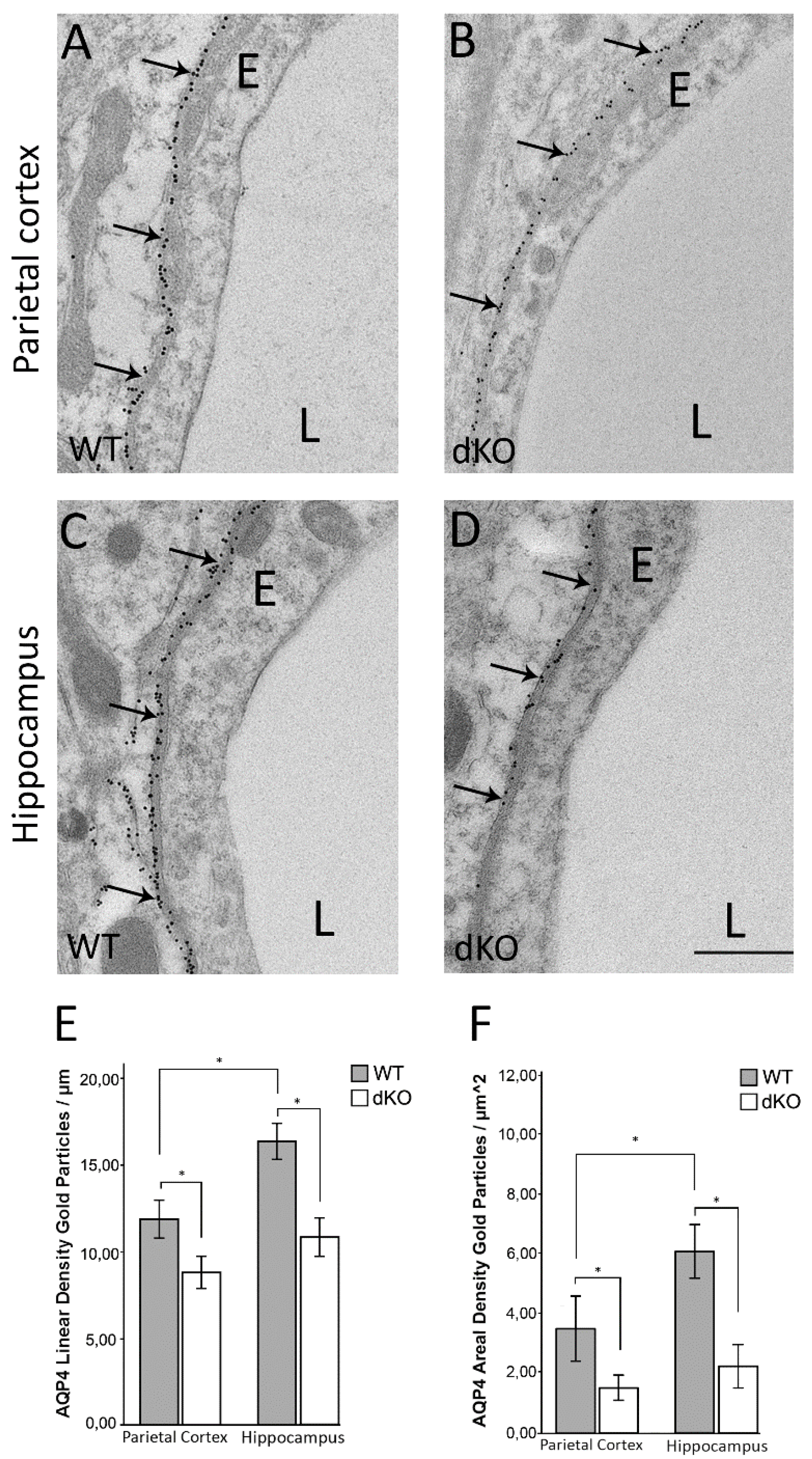
3.1. Deletion of Astroglial Connexins Leads to a Significant Decrease in Perivascular AQP4 and Abolishes the Regional Heterogeneity in AQP4 Distribution
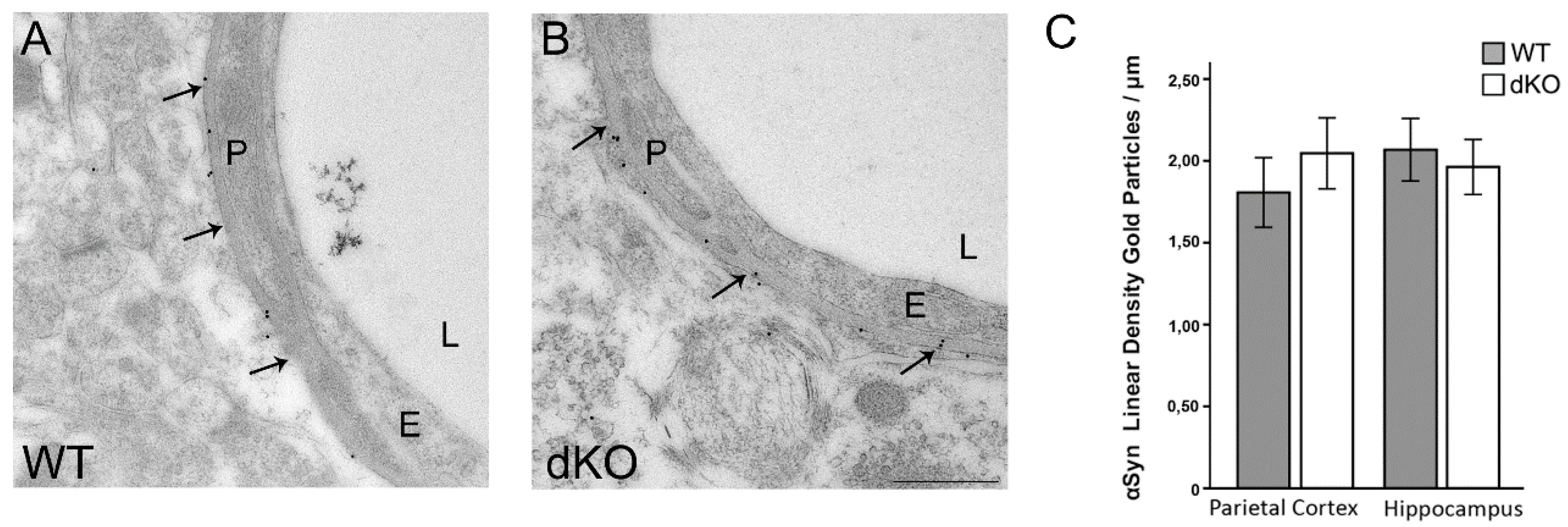
3.2. Reduction in the Perivascular Pool of AQP4 is Independent of α-Syntrophin
3.3. Deletion of Astroglial Connexins Leads to a Decrease in Aqp4 Transcript, but Has a Differential Effect on AQP4 Isoforms at the Protein Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verkhratsky, A.; Sofroniew, M.V.; Messing, A.; DeLanerolle, N.C.; Rempe, D.; Rodriguez, J.J.; Nedergaard, M. Neurological diseases as primary gliopathies: A reassessment of neurocentrism. ASN Neuro 2012, 4. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M.; Hertz, L. Why are astrocytes important? Neurochem. Res. 2015, 40, 389–401. [Google Scholar] [CrossRef]
- Alvestad, S.; Hammer, J.; Hoddevik, E.H.; Skare, Ø.; Sonnewald, U.; Amiry-Moghaddam, M.; Ottersen, O.P. Mislocalization of AQP4 precedes chronic seizures in the kainate model of temporal lobe epilepsy. Epilepsy Res. 2013, 105, 30–41. [Google Scholar] [CrossRef]
- Eid, T.; Lee, T.-S.W.; Thomas, M.J.; Amiry-Moghaddam, M.; Bjørnsen, L.P.; Spencer, D.D.; Agre, P.; Ottersen, O.P.; De Lanerolle, N.C. Loss of perivascular aquaporin 4 may underlie deficient water and K+ homeostasis in the human epileptogenic hippocampus. Proc. Natl. Acad. Sci. USA 2005, 102, 1193–1198. [Google Scholar] [CrossRef]
- Frydenlund, D.S.; Bhardwaj, A.; Otsuka, T.; Mylonakou, M.N.; Yasumura, T.; Davidson, K.G.; Zeynalov, E.; Skare, Ø.; Laake, P.; Haug, F.-M. Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 13532–13536. [Google Scholar] [CrossRef]
- Yang, J.; Lunde, L.K.; Nuntagij, P.; Oguchi, T.; Camassa, L.; Nilsson, L.N.; Lannfelt, L.; Xu, Y.; Amiry-Moghaddam, M.; Ottersen, O.P.; et al. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 27, 711–722. [Google Scholar] [CrossRef]
- Claus, L.; Philippot, C.; Griemsmann, S.; Timmermann, A.; Jabs, R.; Henneberger, C.; Kettenmann, H.; Steinhäuser, C. Barreloid borders and neuronal activity shape panglial gap junction-coupled networks in the mouse thalamus. Cereb. Cortex 2016, 28, 213–222. [Google Scholar] [CrossRef]
- Zhang, J.; Griemsmann, S.; Wu, Z.; Dobrowolski, R.; Willecke, K.; Theis, M.; Steinhäuser, C.; Bedner, P. Connexin43, but not connexin30, contributes to adult neurogenesis in the dentate gyrus. Brain Res. Bull. 2018, 136, 91–100. [Google Scholar] [CrossRef]
- Kielian, T. Glial connexins and gap junctions in CNS inflammation and disease. J. Neurochem. 2008, 106, 1000–1016. [Google Scholar] [CrossRef]
- Nagy, J.I.; Rash, J.E. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res. Rev. 2000, 32, 29–44. [Google Scholar] [CrossRef]
- Quist, A.P.; Rhee, S.K.; Lin, H.; Lal, R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J. Cell Biol. 2000, 148, 1063–1074. [Google Scholar] [CrossRef]
- Matsuuchi, L.; Naus, C.C. Gap junction proteins on the move: Connexins, the cytoskeleton and migration. Biochim. Biophys. Acta 2013, 1828, 94–108. [Google Scholar] [CrossRef]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef]
- Adams, M.E.; Mueller, H.A.; Froehner, S.C. In vivo requirement of the α-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J. Cell Biol. 2001, 155, 113–122. [Google Scholar] [CrossRef]
- Frigeri, A.; Nicchia, G.P.; Nico, B.; Quondamatteo, F.; Herken, R.; Roncali, L.; Svelto, M. Aquaporin-4 deficiency in skeletal muscle and brain of dystrophic mdx mice. FASEB J. 2001, 15, 90–98. [Google Scholar] [CrossRef]
- Nagelhus, E.A.; Ottersen, O.P. Physiological roles of aquaporin-4 in brain. Physiol. Rev. 2013, 93, 1543–1562. [Google Scholar] [CrossRef]
- Neely, J.D.; Amiry-Moghaddam, M.; Ottersen, O.P.; Froehner, S.C.; Agre, P.; Adams, M.E. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc. Natl. Acad. Sci. USA 2001, 98, 14108–14113. [Google Scholar] [CrossRef]
- Amiry-Moghaddam, M.; Frydenlund, D.; Ottersen, O. Anchoring of aquaporin-4 in brain: Molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience 2004, 129, 997–1008. [Google Scholar] [CrossRef]
- Vajda, Z.; Pedersen, M.; Fuchtbauer, E.M.; Wertz, K.; Stodkilde-Jorgensen, H.; Sulyok, E.; Doczi, T.; Neely, J.D.; Agre, P.; Frokiaer, J.; et al. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 13131–13136. [Google Scholar] [CrossRef]
- Amiry-Moghaddam, M.; Xue, R.; Haug, F.M.; Neely, J.D.; Bhardwaj, A.; Agre, P.; Adams, M.E.; Froehner, S.C.; Mori, S.; Ottersen, O.P. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004, 18, 542–544. [Google Scholar] [CrossRef]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Binder, D.K.; Verkman, A.S. Enhanced macromolecular diffusion in brain extracellular space in mouse models of vasogenic edema measured by cortical surface photobleaching. FASEB J. 2005, 19, 425–427. [Google Scholar] [CrossRef]
- Amiry-Moghaddam, M.; Otsuka, T.; Hurn, P.D.; Traystman, R.J.; Haug, F.M.; Froehner, S.C.; Adams, M.E.; Neely, J.D.; Agre, P.; Ottersen, O.P.; et al. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc. Natl. Acad. Sci. USA 2003, 100, 2106–2111. [Google Scholar] [CrossRef]
- Smith, A.J.; Jin, B.-J.; Ratelade, J.; Verkman, A.S. Aggregation state determines the localization and function of M1- and M23-aquaporin-4 in astrocytes. J. Cell Biol. 2014, 204, 559–573. [Google Scholar] [CrossRef]
- Simone, L.; Pisani, F.; Mola, M.G.; De Bellis, M.; Merla, G.; Micale, L.; Frigeri, A.; Vescovi, A.L.; Svelto, M.; Nicchia, G.P. AQP4 aggregation state is a determinant for glioma cell fate. Cancer Res. 2019, 79, 2182–2194. [Google Scholar] [CrossRef]
- Palazzo, C.; Buccoliero, C.; Mola, M.G.; Abbrescia, P.; Nicchia, G.P.; Trojano, M.; Frigeri, A. AQP4ex is crucial for the anchoring of AQP4 at the astrocyte end-feet and for neuromyelitis optica antibody binding. Acta Neuropathol. Commun. 2019, 7, 51. [Google Scholar] [CrossRef]
- De Bellis, M.; Pisani, F.; Mola, M.G.; Rosito, S.; Simone, L.; Buccoliero, C.; Trojano, M.; Nicchia, G.P.; Svelto, M.; Frigeri, A. Translational readthrough generates new astrocyte AQP4 isoforms that modulate supramolecular clustering, glial endfeet localization, and water transport. Glia 2017, 65, 790–803. [Google Scholar] [CrossRef]
- Strohschein, S.; Huttmann, K.; Gabriel, S.; Binder, D.K.; Heinemann, U.; Steinhauser, C. Impact of aquaporin-4 channels on K+ buffering and gap junction coupling in the hippocampus. Glia 2011, 59, 973–980. [Google Scholar] [CrossRef]
- Katoozi, S.; Skauli, N.; Rahmani, S.; Camassa, L.M.A.; Boldt, H.B.; Ottersen, O.P.; Amiry-Moghaddam, M. Targeted deletion of Aqp4 promotes the formation of astrocytic gap junctions. Brain Struct. Funct. 2017, 222, 3959–3972. [Google Scholar] [CrossRef]
- Ezan, P.; Andre, P.; Cisternino, S.; Saubamea, B.; Boulay, A.C.; Doutremer, S.; Thomas, M.A.; Quenech’du, N.; Giaume, C.; Cohen-Salmon, M. Deletion of astroglial connexins weakens the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1457–1467. [Google Scholar] [CrossRef]
- Theis, M.; De Wit, C.; Schlaeger, T.M.; Eckardt, D.; Kruger, O.; Doring, B.; Risau, W.; Deutsch, U.; Pohl, U.; Willecke, K. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis 2001, 29, 1–13. [Google Scholar] [CrossRef]
- Zhuo, L.; Theis, M.; Alvarez-Maya, I.; Brenner, M.; Willecke, K.; Messing, A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 2001, 31, 85–94. [Google Scholar] [CrossRef]
- Teubner, B.; Michel, V.; Pesch, J.; Lautermann, J.; Cohen-Salmon, M.; Sohl, G.; Jahnke, K.; Winterhager, E.; Herberhold, C.; Hardelin, J.P.; et al. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum. Mol. Genet. 2003, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Mathiisen, T.M.; Nagelhus, E.A.; Jouleh, B.; Torp, R.; Frydenlund, D.S.; Mylonakou, M.-N.; Amiry-Moghaddam, M.; Covolan, L.; Utvik, J.K.; Riber, B. Postembedding immunogold cytochemistry of membrane molecules and amino acid transmitters in the central nervous system. In Neuroanatomical Tract-Tracing 3; Springer: Berlin/Heidelberg, Germany, 2006; pp. 72–108. [Google Scholar]
- Amiry-Moghaddam, M.; Lindland, H.; Zelenin, S.; Roberg, B.A.; Gundersen, B.B.; Petersen, P.; Rinvik, E.; Torgner, I.A.; Ottersen, O.P. Brain mitochondria contain aquaporin water channels: Evidence for the expression of a short AQP9 isoform in the inner mitochondrial membrane. FASEB J. 2005, 19, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Hoddevik, E.H.; Khan, F.H.; Rahmani, S.; Ottersen, O.P.; Boldt, H.B.; Amiry-Moghaddam, M. Factors determining the density of AQP4 water channel molecules at the brain–blood interface. Brain Struct. Funct. 2017, 222, 1753–1766. [Google Scholar] [CrossRef]
- Lunde, L.K.; Camassa, L.M.; Hoddevik, E.H.; Khan, F.H.; Ottersen, O.P.; Boldt, H.B.; Amiry-Moghaddam, M. Postnatal development of the molecular complex underlying astrocyte polarization. Brain Struct. Funct. 2015, 220, 2087–2101. [Google Scholar] [CrossRef]
- Prydz, A.; Stahl, K.; Puchades, M.; Davarpaneh, N.; Nadeem, M.; Ottersen, O.P.; Gundersen, V.; Amiry-Moghaddam, M. Subcellular expression of aquaporin-4 in substantia nigra of normal and MPTP-treated mice. Neuroscience 2017, 359, 258–266. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Orntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Nakase, T.; Naus, C.C. Gap junctions and neurological disorders of the central nervous system. Biochim. Biophys. Acta 2004, 1662, 149–158. [Google Scholar] [CrossRef]
- Koulakoff, A.; Mei, X.; Orellana, J.A.; Saez, J.C.; Giaume, C. Glial connexin expression and function in the context of Alzheimer’s disease. Biochim. Biophys. Acta 2012, 1818, 2048–2057. [Google Scholar] [CrossRef]
- Fonseca, C.G.; Green, C.R.; Nicholson, L.F. Upregulation in astrocytic connexin 43 gap junction levels may exacerbate generalized seizures in mesial temporal lobe epilepsy. Brain Res. 2002, 929, 105–116. [Google Scholar] [CrossRef]
- Masaki, K. Early disruption of glial communication via connexin gap junction in multiple sclerosis, Balo’s disease and neuromyelitis optica. Neuropathology 2015, 35, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, W.T.; Cornell-Bell, A.H.; Sontheimer, H. Astrocytes exhibit regional specificity in gap-junction coupling. Glia 1994, 11, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.E.; Zhao, Y.; Gulinello, M.; Lee, S.C.; Raine, C.S.; Brosnan, C.F. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J. Neurosci. 2009, 29, 7743–7752. [Google Scholar] [CrossRef]
- Jullienne, A.; Fukuda, A.M.; Ichkova, A.; Nishiyama, N.; Aussudre, J.; Obenaus, A.; Badaut, J. Modulating the water channel AQP4 alters miRNA expression, astrocyte connectivity and water diffusion in the rodent brain. Sci. Rep. 2018, 8, 4186. [Google Scholar] [CrossRef]
- Ciappelloni, S.; Bouchet, D.; Dubourdieu, N.; Boue-Grabot, E.; Kellermayer, B.; Manso, C.; Marignier, R.; Oliet, S.H.R.; Tourdias, T.; Groc, L. Aquaporin-4 Surface Trafficking Regulates Astrocytic Process Motility and Synaptic Activity in Health and Autoimmune Disease. Cell Rep. 2019, 27, 3860–3872. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]


| Methods | Primary Antibody | Secondary Antibody |
|---|---|---|
| Immunogold | Rabbit anti-AQP4, 1:500, Sigma-Aldrich, A5971 | Goat anti-rabbit 15 nm, 1:20, Abcam |
| Rabbit anti-Cx30, 1:200, Invitrogen, 71-2200 | Goat anti-rabbit 15 nm, 1:20, Abcam | |
| Rabbit anti-Cx43, 1:200, Invitrogen, 71-0700 | Goat anti-rabbit 1 nm, 1:20, Abcam | |
| Rabbit anti-α-syntrophin (SYN259), 1:200, Gift from Dr Marvin E. Adams | Goat anti-rabbit 1 nm, 1:20, Abcam | |
| Western Blotting | Rabbit anti-AQP4, 1:2000, Sigma-Aldrich, A5971 | Donkey anti-rabbit HRP, 1:5000, Amersham, GE Life Sciences |
| Rabbit anti-Cx43, 1:1000, Sigma-Aldrich, C6219 | Donkey anti-rabbit HRP, 1:5000, Amersham, GE Life Sciences | |
| Rabbit anti-Cx30, 1:200, Invitrogen, 71-2200 | Donkey anti-rabbit HRP, 1:5000, Amersham, GE Life Sciences | |
| Rabbit anti-α-tubulin, 1:2000, Abcam, ab4074 | Donkey anti-rabbit HRP, 1:5000 and 1:25,000, Amersham, GE Life Sciences | |
| Custom Rabbit polyclonal anti-mouse AQP4-ex antibody, 1:2000, GeneScript [26] | Donkey anti-rabbit HRP, 1:25,000, Amersham, GE Life Sciences | |
| Custom Rabbit polyclonal anti-AQP4-M1 antibody, 1:2000, GeneScript | Donkey anti-rabbit HRP, 1:25,000, Amersham, GE Life Sciences |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Aqp4 | 5′-TTTGGACCCGCAGTTATCAT-3′ | 5′-GTTGTCCTCCACCTCCATGT-3′ |
| Gja1 | 5′-GTGCCGGCTTCACTTTCATTAAG-3′ | 5′-AAATGAAGAGCACCGACAGC-3′ |
| Gjb6 | 5′-GACATTCCCACTGTGACCCT-3′ | 5′-TCGTGCAGGCTTATTCTGAGT-3′ |
| Gapdh | 5′-TGCGACTTCAACAGCAACTC-3′ | 5′-CTTGCTCAGTGTCCTTGCTG-3′ |
| Hprt1 | 5′-GCCCCAAAATGGTTAAGGTT-3′ | 5′-TTGCGCTCATCTTAGGCTTT-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katoozi, S.; Skauli, N.; Zahl, S.; Deshpande, T.; Ezan, P.; Palazzo, C.; Steinhäuser, C.; Frigeri, A.; Cohen-Salmon, M.; Ottersen, O.P.; et al. Uncoupling of the Astrocyte Syncytium Differentially Affects AQP4 Isoforms. Cells 2020, 9, 382. https://doi.org/10.3390/cells9020382
Katoozi S, Skauli N, Zahl S, Deshpande T, Ezan P, Palazzo C, Steinhäuser C, Frigeri A, Cohen-Salmon M, Ottersen OP, et al. Uncoupling of the Astrocyte Syncytium Differentially Affects AQP4 Isoforms. Cells. 2020; 9(2):382. https://doi.org/10.3390/cells9020382
Chicago/Turabian StyleKatoozi, Shirin, Nadia Skauli, Soulmaz Zahl, Tushar Deshpande, Pascal Ezan, Claudia Palazzo, Christian Steinhäuser, Antonio Frigeri, Martine Cohen-Salmon, Ole Petter Ottersen, and et al. 2020. "Uncoupling of the Astrocyte Syncytium Differentially Affects AQP4 Isoforms" Cells 9, no. 2: 382. https://doi.org/10.3390/cells9020382
APA StyleKatoozi, S., Skauli, N., Zahl, S., Deshpande, T., Ezan, P., Palazzo, C., Steinhäuser, C., Frigeri, A., Cohen-Salmon, M., Ottersen, O. P., & Amiry-Moghaddam, M. (2020). Uncoupling of the Astrocyte Syncytium Differentially Affects AQP4 Isoforms. Cells, 9(2), 382. https://doi.org/10.3390/cells9020382






