A PP2A-B56—Centered View on Metaphase-to-Anaphase Transition in Mouse Oocyte Meiosis I
Abstract
:1. Introduction
2. Protein Phosphatase 2A—One Name for Multiple Phosphatases
2.1. Meiosis: Two Sequential Divisions without Genome Replication
2.2. Meiosis in Oocytes is Highly Error-Prone
3. Stabilization of Kinetochore-Microtubule Attachments
4. The Spindle Assembly Checkpoint, PP2A-B56 and PP1
5. The Spindle Assembly Checkpoint and PP2A-B56 in Oocyte Meiosis I
6. Integrating Error Correction, SAC Control and Anaphase I Onset
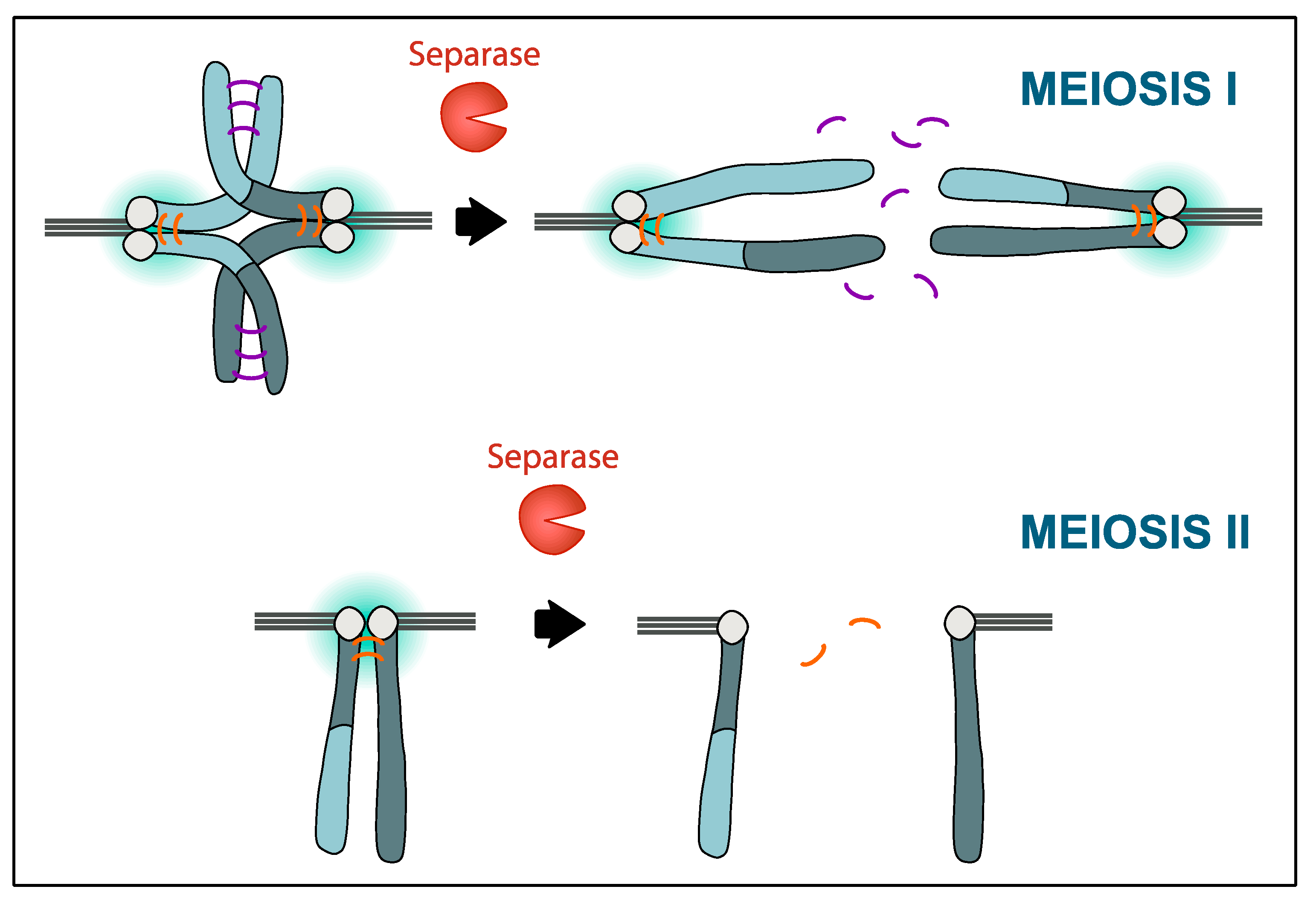
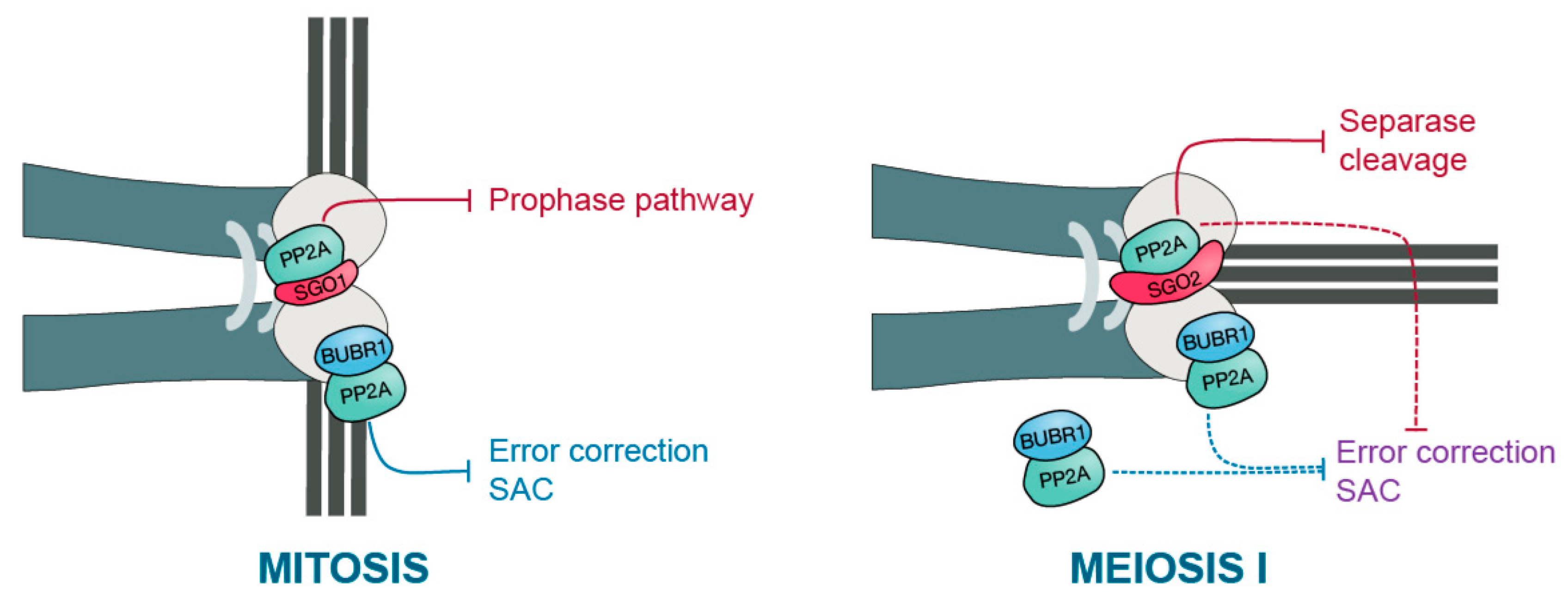
7. Protecting Centromeric Cohesin in Meiosis I but Not Meiosis II
8. Protein Phosphatase 2A—One Name for It All
9. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, K.; D’Souza, R.C.J.; Tyanova, S.; Schaab, C.; Wiśniewski, J.R.; Cox, J.; Mann, M. Ultradeep Human Phosphoproteome Reveals a Distinct Regulatory Nature of Tyr and Ser/Thr-Based Signaling. Cell Rep. 2014, 8, 1583–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlastaridis, P.; Kyriakidou, P.; Chaliotis, A.; Van de Peer, Y.; Oliver, S.G.; Amoutzias, G.D. Estimating the total number of phosphoproteins and phosphorylation sites in eukaryotic proteomes. Gigascience 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Touati, S.A.; Uhlmann, F. A global view of substrate phosphorylation and dephosphorylation during budding yeast mitotic exit. Microb. Cell (Graz, Austria) 2018, 5, 389–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dephoure, N.; Zhou, C.; Villen, J.; Beausoleil, S.A.; Bakalarski, C.E.; Elledge, S.J.; Gygi, S.P. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. USA 2008, 105, 10762–10767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCloy, R.A.; Parker, B.L.; Rogers, S.; Chaudhuri, R.; Gayevskiy, V.; Hoffman, N.J.; Ali, N.; Watkins, D.N.; Daly, R.J.; James, D.E.; et al. Global Phosphoproteomic Mapping of Early Mitotic Exit in Human Cells Identifies Novel Substrate Dephosphorylation Motifs. Mol. Cell. Proteomics 2015, 14, 2194–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, In Vivo, and Site-Specific Phosphorylation Dynamics in Signaling Networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef] [Green Version]
- Kremmer, E.; Ohst, K.; Kiefer, J.; Brewis, N.; Walter, G. Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: Abundant expression of both forms in cells. Mol. Cell. Biol. 1997, 17, 1692–1701. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Fernandes, G.; Lee, C.W. Protein phosphatases involved in regulating mitosis: Facts and hypotheses. Mol. Cells 2016, 39, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Moura, M.; Conde, C. Phosphatases in Mitosis: Roles and regulation. Biomolecules 2019, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, J. Protein phosphatases in the regulation of mitosis. J. Cell Biol. 2019, 218, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Saurin, A.T. Kinase and Phosphatase Cross-Talk at the Kinetochore. Front. Cell Dev. Biol. 2018, 6, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holder, J.; Poser, E.; Barr, F.A. Getting out of mitosis: Spatial and temporal control of mitotic exit and cytokinesis by PP1 and PP2A. FEBS Lett. 2019, 593, 2908–2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, S.; McCloy, R.; Watkins, D.N.; Burgess, A. Mechanisms regulating phosphatase specificity and the removal of individual phosphorylation sites during mitotic exit. Bioessays 2016, 38, S24–S32. [Google Scholar] [CrossRef] [PubMed]
- El Yakoubi, W.; Wassmann, K. Meiotic Divisions: No Place for Gender Equality. Adv. Exp. Med. Biol. 2017, 1002, 1–17. [Google Scholar]
- Nagaoka, S.I.; Hassold, T.J.; Hunt, P.A. Human aneuploidy: Mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 2012, 13, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Volant, S. A First Child at Age 28.5 in 2015: 4.5 Years Later than in 1974. Available online: https://www.insee.fr/en/statistiques/2856712 (accessed on 15 December 2019).
- Davie, E. A First Child at Age 28. Available online: https://www.insee.fr/en/statistiques/1281069 (accessed on 14 January 2020).
- Tachibana-Konwalski, K.; Godwin, J.; van der Weyden, L.; Champion, L.; Kudo, N.R.; Adams, D.J.; Nasmyth, K. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 2010, 24, 2505–2516. [Google Scholar] [CrossRef] [Green Version]
- Chiang, T.; Duncan, F.E.; Schindler, K.; Schultz, R.M.; Lampson, M.A.; Michael, A.; Lampson, M.A.; Michael, A. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr. Biol. 2011, 20, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Jessberger, R.; Chiang, T.; Duncan, F.E.; Schindler, K.; Schultz, R.M.; Lampson, M.A.; Michael, A.; Lampson, M.A.; Michael, A.; Jessberger, R. Age-related aneuploidy through cohesion exhaustion. EMBO Rep. 2012, 13, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Webster, A.; Schuh, M. Mechanisms of Aneuploidy in Human Eggs. Trends Cell Biol. 2017, 27, 55–68. [Google Scholar] [CrossRef]
- Touati, S.A.; Wassmann, K. How oocytes try to get it right: Spindle checkpoint control in meiosis. Chromosoma 2016, 125, 321–335. [Google Scholar] [CrossRef]
- Monda, J.K.; Cheeseman, I.M. The kinetochore-microtubule interface at a glance. J. Cell Sci. 2018, 131, jcs214577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.U. Kinetochore-microtubule interactions: Steps towards bi-orientation. EMBO J. 2010, 29, 4070–4082. [Google Scholar] [CrossRef] [PubMed]
- Wandke, C.; Barisic, M.; Sigl, R.; Rauch, V.; Wolf, F.; Amaro, A.C.; Tan, C.H.; Pereira, A.J.; Kutay, U.; Maiato, H.; et al. Human chromokinesins promote chromosome congression and spindle microtubule dynamics during mitosis. J. Cell Biol. 2012, 198, 847–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, R.L.; Draviam, V.M. Lateral to end-on conversion of chromosome-microtubule attachment requires kinesins CENP-E and MCAK. Curr. Biol. 2013, 23, 1514–1526. [Google Scholar] [CrossRef] [Green Version]
- Kalantzaki, M.; Kitamura, E.; Zhang, T.; Mino, A.; Novák, B.; Tanaka, T.U. Kinetochore–microtubule error correction is driven by differentially regulated interaction modes. Nat. Cell Biol. 2015, 17, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, R.L.; Conti, D.; Tamura, N.; Braun, D.; Ramalingam, R.A.; Cieslinski, K.; Ries, J.; Draviam, V.M. Aurora-B kinase pathway controls the lateral to end-on conversion of kinetochore-microtubule attachments in human cells. Nat. Commun. 2017, 8, 150. [Google Scholar] [CrossRef]
- Doodhi, H.; Kasciukovic, T.; Gierlinski, M.; Li, S.; Clayton, L.; Tanaka, T.U. Error correction is driven by direct competition between microtubules for interaction with a kinetochore. bioRxiv 2019, 455873. [Google Scholar]
- Kim, J.; Ishiguro, K.-I.; Nambu, A.; Akiyoshi, B.; Yokobayashi, S.; Kagami, A.; Ishiguro, T.; Pendas, A.M.; Takeda, N.; Sakakibara, Y.; et al. Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature 2015, 517, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y. Geometry and force behind kinetochore orientation: Lessons from meiosis. Nat. Rev. Mol. Cell Biol. 2012, 13, 370–382. [Google Scholar] [CrossRef]
- Gregan, J.; Polakova, S.; Zhang, L.; Tolić-Nørrelykke, I.M.; Cimini, D. Merotelic kinetochore attachment: Causes and effects. Trends Cell Biol. 2011, 21, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Kitajima, T.S.; Ohsugi, M.; Ellenberg, J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell 2011, 146, 568–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balboula, A.Z.; Schindler, K. Selective disruption of aurora C kinase reveals distinct functions from aurora B kinase during meiosis in mouse oocytes. PLoS Genet. 2014, 10, e1004194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welburn, J.P.I.; Vleugel, M.; Liu, D.; Yates, J.R., 3rd; Lampson, M.A.; Fukagawa, T.; Cheeseman, I.M. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol. Cell 2010, 38, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallot, A.; Leontiou, I.; Cladiere, D.; El Yakoubi, W.; Bolte, S.; Buffin, E.; Wassmann, K. Tension-Induced Error Correction and Not Kinetochore Attachment Status Activates the SAC in an Aurora-B/C-Dependent Manner in Oocytes. Curr. Biol. 2018, 28, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Kaido, M.; Kitajima, T.S. Inherent Instability of Correct Kinetochore-Microtubule Attachments during Meiosis I in Oocytes. Dev. Cell 2015, 33, 589–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.; Shi, P.; Song, A.; Zou, D.; Zhou, Y.; Gu, P.; Huang, Z.; Wang, Q.; Lin, Z.; Gao, X. PP2A regulates kinetochore-microtubule attachment during meiosis I in oocyte. Cell Cycle 2016, 15, 1450–1461. [Google Scholar] [CrossRef] [Green Version]
- Homer, H.; Gui, L.; Carroll, J. A Spindle Assembly Checkpoint Protein Functions in Prophase I Arrest and Prometaphase Progression. Science 2009, 326, 991–994. [Google Scholar] [CrossRef] [Green Version]
- Touati, S.A.; Buffin, E.; Cladière, D.; Hached, K.; Rachez, C.; van Deursen, J.M.; Wassmann, K. Mouse oocytes depend on BubR1 for proper chromosome segregation but not for prophase I arrest. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Musacchio, A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Curr. Biol. 2015, 25, R1002–R1018. [Google Scholar] [CrossRef] [Green Version]
- Foley, E.A.; Kapoor, T.M. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat. Rev. Mol. Cell Biol. 2013, 14, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Marston, A.L.; Wassmann, K. Multiple Duties for Spindle Assembly Checkpoint Kinases in Meiosis. Front. Cell Dev. Biol. 2017, 5, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lischetti, T.; Nilsson, J. Regulation of mitotic progression by the spindle assembly checkpoint. Mol. Cell. Oncol. 2015, 2, e970484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etemad, B.; Kops, G.J.P.L. Attachment issues: Kinetochore transformations and spindle checkpoint silencing. Curr. Opin. Cell Biol. 2016, 39, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, Y.; Sacristan, C.; Pachis, S.T.; Adamopoulos, A.; Kuijt, T.; Ubbink, M.; von Castelmur, E.; Perrakis, A.; Kops, G.J.P.L. Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science 2015, 348, 1264–1267. [Google Scholar] [CrossRef]
- Aravamudhan, P.; Goldfarb, A.A.; Joglekar, A.P. The kinetochore encodes a mechanical switch to disrupt spindle assembly checkpoint signalling. Nat. Cell Biol. 2015, 17, 868–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Z.; Gao, H.; Yu, H. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science 2015, 348, 1260–1264. [Google Scholar] [CrossRef]
- Howell, B.J.; McEwen, B.F.; Canman, J.C.; Hoffman, D.B.; Farrar, E.M.; Rieder, C.L.; Salmon, E.D. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 2001, 155, 1159–1172. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.M.A.; Reis, R.M.; Bolanos-Garcia, V.M.; Florindo, C.; Tavares, A.A.; Bousbaa, H. Dynein-dependent transport of spindle assembly checkpoint proteins off kinetochores toward spindle poles. FEBS Lett. 2014, 588, 3265–3273. [Google Scholar] [CrossRef] [Green Version]
- Vanoosthuyse, V.; Hardwick, K.G. Overcoming inhibition in the spindle checkpoint. Genes Dev. 2009, 23, 2799–2805. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Lischetti, T.; Nilsson, J. A minimal number of MELT repeats supports all the functions of KNL1 in chromosome segregation. J. Cell Sci. 2014, 127, 871–884. [Google Scholar] [CrossRef] [Green Version]
- Nijenhuis, W.; Vallardi, G.; Teixeira, A.; Kops, G.J.P.L.; Saurin, A.T. Negative feedback at kinetochores underlies a responsive spindle checkpoint signal. Nat. Cell Biol. 2014, 16, 1257–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.J.; Cordeiro, M.H.; Davey, N.E.; Vallardi, G.; Ciliberto, A.; Gross, F.; Saurin, A.T. PP1 and PP2A Use Opposite Phospho-dependencies to Control Distinct Processes at the Kinetochore. Cell Rep. 2019, 28, 2206–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, M.H.; Smith, R.J.; Saurin, A.T. Kinetochore phosphatases suppress autonomous kinase activity to control the spindle assembly checkpoint. bioRxiv 2019, 856773. [Google Scholar]
- Dou, Z.; von Schubert, C.; Korner, R.; Santamaria, A.; Elowe, S.; Nigg, E.A. Quantitative mass spectrometry analysis reveals similar substrate consensus motif for human Mps1 kinase and Plk1. PLoS ONE 2011, 6, e18793. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, M.; Tanaka, K. Plk1 bound to Bub1 contributes to spindle assembly checkpoint activity during mitosis. Sci. Rep. 2017, 7, 8794. [Google Scholar] [CrossRef]
- von Schubert, C.; Cubizolles, F.; Bracher, J.M.; Sliedrecht, T.; Kops, G.J.P.L.; Nigg, E.A. Plk1 and Mps1 Cooperatively Regulate the Spindle Assembly Checkpoint in Human Cells. Cell Rep. 2015, 12, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Mihajlović, A.I.; FitzHarris, G. Segregating Chromosomes in the Mammalian Oocyte. Curr. Biol. 2018, 28, R895–R907. [Google Scholar] [CrossRef] [Green Version]
- Sanders, J.R.; Jones, K.T. Regulation of the meiotic divisions of mammalian oocytes and eggs. Biochem. Soc. Trans. 2018, 46, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Levasseur, M.D.; Thomas, C.; Davies, O.R.; Higgins, J.M.G.; Madgwick, S. Aneuploidy in Oocytes Is Prevented by Sustained CDK1 Activity through Degron Masking in Cyclin B1. Dev. Cell 2019, 48, 672–684. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.; Levasseur, M.D.; Harris, R.J.; Davies, O.R.; Madgwick, S. Synchronous chromosome segregation in mouse oocytes is ensured by biphasic securin destruction and cyclin B1-Cdk1. bioRxiv 2019, 824763. [Google Scholar]
- Lane, S.I.R.; Yun, Y.; Jones, K.T. Timing of anaphase-promoting complex activation in mouse oocytes is predicted by microtubule-kinetochore attachment but not by bivalent alignment or tension. Development 2012, 139, 1947–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellmuth, S.; Böttger, F.; Pan, C.; Mann, M.; Stemmann, O. PP2A delays APC/C-dependent degradation of separase-associated but not free securin. EMBO J. 2014, 33, 1134–1147. [Google Scholar] [CrossRef]
- Hindriksen, S.; Lens, S.M.A.; Hadders, M.A. The Ins and Outs of Aurora B Inner Centromere Localization. Front. cell Dev. Biol. 2017, 5, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Vader, G.; Vromans, M.J.M.; Lampson, M.A.; Lens, S.M.A. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 2009, 323, 1350–1353. [Google Scholar] [CrossRef] [Green Version]
- Khodjakov, A.; Pines, J. Centromere tension: A divisive issue. Nat. Cell Biol. 2010, 12, 919–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etemad, B.; Kuijt, T.E.F.; Kops, G.J.P.L. Kinetochore-microtubule attachment is sufficient to satisfy the human spindle assembly checkpoint. Nat. Commun. 2015, 6, 8987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauchman, E.C.; Boehm, F.J.; DeLuca, J.G. Stable kinetochore-microtubule attachment is sufficient to silence the spindle assembly checkpoint in human cells. Nat. Commun. 2015, 6, 10036. [Google Scholar] [CrossRef] [Green Version]
- Remeseiro, S.; Losada, A. Cohesin, a chromatin engagement ring. Curr. Opin. Cell Biol. 2013, 25, 63–71. [Google Scholar] [CrossRef]
- Ishiguro, K. The cohesin complex in mammalian meiosis. Genes to Cells 2019, 24, 6–30. [Google Scholar] [CrossRef] [Green Version]
- Shintomi, K.; Hirano, T. Sister chromatid resolution: A cohesin releasing network and beyond. Chromosoma 2010, 119, 459–467. [Google Scholar] [CrossRef]
- Wassmann, K. Sister chromatid segregation in meiosis II: Deprotection through phosphorylation. Cell Cycle 2013, 12, 1352–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Caballero, C.; Cebollero, L.R.; Pendás, A.M. Shugoshins: From protectors of cohesion to versatile adaptors at the centromere. Trends Genet. 2012, 28, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marston, A.L. Shugoshins: Tension-sensitive pericentromeric adaptors safeguarding chromosome segregation. Mol. Cell. Biol. 2015, 35, 634–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Yakoubi, W.; Buffin, E.; Cladière, D.; Gryaznova, Y.; Berenguer, I.; Touati, S.A.; Gómez, R.; Suja, J.A.; van Deursen, J.M.; Wassmann, K. Mps1 kinase-dependent Sgo2 centromere localisation mediates cohesin protection in mouse oocyte meiosis I. Nat. Commun. 2017, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cetin, B.; Anger, M.; Cho, U.S.; Helmhart, W.; Nasmyth, K.; Xu, W. Structure and function of the PP2A-shugoshin interaction. Mol. Cell 2009, 35, 426–441. [Google Scholar] [CrossRef] [Green Version]
- Rattani, A.; Wolna, M.; Ploquin, M.; Helmhart, W.; Morrone, S.; Mayer, B.; Godwin, J.; Xu, W.; Stemmann, O.; Pendas, A.; et al. Sgol2 provides a regulatory platform that coordinates essential cell cycle processes during meiosis I in oocytes. eLife 2013, 2, e01133. [Google Scholar] [CrossRef] [Green Version]
- Katis, V.L.; Lipp, J.J.; Imre, R.; Bogdanova, A.; Okaz, E.; Habermann, B.; Mechtler, K.; Nasmyth, K.; Zachariae, W. Rec8 phosphorylation by casein kinase 1 and Cdc7-Dbf4 kinase regulates cohesin cleavage by separase during meiosis. Dev. Cell 2010, 18, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Rumpf, C.; Cipak, L.; Dudas, A.; Benko, Z.; Pozgajova, M.; Riedel, C.G.; Ammerer, G.; Mechtler, K.; Gregan, J. Casein kinase 1 is required for efficient removal of Rec8 during meiosis I. Cell Cycle 2010, 9, 2657–2662. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, T.; Tanaka, K.; Sakuno, T.; Watanabe, Y. Shugoshin-PP2A counteracts casein-kinase-1-dependent cleavage of Rec8 by separase. Nat. Cell Biol. 2010, 12, 500–506. [Google Scholar] [CrossRef]
- Rogers, E.; Bishop, J.D.; Waddle, J.A.; Schumacher, J.M.; Lin, R. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J. Cell Biol. 2002, 157, 219–229. [Google Scholar] [CrossRef]
- Kudo, N.R.; Anger, M.; Peters, A.H.F.M.; Stemmann, O.; Theussl, H.-C.; Helmhart, W.; Kudo, H.; Heyting, C.; Nasmyth, K. Role of cleavage by separase of the Rec8 kleisin subunit of cohesin during mammalian meiosis I. J. Cell Sci. 2009, 122, 2686–2698. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kitajima, T.S.; Tanno, Y.; Yoshida, K.; Morita, T.; Miyano, T.; Miyake, M.; Watanabe, Y. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat. Cell Biol. 2008, 10, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.; Valdeolmillos, A.; Parra, M.T.; Viera, A.; Carreiro, C.; Roncal, F.; Rufas, J.S.; Barbero, J.L.; Suja, J.A. Mammalian SGO2 appears at the inner centromere domain and redistributes depending on tension across centromeres during meiosis II and mitosis. EMBO Rep. 2007, 8, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonak, K.; Zagoriy, I.; Oz, T.; Graf, P.; Rojas, J.; Mengoli, V.; Zachariae, W. APC/C-Cdc20 mediates deprotection of centromeric cohesin at meiosis II in yeast. Cell Cycle 2017, 16, 1145–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambon, J.P.; Touati, S.A.; Berneau, S.; Cladière, D.; Hebras, C.; Groeme, R.; McDougall, A.; Wassmann, K. The PP2A inhibitor I2PP2A is essential for sister chromatid segregation in oocyte meiosis II. Curr. Biol. 2013, 23, 485–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, S.T.; Wang, Z.B.; Ouyang, Y.C.; Zhang, Q.H.; Hu, M.W.; Huang, X.; Ge, Z.; Guo, L.; Wang, Y.P.; Hou, Y.; et al. Overexpression of SETbeta, a protein localizing to centromeres, causes precocious separation of chromatids during the first meiosis of mouse oocytes. J. Cell Sci. 2013, 126, 1595–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moshkin, Y.M.; Doyen, C.M.; Kan, T.W.; Chalkley, G.E.; Sap, K.; Bezstarosti, K.; Demmers, J.A.; Ozgur, Z.; van Ijcken, W.F.J.; Verrijzer, C.P. Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A. PLoS Genet. 2013, 9. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Bulger, M.; Kobayashi, R.; Kadonaga, J.T. Drosophila NAP-1 is a core histone chaperone that functions in ATP-facilitated assembly of regularly spaced nucleosomal arrays. Mol. Cell. Biol. 1996, 16, 3112–3124. [Google Scholar] [CrossRef] [Green Version]
- Muto, S.; Senda, M.; Akai, Y.; Sato, L.; Suzuki, T.; Nagai, R.; Senda, T.; Horikoshi, M. Relationship between the structure of SET/TAF-Iβ/INHAT and its histone chaperone activity. Proc. Natl. Acad. Sci. USA 2007, 104, 4285–4290. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Makkinje, A.; Damuni, Z. The Myeloid Leukemia- associated Protein SET Is a Potent Inhibitor of Protein Phosphatase 2A*. J. Biol. Chem. 1996, 271, 11059–11063. [Google Scholar] [CrossRef] [Green Version]
- Hertz, E.P.T.; Kruse, T.; Davey, N.E.; Montoya, G.; Olsen, J.V.; Nilsson, J.; Peter Thrane Hertz, E.; López-Méndez, B.; Otti Sigurðsson, J. A Conserved Motif Provides Binding Specificity to the PP2A-B56 Phosphatase Molecular Cell. Mol. Cell 2016, 63, 686–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cundell, M.J.; Hutter, L.H.; Bastos, R.N.; Poser, E.; Holder, J.; Mohammed, S.; Novak, B.; Barr, F.A. A PP2A-B55 recognition signal controls substrate dephosphorylation kinetics during mitotic exit. J. Cell Biol. 2016, 214, 539–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, Z.; Yu, T.; Yang, H.; Virshup, D.M.; Kops, G.J.P.L.; Lee, S.H.; Zhou, W.; Li, X.; Xu, W.; et al. Crystal structure of a PP2A B56-BubR1 complex and its implications for PP2A substrate recruitment and localization. Protein Cell 2016, 7, 516–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, T.; Biedenkopf, N.; Hertz, E.P.T.; Dietzel, E.; Stalmann, G.; Lopez-Mendez, B.; Davey, N.E.; Nilsson, J.; Becker, S. The Ebola Virus Nucleoprotein Recruits the Host PP2A-B56 Phosphatase to Activate Transcriptional Support Activity of VP30. Mol. Cell 2018, 69, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Karasu, M.E.; Bouftas, N.; Keeney, S.; Wassmann, K. Cyclin B3 promotes anaphase I onset in oocyte meiosis. J. Cell Biol. 2019, 218, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Zhang, L.; He, Z.; Feng, G.; Sun, H.; Wang, J.; Li, Z.; Liu, C.; Han, J.; et al. Cyclin B3 is required for metaphase to anaphase transition in oocyte meiosis I. J. Cell Biol. 2019, 218, 1553–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfrey, M.; Touati, S.A.; Kataria, M.; Jones, A.; Snijders, A.P.; Uhlmann, F. PP2A(Cdc55) Phosphatase Imposes Ordered Cell-Cycle Phosphorylation by Opposing Threonine Phosphorylation. Mol. Cell 2017, 65, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Touati, S.A.; Hofbauer, L.; Jones, A.W.; Snijders, A.P.; Kelly, G.; Uhlmann, F. Cdc14 and PP2A Phosphatases Cooperate to Shape Phosphoproteome Dynamics during Mitotic Exit. Cell Rep. 2019, 29, 2105–2119. [Google Scholar] [CrossRef]
- Baro, B.; Jativa, S.; Calabria, I.; Vinaixa, J.; Bech-Serra, J.-J.; de LaTorre, C.; Rodrigues, J.; Hernaez, M.L.; Gil, C.; Barcelo-Batllori, S.; et al. SILAC-based phosphoproteomics reveals new PP2A-Cdc55-regulated processes in budding yeast. Gigascience 2018, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Hein, J.B.; Hertz, E.P.T.; Garvanska, D.H.; Kruse, T.; Nilsson, J. Distinct kinetics of serine and threonine dephosphorylation are essential for mitosis. Nat. Cell Biol. 2017, 19, 1433–1440. [Google Scholar] [CrossRef]
- Holt, L.J.; Hutti, J.E.; Cantley, L.C.; Morgan, D.O. Evolution of Ime2 phosphorylation sites on Cdk1 substrates provides a mechanism to limit the effects of the phosphatase Cdc14 in meiosis. Mol. Cell 2007, 25, 689–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Guo, J.; Choi, J.W.; Shin, K.T.; Wang, H.Y.; Jo, Y.J.; Kim, N.H.; Cui, X.S. Protein phosphatase 2A regulatory subunit B55α functions in mouse oocyte maturation and early embryonic development. Oncotarget 2017, 8, 26979–26991. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keating, L.; Touati, S.A.; Wassmann, K. A PP2A-B56—Centered View on Metaphase-to-Anaphase Transition in Mouse Oocyte Meiosis I. Cells 2020, 9, 390. https://doi.org/10.3390/cells9020390
Keating L, Touati SA, Wassmann K. A PP2A-B56—Centered View on Metaphase-to-Anaphase Transition in Mouse Oocyte Meiosis I. Cells. 2020; 9(2):390. https://doi.org/10.3390/cells9020390
Chicago/Turabian StyleKeating, Leonor, Sandra A. Touati, and Katja Wassmann. 2020. "A PP2A-B56—Centered View on Metaphase-to-Anaphase Transition in Mouse Oocyte Meiosis I" Cells 9, no. 2: 390. https://doi.org/10.3390/cells9020390
APA StyleKeating, L., Touati, S. A., & Wassmann, K. (2020). A PP2A-B56—Centered View on Metaphase-to-Anaphase Transition in Mouse Oocyte Meiosis I. Cells, 9(2), 390. https://doi.org/10.3390/cells9020390





