Dynamics and Regulations of BimEL Ser65 and Thr112 Phosphorylation in Porcine Granulosa Cells during Follicular Atresia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Classification of Healthy, Slightly Atretic, and Atretic Follicles and Recovery of Granulosa Cells
2.2. Granulosa Cell Culture and Experimental Design
2.3. Construction of BIM DNA Mutant Vectors and Transfection
2.4. Observation of Cell Morphologies in Porcine Granulosa Cells after Transfection with Different BIM Mutant Vectors
2.5. Protein Extraction and Immunoblotting
2.6. Histology
2.7. Apoptosis Assay by Fluorescence-Activated Cell Sorter
2.8. Statistical Analyses
3. Results
3.1. Subsection
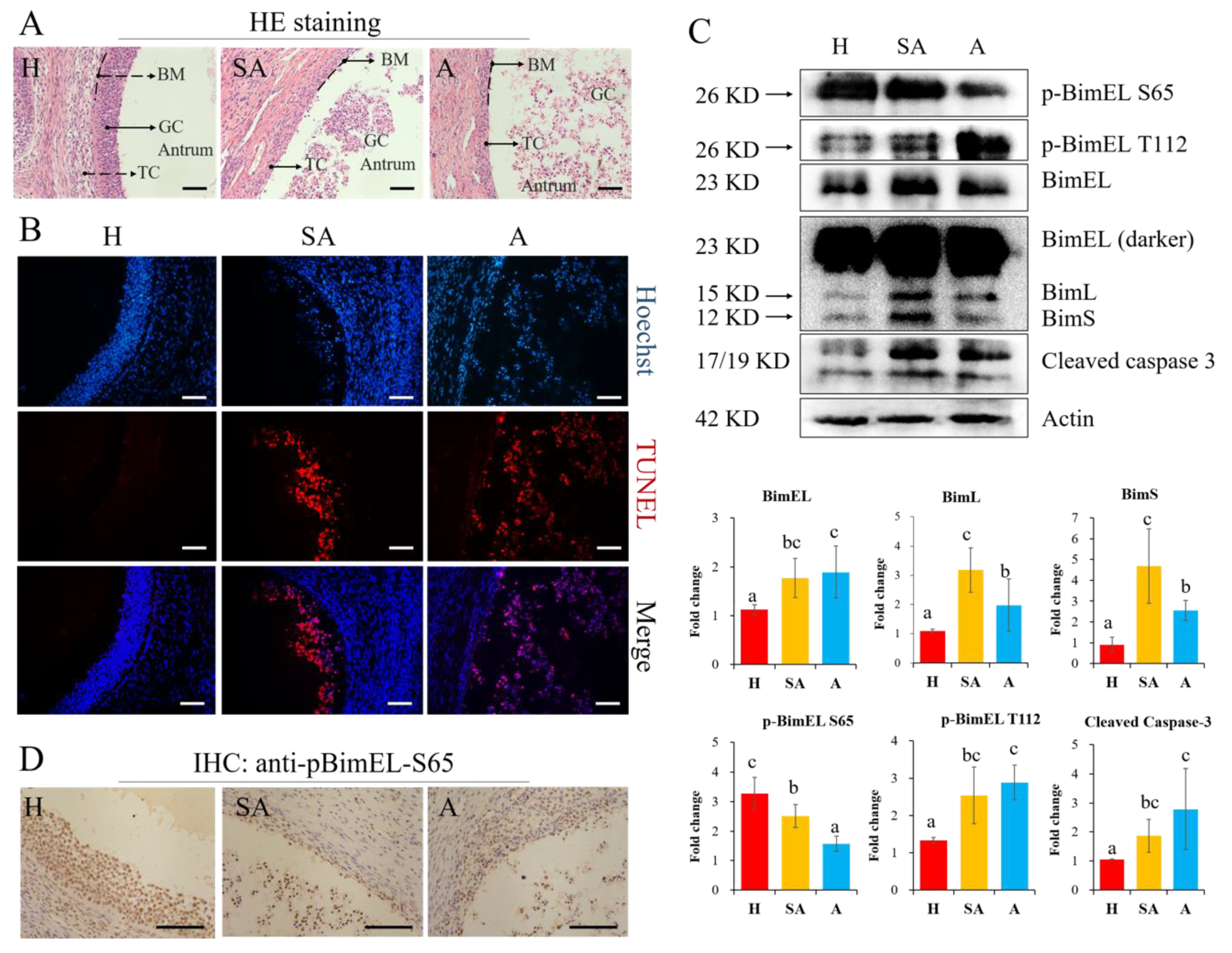
3.1.1. The levels of P-BimEL-S65 were Decreased while P-BimEL-T112 were Increased in Granulosa Cells during Follicular Atresia
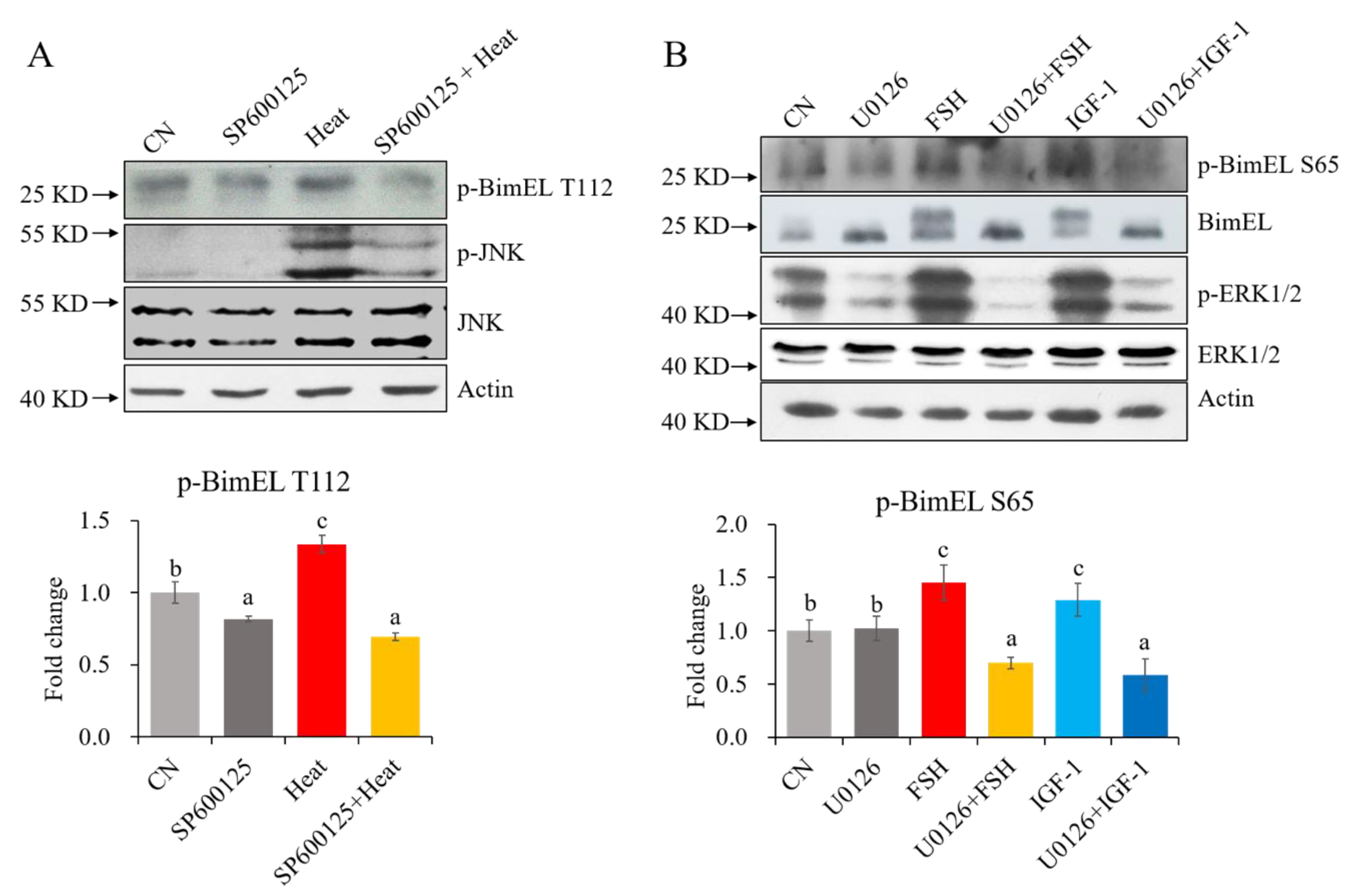
3.1.2. Effects of Proapoptotic and Survival Factors on the Levels of P-BimEL S65 and P-BimEL T112 in Cultured Granulosa Cells
3.1.3. Effects of BimEL Phosphorylation Site Mutation on the Apoptosis of Porcine Granulosa Cells
3.1.4. Regulations of P-BimEL S65 and P-BimEL T112 in Porcine Granulosa Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hughes, F.M., Jr.; Gorospe, W.C. Biochemical identification of apoptosis (programmed cell death) in granulosa cells: Evidence for a potential mechanism underlying follicular atresia. Endocrinology 1991, 129, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Tilly, J.L.; Kowalski, K.I.; Johnson, A.L.; Hsueh, A.J. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 1991, 129, 2799–2801. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Eisenhauer, K.M.; Minami, S.; Billig, H.; Perlas, E.; Hsueh, A.J. Hormonal regulation of apoptosis in early antral follicles: Follicle-stimulating hormone as a major survival factor. Endocrinology 1996, 137, 1447–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsueh, A.J.; Billig, H.; Tsafriri, A. Ovarian follicle atresia: A hormonally controlled apoptotic process. Endocr. Rev. 1994, 15, 707–724. [Google Scholar]
- Guthrie, H.D.; Garrett, W.M. Apoptosis during folliculogenesis in pigs. Reprod. Suppl. 2001, 58, 17. [Google Scholar]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.-A.; Dupont, J.; Fortune, J.E.; Gilchrist, R.B.; Martin, G.B.; McNatty, K.P.; McNeilly, A.S.; et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 2011, 23, 444–467. [Google Scholar] [CrossRef] [Green Version]
- Terenina, E.; Fabre, S.; Bonnet, A.; Monniaux, D.; Robert-Granie, C.; Sancristobal, M.; Sarry, J.; Vignoles, F.; Gondret, F.; Monget, P. Differentially expressed genes and gene networks involved in pig ovarian follicular atresia. Physiol. Genom. 2016, 49, 67–80. [Google Scholar] [CrossRef]
- Cao, R.; Wu, W.J.; Zhou, X.L.; Xiao, P.; Wang, Y.; Liu, H.L. Expression and preliminary functional profiling of the let-7 family during porcine ovary follicle atresia. Mol. Cells 2015, 38, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Strasser, A. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 2005, 5, 189–200. [Google Scholar] [CrossRef]
- Sionov, R.V.; Vlahopoulos, S.A.; Granot, Z. Regulation of Bim in Health and Disease. Oncotarget 2015, 6, 23058–23134. [Google Scholar] [CrossRef] [Green Version]
- Hübner, A.; Barrett, T.; Flavell, R.A.; Davis, R.J. Multisite Phosphorylation Regulates Bim Stability and Apoptotic Activity. Mol. Cell 2008, 30, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewings, K.E.; Kathryn, H.M.; Wiggins, C.M.; Wickenden, J.A.; Kathryn, B.; Rebecca, G.; Kurt, D.; Eileen, W.; Cook, S.J. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 2014, 26, 2856–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zeng, S. Melatonin Promotes Ubiquitination of Phosphorylated Pro-Apoptotic Protein Bcl-2-Interacting Mediator of Cell Death-Extra Long (BimEL) in Porcine Granulosa Cells. Int. J. Mol. Sci. 2018, 19, 3431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luciano, F.; Jacquel, A.; Colosetti, P.; Herrant, M.; Cagnol, S.; Pages, G.; Auberger, P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 2003, 22, 6785–6793. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.; Balmanno, K.; Hadfield, K.; Weston, C.; Cook, S.J. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 2003, 278, 18811–18816. [Google Scholar] [CrossRef] [Green Version]
- Geissler, A.; Haun, F.; Frank, D.O.; Wieland, K.; Simon, M.M.; Idzko, M.; Davis, R.J.; Maurer, U.; Borner, C. Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK. Cell Death Differ. 2013, 20, 1317–1329. [Google Scholar] [CrossRef] [Green Version]
- Beth, L.; Sangita, S.; Guido, K. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy 2008, 4, 600–606. [Google Scholar]
- Ley, R.; Ewings, K.E.; Hadfield, K.; Cook, S.J. Regulatory phosphorylation of Bim: Sorting out the ERK from the JNK. Cell Death Differ. 2005, 12, 1008–1014. [Google Scholar] [CrossRef]
- Bonora, M.; Wieckowsk, M.R.; Chinopoulos, C.; Kepp, O.; Kroemer, G.; Galluzzi, L.; Pinton, P. Molecular mechanisms of cell death: Central implication of ATP synthase in mitochondrial permeability transition. Oncogene 2015, 34, 1475–1486. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Liu, Q.; Zhu, L.J.; Gao, H.; Cui, M.; Liu, J.; Zhao, P.; Liu, J.; Chen, L. Conserved microRNA mediates heating tolerance in germ cells versus surrounding somatic cells. Rna Biol. 2019, 16, 1494–1503. [Google Scholar] [CrossRef]
- Han, Y.; Wang, S.; Wang, Y.; Zeng, S. IGF-1 Inhibits Apoptosis of Porcine Primary Granulosa Cell by Targeting Degradation of BimEL. Int. J. Mol. Sci. 2019, 20, 5356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Wang, S.; Lin, L.; Gao, H.; Wang, Y.; Ji, Z.; Zeng, S. Insulin mitigates apoptosis of porcine follicular granulosa cells by downregulating BimEL. Reprod. Biol. 2019, 19, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wu, Y.; Tan, L.B.; Tian, Z.; Liu, J.H.; Zhu, D.S.; Zeng, S.M. Follicle-stimulating hormone regulates pro-apoptotic protein Bcl-2-interacting mediator of cell death-extra long (BimEL)-induced porcine granulosa cell apoptosis. J. Biol. Chem. 2012, 287, 10166–10177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, P.D.; Smith, P.R.; Heath, D.A.; Hudson, N.L.; Lun, S.; Still, L.A.; Watts, C.H.; Mcnatty, K.P. Morphological evidence of apoptosis and the prevalence of apoptotic versus mitotic cells in the membrana granulosa of ovarian follicles during spontaneous and induced atresia in ewes. Biol. Reprod. 1997, 56, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Gao, H.; Tian, Z.; Wu, Y.; Wang, Y.; Fang, Y.; Lin, L.; Han, Y.; Wu, S.; Haq, I.; et al. Effects of chronic heat stress on granulosa cell apoptosis and follicular atresia in mouse ovary. J. Anim. Sci. Biotechnol. 2016, 7, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Lei, K.; Davis, R.J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 2432–2437. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Zhang, Z.; Xu, Z.; Wang, B.; Li, X.; Cao, H.; Wang, Y.; Zheng, S.J. The association of receptor of activated protein kinase C 1(RACK1) with infectious bursal disease virus viral protein VP5 and voltage-dependent anion channel 2 (VDAC2) inhibits apoptosis and enhances viral replication. J. Biol. Chem. 2015, 290, 8500–8510. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Gong, L.; Arnold, E.; Shanmugam, S.; Fort, P.E.; Gardner, T.W.; Abcouwer, S.F. Insulin-like growth factor 1 rescues R28 retinal neurons from apoptotic death through ERK-mediated BimEL phosphorylation independent of Akt. Exp. Eye Res. 2016, 151, 82–95. [Google Scholar] [CrossRef] [Green Version]
- Tilly, J.L.; Tilly, K.I.; Perez, G.I. The genes of cell death and cellular susceptibility to apoptosis in the ovary: A hypothesis. Cell Death Differ. 1997, 4, 180–187. [Google Scholar] [CrossRef]
- Mann, J.J.; Fraker, P.J. Zinc pyrithione induces apoptosis and increases expression of Bim. Apoptosis 2005, 10, 369–379. [Google Scholar] [CrossRef]
- Akiyama, T.; Dass, C.R.; Choong, P.F.M. Bim-targeted cancer therapy: A link between drug action and underlying molecular changes. Mol. Cancer Ther. 2009, 8, 3173–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zuo, Z.; Lu, X.; Wang, L.; Wang, H.; Zhu, Z. MiR-25 regulates apoptosis by targeting Bim in human ovarian cancer. Oncol. Rep. 2012, 27, 594–598. [Google Scholar] [CrossRef]
- Shinjyo, T.; Kuribara, R.; Inukai, T.; Hosoi, H.; Kinoshita, T.; Miyajima, A.; Houghton, P.J.; Look, A.T.; Ozawa, K.; Inaba, T. Downregulation of Bim, a proapoptotic relative of Bcl-2, is a pivotal step in cytokine-initiated survival signaling in murine hematopoietic progenitors. Mol. Cell. Biol. 2001, 21, 854–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elke, D.B.; Bos, T.J.; Frans, S.; Els, V.V.; Eline, M.; Lieven, T.; Peter, A.; Helena, J.W.; Karin, V. IGF-1 suppresses Bim expression in multiple myeloma via epigenetic and posttranslational mechanisms. Blood 2010, 115, 2430–2440. [Google Scholar]
- Hansen, P.J. Effect of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. 2009, 364, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Tabayashi, D.; Latief, T.A.; Shimizu, T.; Oshima, I.; Kanai, Y. Alterations in follicular dynamics and steroidogenic abilities induced by heat stress during follicular recruitment in goats. Reproduction 2005, 129, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ohshima, I.; Ozawa, M.; Takahashi, S.; Tajima, A.; Shiota, M.; Miyazaki, H.; Kanai, Y. Heat stress diminishes gonadotropin receptor expression and enhances susceptibility to apoptosis of rat granulosa cells. Reproduction 2005, 129, 463–472. [Google Scholar] [CrossRef]
- Yao, F.; Chang-Jiu, H.; Peng-Yun, J.; Zhi-Yong, Z.; Xiu-Zhi, T.; Feng, W.; Dun-Xian, T.; Guo-Shi, L. Effects of melatonin on the proliferation and apoptosis of sheep granulosa cells under thermal stress. Int. J. Mol. Sci. 2014, 15, 21090. [Google Scholar]
- Mustafi, S.B.; Chakraborty, P.K.; Dey, R.S.; Raha, S. Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones 2009, 14, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.S.; Fan, Y.P.; Wu, Y.; Hao, Z.D.; Liu, S.; Chen, X.J.; Zeng, S.M. Porcine cumulus cell influences ooplasmic mitochondria-lipid distributions, GSH-ATP contents and calcium release pattern after electro-activation. Theriogenology 2009, 71, 412–421. [Google Scholar] [CrossRef]
- Lu, X.; Kambe, F.; Cao, X.; Yamauchi, M.; Seo, H. Insulin-like growth factor-I activation of Akt survival cascade in neuronal cells requires the presence of its cognate receptor in caveolae. Exp. Cell Res. 2008, 314, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Quearry, B.; Ruizvela, A.; Korsmeyer, S.J. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc. Natl. Acad. Sci. USA 2004, 101, 15313–15317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, S.N.; Fletcher, J.I.; Kaufmann, T.; Delft, M.F.V.; Huang, D.C.S. Apoptosis Initiated When BH3 Ligands Engage Multiple Bcl-2 Homologs, Not Bax or Bak. Science 2007, 315, 856–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustafa-Kamal, M.; Gamache, I.; Lu, Y.; Li, S.; Teodoro, J.G. BimEL is phosphorylated at mitosis by Aurora A and targeted for degradation by betaTrCP1. Cell Death Differ. 2013, 20, 1393–1403. [Google Scholar] [CrossRef] [PubMed]




| Name | Primer Sequence | |
|---|---|---|
| BimEL | Forward | gaattcatggcaaagcaaccttccgatg |
| Reverse | ggatccgcaatgtaagggggagggagggt | |
| BimEL-S65A | Forward | ccactggccccaccgaccGCCcctggcccctttgctacc |
| Reverse | GGCggtcggtggggccagtgggccctgggggctgccttg | |
| BimEL-T112A | Forward | aaatcaacacaaGCCccaagtcctccttgccaagcc |
| Reverse | acttggGGCttgtgttgatttgtcacaactcatggg | |
| BimEL-S55A65A73A | S55A-Forward | gaccgctgcccccaaggcGCCccccagggcccactg |
| S55A-Reverse | GGCgccttgggggcagcggtccccttctccttccgg | |
| S73A-Forward | tggcccctttgctaccagaGCCccgcttttcatcttcgtg | |
| S73A-Reverse | GGCtctggtagcaaaggggccaggggcggtcggtgg | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Chen, Y.; Liu, Q.; Dai, S.; Zeng, S. Dynamics and Regulations of BimEL Ser65 and Thr112 Phosphorylation in Porcine Granulosa Cells during Follicular Atresia. Cells 2020, 9, 402. https://doi.org/10.3390/cells9020402
Yang F, Chen Y, Liu Q, Dai S, Zeng S. Dynamics and Regulations of BimEL Ser65 and Thr112 Phosphorylation in Porcine Granulosa Cells during Follicular Atresia. Cells. 2020; 9(2):402. https://doi.org/10.3390/cells9020402
Chicago/Turabian StyleYang, Feng, Yanhong Chen, Qiang Liu, Shizhen Dai, and Shenming Zeng. 2020. "Dynamics and Regulations of BimEL Ser65 and Thr112 Phosphorylation in Porcine Granulosa Cells during Follicular Atresia" Cells 9, no. 2: 402. https://doi.org/10.3390/cells9020402
APA StyleYang, F., Chen, Y., Liu, Q., Dai, S., & Zeng, S. (2020). Dynamics and Regulations of BimEL Ser65 and Thr112 Phosphorylation in Porcine Granulosa Cells during Follicular Atresia. Cells, 9(2), 402. https://doi.org/10.3390/cells9020402





