Increase in Leptin and PPAR-γ Gene Expression in Lipedema Adipocytes Differentiated in vitro from Adipose-Derived Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. ASCs Isolation and Cell Culture
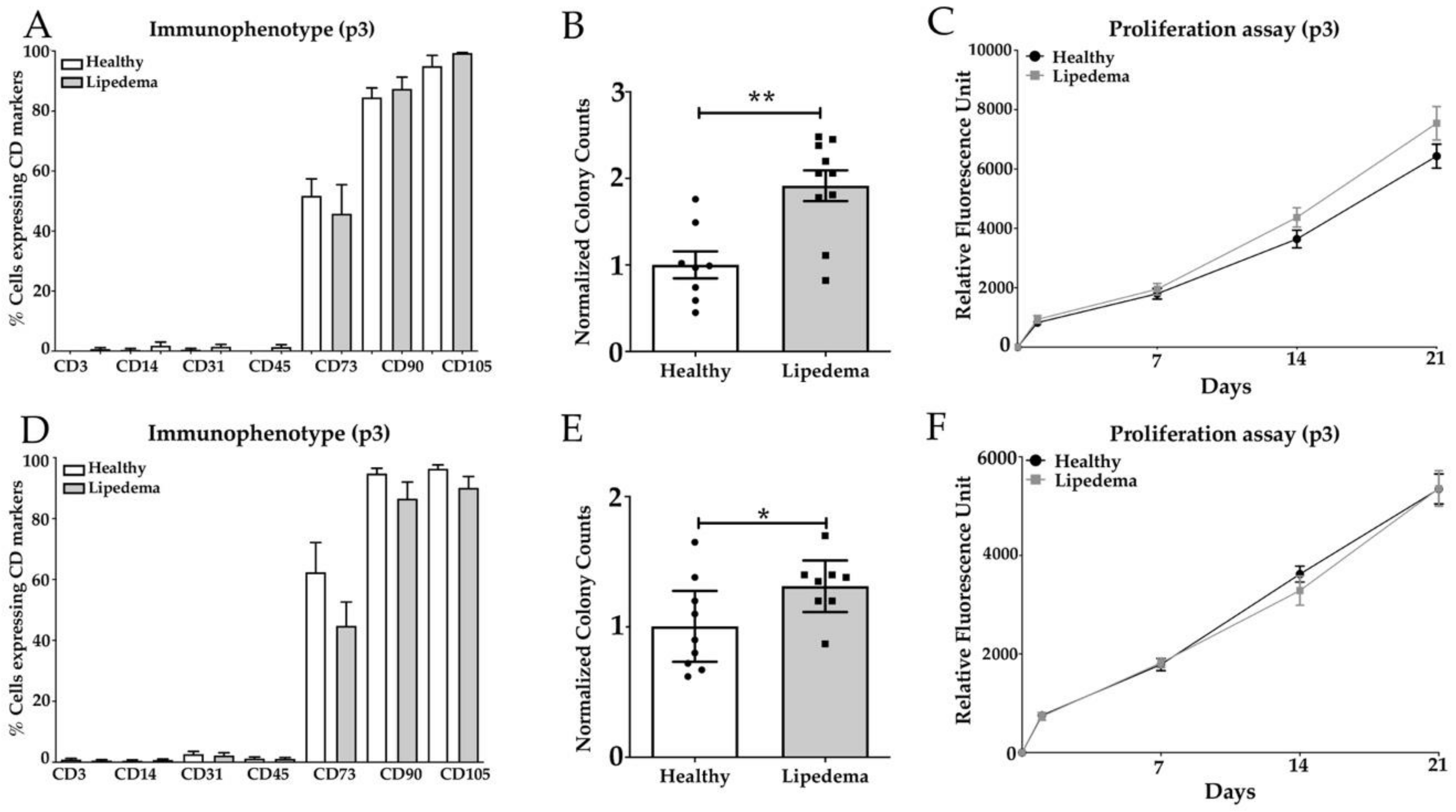
2.3. Alamar Blue Cell Proliferation Assay
2.4. Colony-Forming Unit (CFU) Fibroblast Assay
2.5. Flow Cytometry
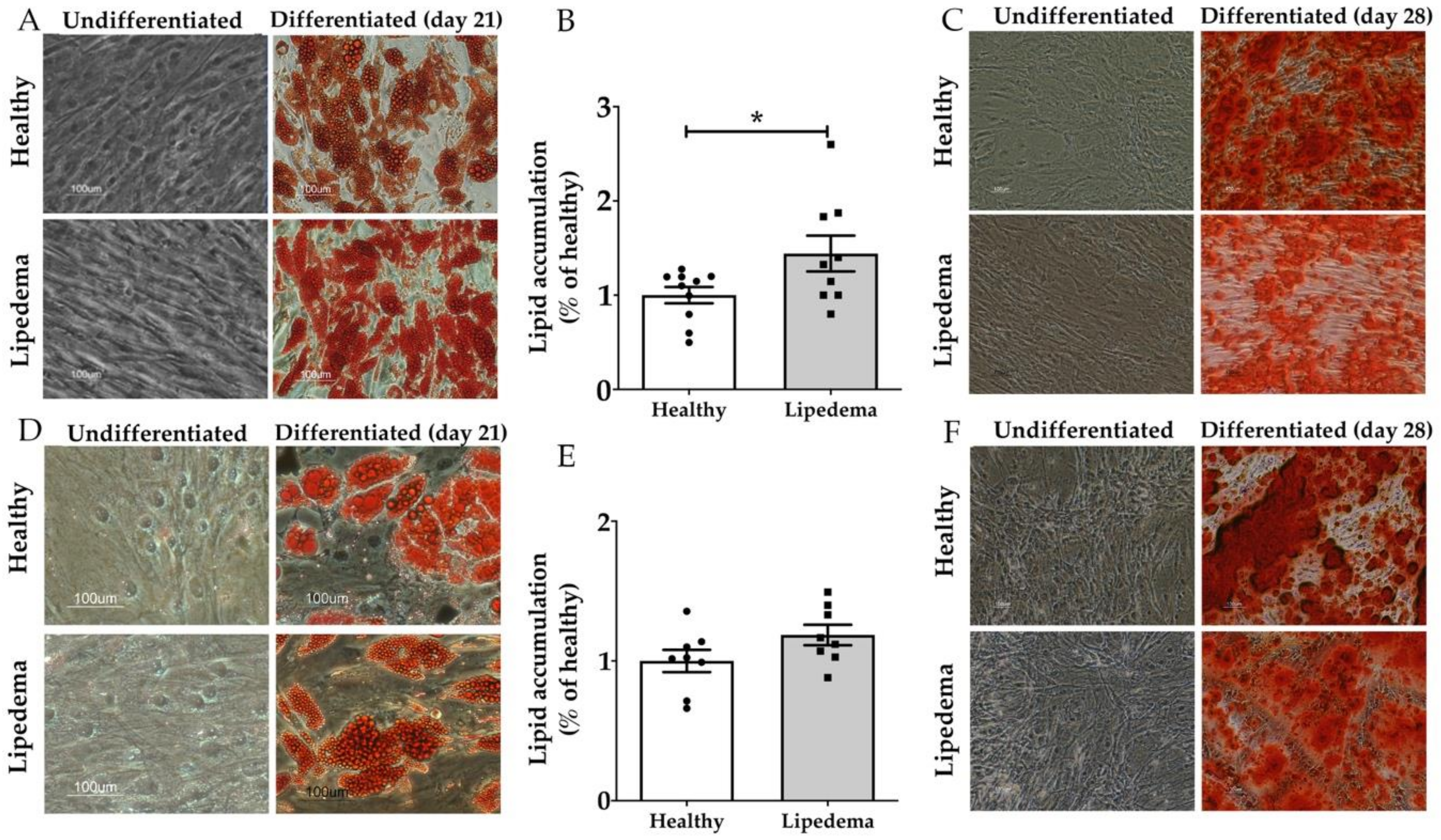
2.6. Adipogenic Differentiation and Oil Red O Staining
2.7. Osteogenic Differentiation and Alizarin Red Staining
2.8. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Lipedema ASCs Isolated from SVF-T and SVF-A Showed a Significant Increase in CFU with no Change in Stemness Makers or Proliferation Rate Compared to Healthy ASCs
3.2. Lipedema ASCs Showed Increased Adipogenic but not Osteogenic Differentiation Potential as Compared to Healthy ASCs
3.3. Significant Increase in Leptin and PPAR-γ Gene Expression in Lipedema Differentiated Adipocytes Compared to Healthy Adipocytes
3.4. Inflammatory Genes Expression Was Similar for Lipedema ASCs and Adipocytes Compared to Healthy Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buso, G.; Depairon, M.; Tomson, D.; Raffoul, W.; Vettor, R.; Mazzolai, L. Lipedema: A Call to Action! Obesity 2019, 27, 1567–1576. [Google Scholar] [CrossRef]
- Herbst, K.L.; Mirkovskaya, L.; Bharhagava, A.; Chava, Y.; Te, C.H.T. Lipedema fat and signs and symptoms of illness, increase with advancing stage. Arch. Med. 2015, 7, 1–8. [Google Scholar]
- Herbst, K.L. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol. Sin. 2012, 33, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Wold, L.E.; Hines, E.A.; Allen, E.V. Lipedema of the legs: A syndrome characterized by fat legs and edema. Ann. Intern. Med. 1951, 34, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Herbst, K.L.; Bunnell, B.A. Lipedema: A Painful Adipose Tissue Disorder. In Adipose Tissue—An Update; IntechOpen: London, UK, 2019. [Google Scholar]
- Shavit, E.; Wollina, U.; Alavi, A. Lipoedema is not lymphoedema: A review of current literature. Int. Wound J. 2018, 15, 921–928. [Google Scholar] [CrossRef]
- Buck, D.W.; Herbst, K.L. Lipedema: A relatively common disease with extremely common misconceptions. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1043. [Google Scholar] [CrossRef]
- Fife, E.C.; Maus, A.E.; Carter, J.M. Lipedema: A frequently misdiagnosed and misunderstood fatty deposition syndrome. Adv. Skin Wound Care 2010, 23, 81–92. [Google Scholar] [CrossRef]
- Beltran, K.; Herbst, K.L. Differentiating lipedema and dercum’s disease. Int. J. Obes. 2017, 41, 240–245. [Google Scholar] [CrossRef]
- Crescenzi, R.; Donahue, P.M.; Weakley, S.; Garza, M.; Donahue, M.J.; Herbst, K.L. Lipedema and Dercum’s Disease: A New Application of Bioimpedance. Lymphat. Res. Boil. 2019, 17, 671–679. [Google Scholar] [CrossRef]
- Pascucci, A.; Lynch, P.J. Lipedema with multiple lipomas. Dermatol. Online J. 2010, 16, 4. [Google Scholar] [PubMed]
- La Torre, Y.S.-D.; Wadeea, R.; Rosas, V.; Herbst, K.L. Lipedema: Friend and foe. Horm. Mol. Boil. Clin. Investig. 2018, 33. [Google Scholar] [CrossRef]
- Langendoen, S.; Habbema, L.; Nijsten, T.; Neumann, H. Lipoedema: From clinical presentation to therapy. A review of the literature. Br. J. Dermatol. 2009, 161, 980–986. [Google Scholar] [CrossRef]
- Shin, B.W.; Sim, Y.-J.; Jeong, H.J.; Kim, G.C. Lipedema, a Rare Disease. Ann. Rehabilitation Med. 2011, 35, 922–927. [Google Scholar] [CrossRef]
- Dudek, J.E.; Białaszek, W.; Ostaszewski, P.; Smidt, T. Depression and appearance-related distress in functioning with lipedema. Psychol. Heal. Med. 2018, 23, 846–853. [Google Scholar] [CrossRef]
- Szél, E.; Kemény, L.; Groma, G.; Szolnoky, G. Pathophysiological dilemmas of lipedema. Med. Hypotheses 2014, 83, 599–606. [Google Scholar] [CrossRef]
- Alwardat, N.; Di Renzo, L.; Alwardat, M.; Romano, L.; De Santis, G.L.; Gualtieri, P.; Carrano, E.; Nocerino, P.; De Lorenzo, A. The effect of lipedema on health-related quality of life and psychological status: A narrative review of the literature. Eat. Weight. Disord. Stud. Anorexia Bulim. Obes. 2019, 1–6. [Google Scholar] [CrossRef]
- Schmeller, W.; Hueppe, M.; Meier-Vollrath, I. Tumescent liposuction in lipoedema yields good long-term results. Brit. J. Derm. 2012, 166, 161–168. [Google Scholar] [CrossRef]
- Dadras, M.; Mallinger, P.J.; Corterier, C.C.; Theodosiadi, S.; Ghods, M. Liposuction in the Treatment of Lipedema: A Longitudinal Study. Arch. Plast. Surg. 2017, 44, 324–331. [Google Scholar] [CrossRef]
- Baum, S.; Kaak, I.; Kottmann, T.; Podda, M.; Rapprich, S. Treatment of lipoedema using liposuction: Results of our own surveys. Phlebologie 2015, 44, 121–132. [Google Scholar] [CrossRef]
- Peled, A.W.; Kappos, E.A. Lipedema: Diagnostic and management challenges. Int. J. Women’s Heal. 2016, 8, 389–395. [Google Scholar] [CrossRef]
- Herbst, K.L.; Ussery, C.; Eekema, A. Pilot study: Whole body manual subcutaneous adipose tissue (SAT) therapy improved pain and SAT structure in women with lipedema. Horm. Mol. Boil. Clin. Investig. 2017, 33. [Google Scholar] [CrossRef]
- Szolnoky, G.; Borsos, B.; Bársony, K.; Balogh, M.; Kemény, L. Complete decongestive physiotherapy with and without pneumatic compression for treatment of lipedema: A pilot study. Lymphology 2008, 41, 40–41. [Google Scholar]
- Siems, W.; Grune, T.; Voss, P.; Brenke, R. Anti-fibrosclerotic effects of shock wave therapy in lipedema and cellulite. BioFactors 2005, 24, 275–282. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Cromer, W.; Allen, M.; Ussery, C.; Badowski, M.; Harris, D.; Herbst, K.L. Dilated Blood and Lymphatic Microvessels, Angiogenesis, Increased Macrophages and Adipocyte Hypertrophy in Lipedema Thigh Skin and Fat Tissue. J. Obes. 2019, 2019. [Google Scholar] [CrossRef]
- Child, A.H.; Gordon, K.D.; Sharpe, P.; Brice, G.; Ostergaard, P.; Jeffery, S.; Mortimer, P.S. Lipedema: An inherited condition. Am. J. Med Genet. Part A 2010, 152, 970–976. [Google Scholar] [CrossRef]
- Precone, V.; Barati, S.; Paolacci, S.; Salgarello, M.; Visconti, G.; Gentileschi, S.; Guerri, G.; Gagliardi, L.; Aquilanti, B.; Matera, G.; et al. Genetic syndromes with localized subcutaneous fat tissue accumulation. Acta Bio-Med. Atenei Parmensis 2019, 90, 90–92. [Google Scholar] [CrossRef]
- Suga, H.; Araki, J.; Aoi, N.; Kato, H.; Higashino, T.; Yoshimura, K. Adipose tissue remodeling in lipedema: Adipocyte death and concurrent regeneration. J. Cutan. Pathol. 2009, 36, 1293–1298. [Google Scholar] [CrossRef]
- Priglinger, E.; Wurzer, C.; Steffenhagen, C.; Maier, J.; Hofer, V.; Peterbauer, A.; Nuernberger, S.; Redl, H.; Wolbank, S.; Sandhofer, M. The adipose tissue–derived stromal vascular fraction cells from lipedema patients: Are they different? Cytotherapy 2017, 19, 849–860. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Vishvanath, L.; Gupta, R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef]
- White, U.; Ravussin, E. Dynamics of adipose tissue turnover in human metabolic health and disease. Diabetologia 2019, 62, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Cubedo, J. Adipose tissue depots and inflammation: Effects on plasticity and resident mesenchymal stem cell function. Cardiovasc. Res. 2017, 113, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Louwen, F.; Ritter, A.; Kreis, N.N.; Yuan, J. Insight into the development of obesity: Functional alterations of adipose-derived mesenchymal stem cells. Obes. Rev. 2018, 19, 888–904. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C.; Geiger, H. Adipose-Derived Mesenchymal Stromal/Stem Cells: Tissue Localization, Characterization and Heterogeneity. Stem Cells Int. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Guo, J.; Nguyen, A.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 180–188. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Bauer, A.-T.; Von Lukowicz, D.; Lossagk, K.; Hopfner, U.; Kirsch, M.; Moog, P.; Bauer, H.; Machens, H.-G.; Schmauss, D. Adipose Stem Cells from Lipedema and Control Adipose Tissue Respond Differently to Adipogenic Stimulation In Vitro. Plast. Reconstr. Surg. 2019, 144, 623–632. [Google Scholar] [CrossRef]
- Patrikoski, M.; Mannerström, B.; Miettinen, S. Perspectives for Clinical Translation of Adipose Stromal/Stem Cells. Stem Cells Int. 2019, 2019, 1–21. [Google Scholar] [CrossRef]
- Cignarelli, A.; Perrini, S.; Ficarella, R.; Peschechera, A.; Nigro, P.; Giorgino, F. Human adipose tissue stem cells: Relevance in the pathophysiology of obesity and metabolic diseases and therapeutic applications. Expert Rev. Mol. Med. 2012, 14, e19. [Google Scholar] [CrossRef]
- Levi, B.; James, A.; Glotzbach, J.P.; Wan, D.C.; Commons, G.; Longaker, M. Depot-specific variation in the osteogenic and adipogenic potential of human adipose-derived stromal cells. Plast. Reconstr. Surg. 2010, 126, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Unger, M.; Van Griensven, M.; Balmayor, E.R. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur. J. Med Res. 2017, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Konno, M.; Hamabe, A.; Hasegawa, S.; Ogawa, H.; Fukusumi, T.; Nishikawa, S.; Ohta, K.; Kano, Y.; Ozaki, M.; Noguchi, Y.; et al. Adipose-derived mesenchymal stem cells and regenerative medicine. Dev. Growth Differ. 2013, 55, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zimmerlin, L.; Marra, K.G.; Donnenberg, V.S.; Donnenberg, A.D.; Rubin, J.P. Adipogenic potential of adipose stem cell subpopulations. Plast. Reconstr. Surg. 2011, 128, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Crosstalk between PPARγ and insulin signaling and modulation of insulin sensitivity (report). PPAR Res. 2009, 2009. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Strong, A.L.; Ohlstein, J.F.; Biagas, B.A.; Rhodes, L.V.; Pei, D.T.; Tucker, H.A.; Llamas, C.; Bowles, A.C.; DuTreil, M.F.; Zhang, S.; et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015, 17, 112. [Google Scholar] [CrossRef]
- Scheller, E.L.; Song, J.; Dishowitz, M.I.; Soki, F.N.; Hankenson, K.D.; Krebsbach, P.H. Leptin Functions Peripherally to Regulate Differentiation of Mesenchymal Progenitor Cells. Stem Cells 2010, 28, 1071–1080. [Google Scholar] [CrossRef]
- Taylor, N.E.; Foster, W.C.; Wick, M.R.; Patterson, J.W. Tumefactive lipedema with pseudoxanthoma elasticum-like microscopic changes. J. Cutan. Pathol. 2004, 31, 205–209. [Google Scholar] [CrossRef]


| Characteristics | Healthy | Lipedema |
|---|---|---|
| Number | 20 | 20 |
| Sex (M/F) | Female | Female |
| Age (years) | 46.9 ± 2.2 | 44.1 ± 2.2 |
| BMI (kg/m2) | 28.5 ± 0.86 | 29.9 ± 0.97 |
| Stage 1 | − | 15% |
| Stage 2 | 70% | |
| Stage 2–3 | − | 5% |
| Stage 3 | − | 10% |
| Name | Forward | Reverse |
|---|---|---|
| IL-6 | 5′-GTAGCCGCCCCACACAGACAGCC-3′ | 5′-GCCATCTTTGGAAGGTTC-3′ |
| IL-1β | 5′-TCCCCAGCCCTTTTGTTGA-3′ | 5′-TTAGAACCAAATGTGGCCGTG-3′ |
| TNF-α | 5′-TCTTCTCGAACCCCGAGTGA-3′ | 5′-CCTCTGATGGCACCACCAG-3′ |
| VEGF | 5′-CCTTGCTGCTCTACCTCCAC-3′ | 5′-CACACAGGATGGCTTGAAGA-3′ |
| Glut4 | 5′-AGC AGC TCT CTG GCA TCA AT-3′ | 5′-CAA TGG AGA CGT AGC ACA TG-3′ |
| CD36 | 5′-GAGACCTGCTTATCCAGAAGACAAT-3′ | 5′-TTCTGTGCCTGTTTTAACCCAATTTTT-3′ |
| LPL | 5′-GAGATTTCTCTGTATGGCACTG-3′ | 5′-CTGCAAATGAGACACTTTCTC-3′ |
| Leptin | 5′-GAAGACCACATCCACACACG-3′ | 5′-AGCTCAGCCAGACCCATCTA-3′ |
| PPAR-γ | 5′-AGGCGAGGGCGATCTTG-3′ | 5′-CCCATCATTAAGGAATTCATGTCATA-3′ |
| ADIPOQ | 5′-AACATGCCCATTCGCTTTAC-3′ | 5′-AGAGGCTGACCTTCACATCC-3′ |
| GAPDH | 5′-CGCTGAGTACGTCGTGGAGTC-3′ | 5′-GCAGGAGGCATTGCAGATGA-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ghadban, S.; Diaz, Z.T.; Singer, H.J.; Mert, K.B.; Bunnell, B.A. Increase in Leptin and PPAR-γ Gene Expression in Lipedema Adipocytes Differentiated in vitro from Adipose-Derived Stem Cells. Cells 2020, 9, 430. https://doi.org/10.3390/cells9020430
Al-Ghadban S, Diaz ZT, Singer HJ, Mert KB, Bunnell BA. Increase in Leptin and PPAR-γ Gene Expression in Lipedema Adipocytes Differentiated in vitro from Adipose-Derived Stem Cells. Cells. 2020; 9(2):430. https://doi.org/10.3390/cells9020430
Chicago/Turabian StyleAl-Ghadban, Sara, Zaidmara T. Diaz, Hallie J. Singer, Karya B. Mert, and Bruce A. Bunnell. 2020. "Increase in Leptin and PPAR-γ Gene Expression in Lipedema Adipocytes Differentiated in vitro from Adipose-Derived Stem Cells" Cells 9, no. 2: 430. https://doi.org/10.3390/cells9020430
APA StyleAl-Ghadban, S., Diaz, Z. T., Singer, H. J., Mert, K. B., & Bunnell, B. A. (2020). Increase in Leptin and PPAR-γ Gene Expression in Lipedema Adipocytes Differentiated in vitro from Adipose-Derived Stem Cells. Cells, 9(2), 430. https://doi.org/10.3390/cells9020430







