The Phenotype and Functional Activity of Mesenchymal Stromal Cells in Pediatric Patients with Non-Malignant Hematological Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Isolation of Bone Marrow Mononuclear Cells (BM-MNCs)
2.3. Generation of Mesenchymal Stromal Cells (MSCs)
2.4. Estimation of the Frequency of Progenitor Cells for MSCs Using Colony Forming Units-Fibroblast (CFU-F) Assay
2.5. Immunophenotyping of Mesenchymal Stromal Cells (MSC)
2.6. Differentiation Potential of MSCs of Patients with Non-Malignant Hematological Diseases (NMHD)
2.6.1. Differentiation into Osteoblasts
2.6.2. Differentiation into Adipocytes
2.6.3. Differentiation into Chondrocytes
2.7. Mixed Lymphocyte Reaction (MLR)
2.8. Data Analysis
3. Results
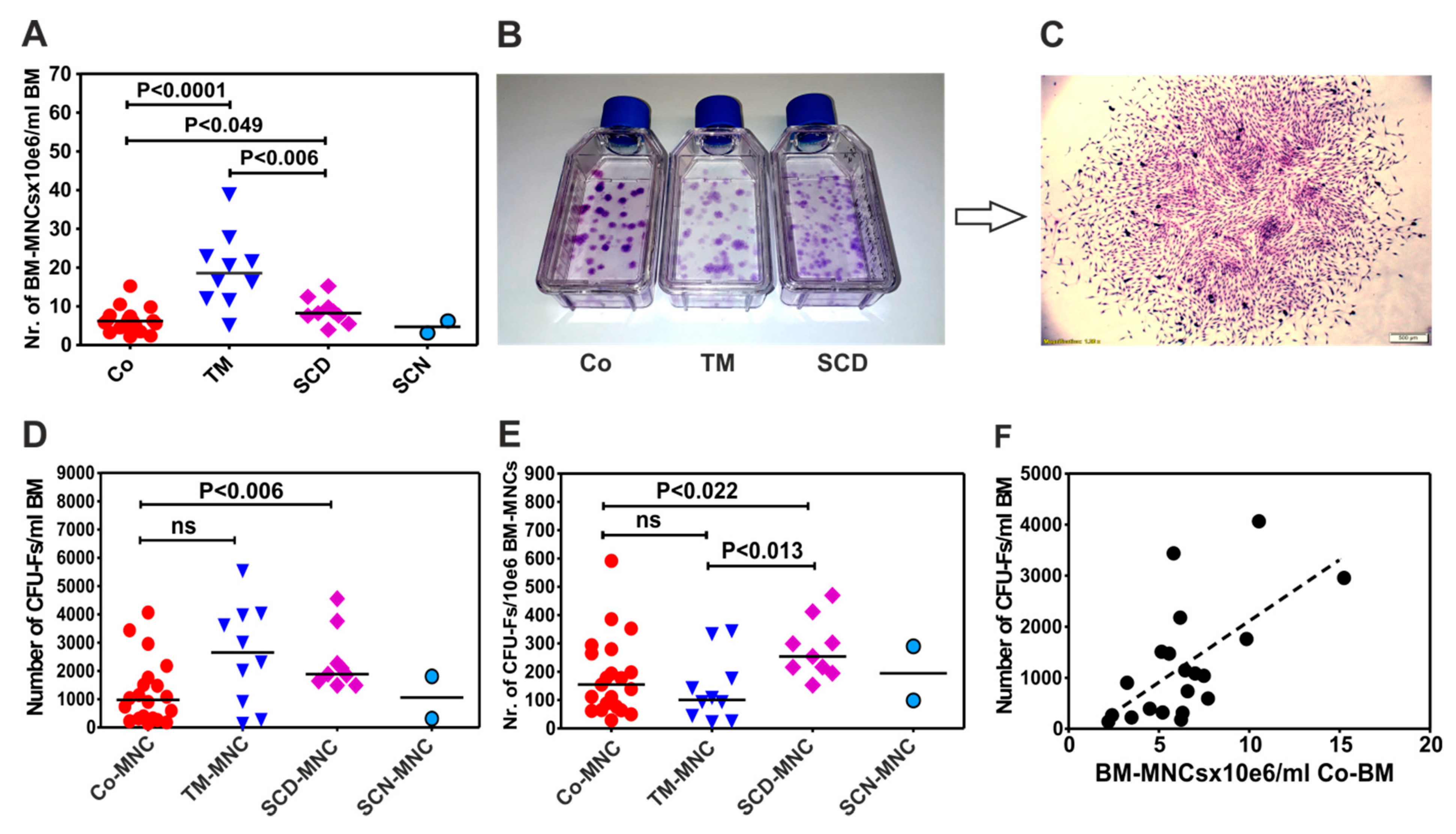
3.1. Patients with Thalassemia Contain more BM-MNCs per Milliliter Bone Marrow than Patients with Sickle Cell Disease (SCD) and Control Group
3.2. BM-MNCs of Patients with SCD Generate More CFU-Fs per 1×106 BM-MNCs Compared to Patients with Thalassemia or Healthy Donors
3.3. BM-MNCs of Patients with NMHD Demonstrate a Normal Generation and Proliferation Potential of Mesenchymal Stromal Cells (MSCs)
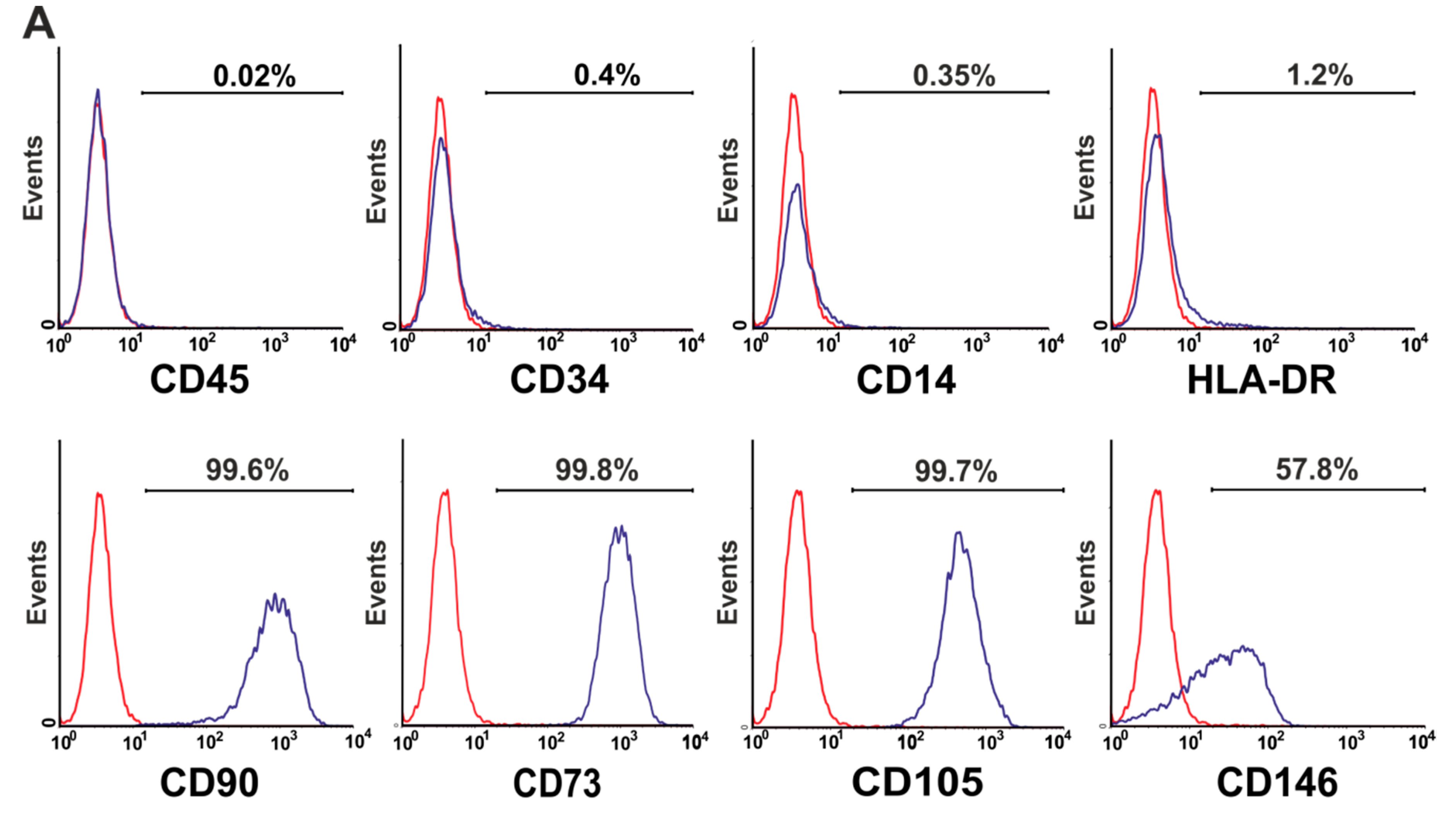
3.4. Phenotype of Mesenchymal Stromal Cells (MSCs) Generated by BM-MNCs of Patients with Non-Malignant Hematological Diseases (NMHD)
3.5. Trilineage Differentiation Potential of Mesenchymal Stromal Cells (MSCs) of Patients with Non-Malignant Hematological Diseases (NMHD)
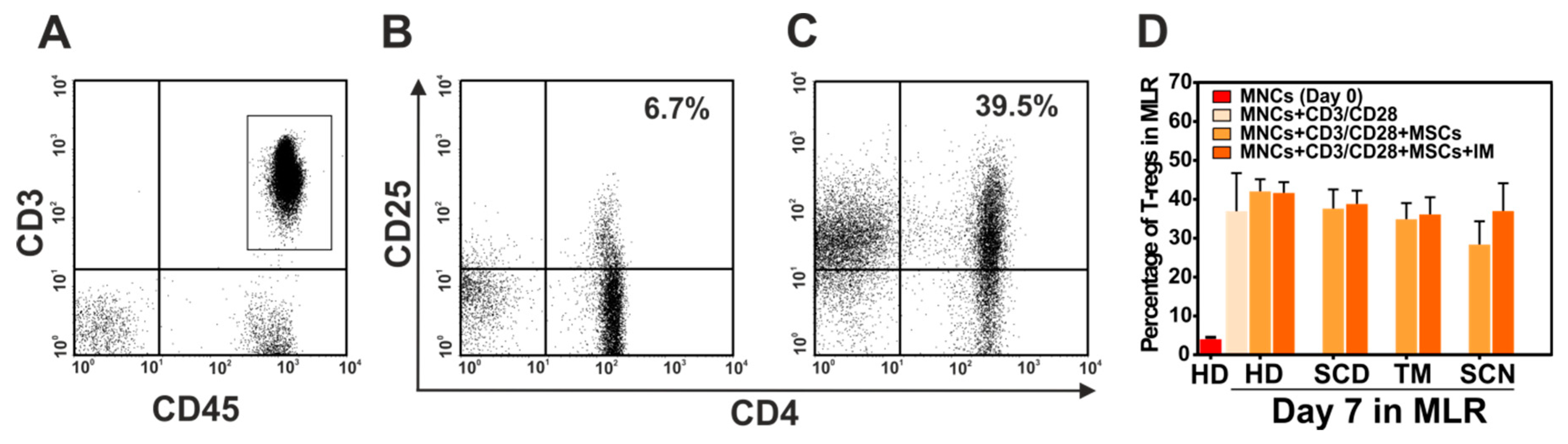
3.6. Immunosuppressive Effect of Mesenchymal Stromal Cells (MSCs) of Patients with Non-Malignant Hematological Diseases (NMHD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Reilly, R.J. Allogenic Bone Marrow Transplantation: Current Status and Future Directions. Blood 1983, 62, 941–964. [Google Scholar] [CrossRef] [Green Version]
- Gluckman, E.; Cappelli, B.; Bernaudin, F.; Labopin, M.; Volt, F.; Carreras, J.; Pinto, S.B.; Ferster, A.; Dupont, S.; de la Fuente, J.; et al. Sickle Cell Disease: An International Survey of Results of HLA-Identical Sibling Hematopoietic Stem Cell Transplantation. Blood 2017, 129, 1548–1556. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, A.; Eapen, M.; Scaravadou, A.; Cairo, M.S.; Bhatia, M.; Kurtzberg, J.; Wingard, J.R.; Fasth, A.; Lo, N.L.; Ayas, M.; et al. Umbilical Cord Blood Transplantation for Children With Thalassemia and Sickle Cell Disease. Biol. Blood Marrow Transplant. 2011, 17, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, H.K.; Elhaddad, A.M.; Fahmy, O.A.; Samra, M.A.; Abdelfattah, R.M.; El-Nahass, Y.H.; Fathy, G.M.; Abdelhady, M.S. Allogeneic Hematopoietic Stem Cell Transplantation for Non-Malignant Hematological Disorders. J. Adv. Res. 2015, 6, 449–458. [Google Scholar] [CrossRef]
- Fioredda, F.; Iacobelli, S.; van Biezen, A.; Gaspar, B.; Ancliff, P.; Donadieu, J.; Aljurf, M.; Peters, C.; Calvillo, M.; Matthes-Martin, S.; et al. Stem Cell Transplantation in Severe Congenital Neutropenia: An Analysis From the European Society for Blood and Marrow Transplantation. Blood 2015, 126, 1885–1892. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and Hematopoietic Stem Cells Form a Unique Bone Marrow Niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Haynesworth, S.E.; Baber, M.A.; Caplan, A.I. Cytokine Expression by Human Marrow-Derived Mesenchymal Progenitor Cells in Vitro: Effects of Dexamethasone and IL-1 Alpha. J. Cell Physiol. 1996, 166, 585–592. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Thiede, M.A.; Mosca, J.D.; Moorman, M.; Gerson, S.L. Phenotypic and Functional Comparison of Cultures of Marrow-Derived Mesenchymal Stem Cells (MSCs) and Stromal Cells. J. Cell Physiol. 1998, 176, 57–66. [Google Scholar] [CrossRef]
- Koc, O.N.; Gerson, S.L.; Cooper, B.W.; Dyhouse, S.M.; Haynesworth, S.E.; Caplan, A.I.; Lazarus, H.M. Rapid Hematopoietic Recovery After Coinfusion of Autologous-Blood Stem Cells and Culture-Expanded Marrow Mesenchymal Stem Cells in Advanced Breast Cancer Patients Receiving High-Dose Chemotherapy. J. Clin. Oncol. 2000, 18, 307–316. [Google Scholar] [CrossRef]
- Lazarus, H.M.; Koc, O.N.; Devine, S.M.; Curtin, P.; Maziarz, R.T.; Holland, H.K.; Shpall, E.J.; McCarthy, P.; Atkinson, K.; Cooper, B.W.; et al. Cotransplantation of HLA-Identical Sibling Culture-Expanded Mesenchymal Stem Cells and Hematopoietic Stem Cells in Hematologic Malignancy Patients. Biol. Blood Marrow Transplant. 2005, 11, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, Y.; Xiao, H.; Yao, Z.; Zhang, H.; Liu, Q.; Wu, B.; Nie, D.; Li, Y.; Pang, Y.; et al. Cotransplantation of Bone Marrow-Derived Mesenchymal Stem Cells in Haploidentical Hematopoietic Stem Cell Transplantation in Patients With Severe Aplastic Anemia: An Interim Summary for a Multicenter Phase II Trial Results. Bone Marrow Transplant. 2017, 52, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.; Arora, M.; Flowers, M.E.; Chao, N.J.; McCarthy, P.L.; Cutler, C.S.; Urbano-Ispizua, A.; Pavletic, S.Z.; Haagenson, M.D.; Zhang, M.J.; et al. Risk Factors for Acute GVHD and Survival After Hematopoietic Cell Transplantation. Blood 2012, 119, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Pittenger, M.F. Human Mesenchymal Stem Cells Modulate Allogeneic Immune Cell Responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Jiang, E.; Yao, J.; Wang, M.; Chen, S.; Zhou, Z.; Zhai, W.; Ma, Q.; Feng, S.; Han, M. Interferon-Gamma Mediates the Immunosuppression of Bone Marrow Mesenchymal Stem Cells on T-Lymphocytes in Vitro. Hematology 2018, 23, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Le, B.K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal Stem Cells for Treatment of Steroid-Resistant, Severe, Acute Graft-Versus-Host Disease: A Phase II Study. Lancet. 2008, 371, 1579–1586. [Google Scholar]
- Jackson, T.J.; Mostoufi-Moab, S.; Hill-Kayser, C.; Balamuth, N.J.; Arkader, A. Musculoskeletal Complications Following Total Body Irradiation in Hematopoietic Stem Cell Transplant Patients. Pediatr. Blood Cancer 2017, e26905. [Google Scholar] [CrossRef]
- Mont, M.A.; Pivec, R.; Banerjee, S.; Issa, K.; Elmallah, R.K.; Jones, L.C. High-Dose Corticosteroid Use and Risk of Hip Osteonecrosis: Meta-Analysis and Systematic Literature Review. J. Arthroplasty 2015, 30, 1506–1512. [Google Scholar] [CrossRef]
- Mahadeo, K.M.; Oyeku, S.; Taragin, B.; Rajpathak, S.N.; Moody, K.; Santizo, R.; Driscoll, M.C. Increased Prevalence of Osteonecrosis of the Femoral Head in Children and Adolescents With Sickle-Cell Disease. Am. J. Hematol. 2011, 86, 806–808. [Google Scholar] [CrossRef]
- Hernigou, P.; Daltro, G.; Filippini, P.; Mukasa, M.M.; Manicom, O. Percutaneous Implantation of Autologous Bone Marrow Osteoprogenitor Cells As Treatment of Bone Avascular Necrosis Related to Sickle Cell Disease. Open. Orthop. J. 2008, 2, 62–65. [Google Scholar] [CrossRef]
- Muller, I.; Vaegler, M.; Holzwarth, C.; Tzaribatchev, N.; Pfister, S.M.; Schutt, B.; Reize, P.; Greil, J.; Handgretinger, R.; Rudert, M. Secretion of Angiogenic Proteins by Human Multipotent Mesenchymal Stromal Cells and Their Clinical Potential in the Treatment of Avascular Osteonecrosis. Leukemia 2008, 22, 2054–2061. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Cui, D.; Wang, B.; Tian, F.; Guo, L.; Yang, L.; Liu, B.; Yu, X. Treatment of Early Stage Osteonecrosis of the Femoral Head With Autologous Implantation of Bone Marrow-Derived and Cultured Mesenchymal Stem Cells. Bone 2012, 50, 325–330. [Google Scholar] [CrossRef]
- Chao, Y.H.; Peng, C.T.; Harn, H.J.; Chan, C.K.; Wu, K.H. Poor Potential of Proliferation and Differentiation in Bone Marrow Mesenchymal Stem Cells Derived From Children With Severe Aplastic Anemia. Ann. Hematol. 2010, 89, 715–723. [Google Scholar] [CrossRef]
- Hamzic, E.; Whiting, K.; Gordon, S.E.; Pettengell, R. Characterization of Bone Marrow Mesenchymal Stromal Cells in Aplastic Anaemia. Br. J. Haematol. 2015, 169, 804–813. [Google Scholar] [CrossRef]
- El-Mahgoub, E.R.; Ahmed, E.; Afifi, R.A.; Kamal, M.A.; Mousa, S.M. Mesenchymal Stem Cells From Pediatric Patients With Aplastic Anemia: Isolation, Characterization, Adipogenic, and Osteogenic Differentiation. Fetal Pediatr. Pathol. 2014, 33, 9–15. [Google Scholar] [CrossRef]
- Bueno, C.; Roldan, M.; Anguita, E.; Romero-Moya, D.; Martin-Antonio, B.; Rosu-Myles, M.; del Cañizo, C.; Campos, F.; Garcia, R.; Gomez-Casares, M.; et al. Bone Marrow Mesenchymal Stem Cells From Patients With Aplastic Anemia Maintain Functional and Immune Properties and Do Not Contribute to the Pathogenesis of the Disease. Haematologica 2014, 99, 1168–1175. [Google Scholar] [CrossRef] [Green Version]
- Mantelli, M.; Avanzini, M.A.; Rosti, V.; Ingo, D.M.; Conforti, A.; Novara, F.; Arrigo, G.; Boni, M.; Zappatore, R.; Lenta, E.; et al. Comprehensive Characterization of Mesenchymal Stromal Cells From Patients With Fanconi Anaemia. Br. J. Haematol. 2015, 170, 826–836. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yang, S.; Lu, S.; Zhao, H.; Feng, J.; Li, W.; Ma, F.; Ren, Q.; Liu, B.; Zhang, L.; et al. Differential Gene Expression Profile Associated With the Abnormality of Bone Marrow Mesenchymal Stem Cells in Aplastic Anemia. PLoS ONE 2012, 7, e47764. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, N.K.; Singh, S.P.; Nityanand, S. Enhanced Adipogenicity of Bone Marrow Mesenchymal Stem Cells in Aplastic Anemia. Stem Cells Int. 2014, 2014, 276862. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.S.; Kim, E.S.; Hwang, D.W.; Choi, J.Y.; Kim, B.K.; Park, B.B.; Choi, J.H.; Lee, Y.Y. Biologic Characteristics of Bone Marrow-Derived Mesenchymal Stem Cells From a Patient With Thalassemia Syndrome. Int. J. Lab. Hematol. 2011, 33, 281–289. [Google Scholar] [CrossRef]
- Aksoy, C.; Guliyev, A.; Kilic, E.; Uckan, D.; Severcan, F. Bone Marrow Mesenchymal Stem Cells in Patients With Beta Thalassemia Major: Molecular Analysis With Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy Study As a Novel Method. Stem Cells Dev. 2012, 21, 2000–2011. [Google Scholar] [CrossRef]
- Crippa, S.; Rossella, V.; Aprile, A.; Silvestri, L.; Rivis, S.; Scaramuzza, S.; Pirroni, S.; Avanzini, M.A.; Basso-Ricci, L.; Hernandez, R.J.; et al. Bone Marrow Stromal Cells From β-Thalassemia Patients Have Impaired Hematopoietic Supportive Capacity. J Clin. Invest. 2019, 129, 1566–1580. [Google Scholar] [CrossRef] [Green Version]
- Lebouvier, A.; Poignard, A.; Coquelin-Salsac, L.; Leotot, J.; Homma, Y.; Jullien, N.; Bierling, P.; Galacteros, F.; Hernigou, P.; Chevallier, N.; et al. Autologous Bone Marrow Stromal Cells Are Promising Candidates for Cell Therapy Approaches to Treat Bone Degeneration in Sickle Cell Disease. Stem Cell Res. 2015, 15, 584–594. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, S.A.; Mankani, M.H.; Bianco, P.; Robey, P.G. Enumeration of the Colony-Forming Units-Fibroblast From Mouse and Human Bone Marrow in Normal and Pathological Conditions. Stem Cell Res. 2009, 2, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Daltro, G.C.; Fortuna, V.; de Souza, E.S.; Salles, M.M.; Carreira, A.C.; Meyer, R.; Freire, S.M.; Borojevic, R. Efficacy of Autologous Stem Cell-Based Therapy for Osteonecrosis of the Femoral Head in Sickle Cell Disease: A Five-Year Follow-Up Study. Stem Cell Res. Ther. 2015, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Stenger, E.O.; Chinnadurai, R.; Yuan, S.; Garcia, M.; Arafat, D.; Gibson, G.; Krishnamurti, L.; Galipeau, J. Bone Marrow-Derived Mesenchymal Stromal Cells From Patients With Sickle Cell Disease Display Intact Functionality. Biol. Blood Marrow Transplant. 2017, 23, 736–745. [Google Scholar] [CrossRef]
- Donadieu, J.; Beaupain, B.; Mahlaoui, N.; Bellanne-Chantelot, C. Epidemiology of Congenital Neutropenia. Hematol. Oncol. Clin. North Am. 2013, 27, 1–17. [Google Scholar] [CrossRef]
- Stavroulaki, E.; Kastrinaki, M.C.; Pontikoglou, C.; Eliopoulos, D.; Damianaki, A.; Mavroudi, I.; Pyrovolaki, K.; Katonis, P.; Papadaki, H.A. Mesenchymal Stem Cells Contribute to the Abnormal Bone Marrow Microenvironment in Patients With Chronic Idiopathic Neutropenia by Overproduction of Transforming Growth Factor-Beta1. Stem Cells Dev. 2011, 20, 1309–1318. [Google Scholar] [CrossRef]
- Dominici, M.; Le, B.K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Sun, Y.; Deng, W.; Geng, L.; Zhang, L.; Liu, R.; Chen, W.; Yao, G.; Zhang, H.; Feng, X.; Gao, X.; et al. Mesenchymal Stem Cells From Patients With Rheumatoid Arthritis Display Impaired Function in Inhibiting Th17 Cells. J. Immunol. Res. 2015, 2015, 284215. [Google Scholar] [CrossRef]
- Codinach, M.; Blanco, M.; Ortega, I.; Lloret, M.; Reales, L.; Coca, M.I.; Torrents, S.; Doral, M.; Oliver-Vila, I.; Requena-Montero, M.; et al. Design and Validation of a Consistent and Reproducible Manufacture Process for the Production of Clinical-Grade Bone Marrow-Derived Multipotent Mesenchymal Stromal Cells. Cytotherapy 2016, 18, 1197–1208. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Copland, I.B.; Ng, S.; Garcia, M.; Prasad, M.; Arafat, D.; Gibson, G.; Kugathasan, S.; Galipeau, J. Mesenchymal Stromal Cells Derived From Crohn’s Patients Deploy Indoleamine 2,3-Dioxygenase-Mediated Immune Suppression, Independent of Autophagy. Mol. Ther. 2015, 23, 1248–1261. [Google Scholar] [CrossRef] [Green Version]
- Copland, I.B.; Qayed, M.; Garcia, M.A.; Galipeau, J.; Waller, E.K. Bone Marrow Mesenchymal Stromal Cells From Patients With Acute and Chronic Graft-Versus-Host Disease Deploy Normal Phenotype, Differentiation Plasticity, and Immune-Suppressive Activity. Biol. Blood Marrow Transplant. 2015, 21, 934–940. [Google Scholar] [CrossRef] [Green Version]
- Tormin, A.; Li, O.; Brune, J.C.; Walsh, S.; Schutz, B.; Ehinger, M.; Ditzel, N.; Kassem, M.; Scheding, S. CD146 Expression on Primary Nonhematopoietic Bone Marrow Stem Cells Is Correlated With in Situ Localization. Blood 2011, 117, 5067–5077. [Google Scholar] [CrossRef] [Green Version]
- Islam, A. Do Bone Marrow Fat Cells or Their Precursors Have a Pathogenic Role in Idiopathic Aplastic Anaemia? Med. Hypotheses 1988, 25, 209–217. [Google Scholar] [CrossRef]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.; Daley, G.Q. Bone-Marrow Adipocytes As Negative Regulators of the Hematopoietic Microenvironment. Nature 2009, 460, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs Inhibit Monocyte-Derived DC Maturation and Function by Selectively Interfering With the Generation of Immature DCs: Central Role of MSC-Derived Prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Yanez, R.; Oviedo, A.; Aldea, M.; Bueren, J.A.; Lamana, M.L. Prostaglandin E2 Plays a Key Role in the Immunosuppressive Properties of Adipose and Bone Marrow Tissue-Derived Mesenchymal Stromal Cells. Exp. Cell Res. 2010, 316, 3109–3123. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Zale, E.A. Prostaglandin E2: A Putative Potency Indicator of the Immunosuppressive Activity of Human Mesenchymal Stem Cells. Am. J. Stem Cells 2012, 1, 138–145. [Google Scholar]
- Kota, D.J.; Prabhakara, K.S.; Toledano-Furman, N.; Bhattarai, D.; Chen, Q.; DiCarlo, B.; Smith, P.; Triolo, F.; Wenzel, P.L.; Cox, C.S., Jr.; et al. Prostaglandin E2 Indicates Therapeutic Efficacy of Mesenchymal Stem Cells in Experimental Traumatic Brain Injury. Stem Cells 2017, 35, 1416–1430. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, P.; Eder, R.; Kunz-Schughart, L.A.; Andreesen, R.; Edinger, M. Large-scale in vitro expansion of polyclonal human CD4(+) CD25high regulatory T cells. Blood 2004, 104, 895–903. [Google Scholar] [CrossRef]
- He, X.; Smeets, R.L.; van Rijssen, E.; Boots, A.M.; Joosten, I.; Koenen, H.J. Single CD28 stimulation induces stable and polyclonal expansion of human regulatory T cells. Sci. Rep. 2017, 7, 43003. [Google Scholar] [CrossRef] [Green Version]




| Gender | Healthy donors | TM | SCD | SCN-CN |
|---|---|---|---|---|
| N | 21 | 10 | 9 | 2 |
| Female, N (%) | 12 (60) | 5 (50) | 5 (56) | 2 (100) |
| Male, N (%) | 9 (40) | 5 (50) | 4 (44) | 0 |
| Age in years: median (range) | 12 (3–16) | 10.5 (4–14) | 14 (9–15) | 7–11 |
| Patient’s ID. | Disease | Age (Years)/Gender | Osteoblasts | Adipocytes | Chondrocytes |
|---|---|---|---|---|---|
 |  |  | |||
| 1 | TM | 9/f | *** | * | *** |
| 2 | TM | 5/m | *** | * | *** |
| 3 | TM | 13/m | ** | * | *** |
| 4 | TM | 4/f | *** | * | *** |
| 11 | SCD | 14/m | ** | *** | *** |
| 12 | SCD | 14/f | *** | ** | *** |
| 13 | SCD | 15/f | *** | *** | ** |
| 20 | SCN | 11/f | *** | *** | *** |
| 21 | CN | 7/f | *** | ** | *** |
| Healthy Donors’ ID | |||||
| 22 | HD | 8/m | ** | *** | *** |
| 23 | HD | 6/m | *** | *** | *** |
| 24 | HD | 13/m | *** | *** | *** |
| 25 | HD | 4/f | * | *** | *** |
| 32 | HD | 12/f | *** | *** | *** |
| 33 | HD | 14/f | *** | * | |
| 34 | HD | 16/m | *** | ** | *** |
| 35 | HD | 13/m | *** | ** | *** |
| 41 | HD | 12/f | ** | *** | *** |
| 42 | HD | 8/f | *** | *** | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuҫi, Z.; Jordan, C.; Wehner, S.; Sörensen, J.; Jarisch, A.; Salzmann-Manrique, E.; Pfeffermann, L.-M.; Klingebiel, T.; Bader, P.; Kuҫi, S. The Phenotype and Functional Activity of Mesenchymal Stromal Cells in Pediatric Patients with Non-Malignant Hematological Diseases. Cells 2020, 9, 431. https://doi.org/10.3390/cells9020431
Kuҫi Z, Jordan C, Wehner S, Sörensen J, Jarisch A, Salzmann-Manrique E, Pfeffermann L-M, Klingebiel T, Bader P, Kuҫi S. The Phenotype and Functional Activity of Mesenchymal Stromal Cells in Pediatric Patients with Non-Malignant Hematological Diseases. Cells. 2020; 9(2):431. https://doi.org/10.3390/cells9020431
Chicago/Turabian StyleKuҫi, Zyrafete, Christiane Jordan, Sibylle Wehner, Jan Sörensen, Andrea Jarisch, Emilia Salzmann-Manrique, Lisa-Marie Pfeffermann, Thomas Klingebiel, Peter Bader, and Selim Kuҫi. 2020. "The Phenotype and Functional Activity of Mesenchymal Stromal Cells in Pediatric Patients with Non-Malignant Hematological Diseases" Cells 9, no. 2: 431. https://doi.org/10.3390/cells9020431





