A Systematic Review on the Implications of O-linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis
Abstract
1. Introduction
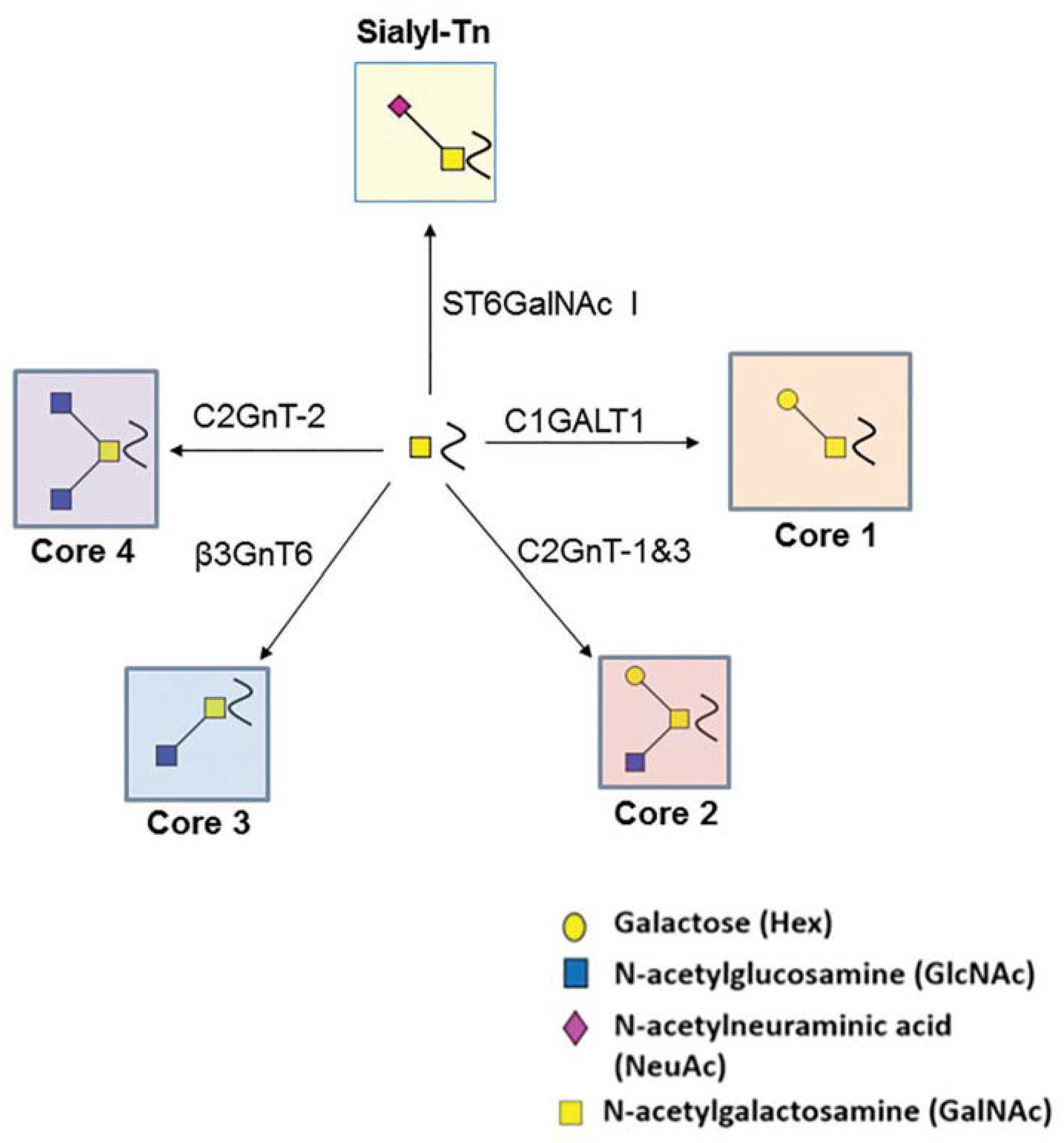
2. O-Glycosylation
3. Implications of Key O-Glycosyltransferases
4. Biological Function of TF-Antigen
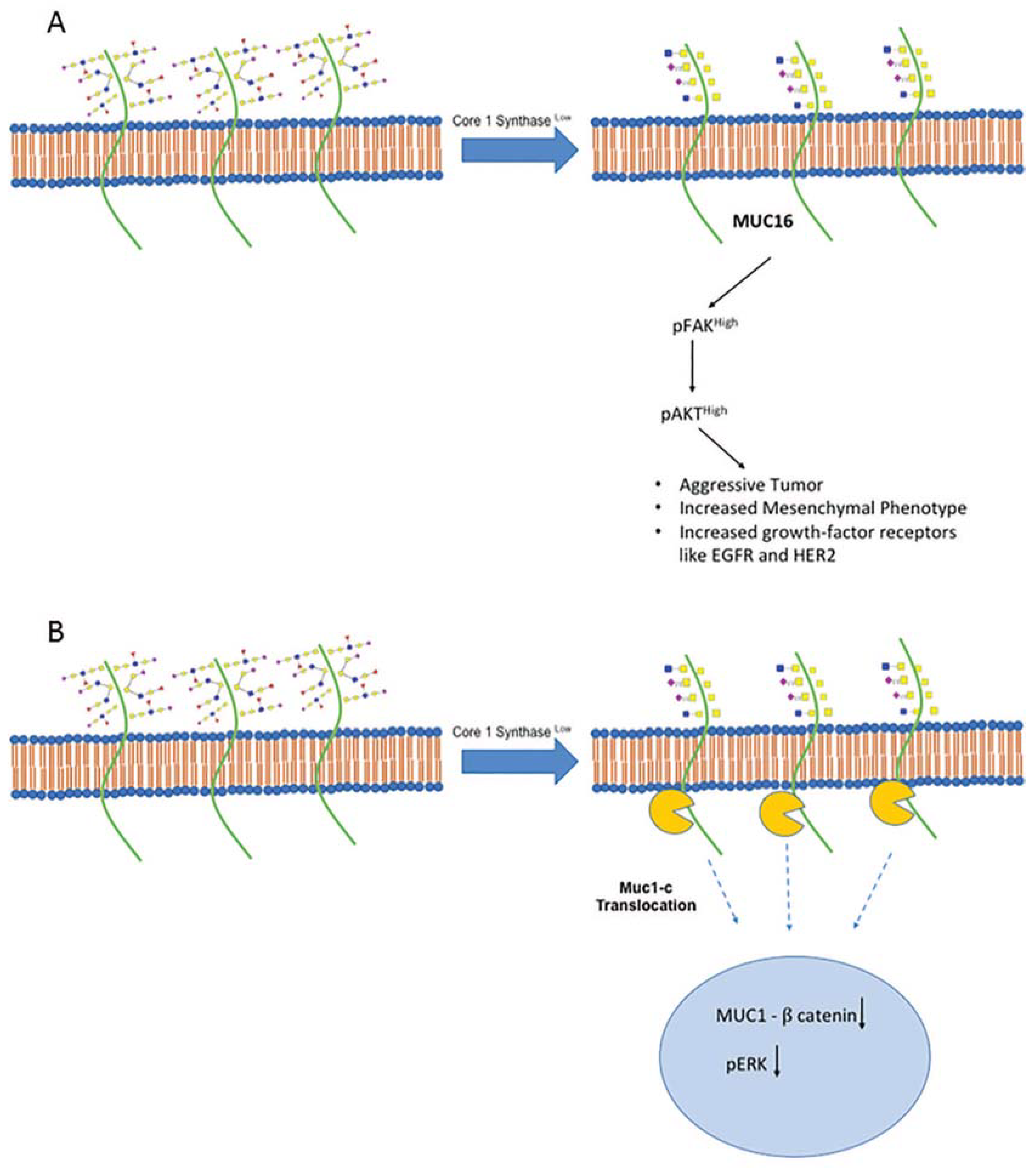
5. Core-1 Synthase Loss Aggravates Pancreatic Cancer
6. Historical Perspective of Core-1 Synthase in Breast Cancer
7. Core-1 Synthase Regulates Intestinal Cancer by Different Mechanisms
8. Mechanistic Insights of Core-1 Synthase in Hepatocellular Carcinoma
9. Interaction of Core-1 Synthase and Galectin in Prostate Cancer
10. Core-1 Synthase Behaves Differently in Different Cancers
11. Unique Study on Core-3 Synthase in Pancreatic Cancer
12. Implications of Core-3 Synthase in Colon and Colorectal Cancer
13. Core-3 Synthase Overexpression Study in Prostate Cancer
14. In Vitro and In Vivo Role of ST6GalNAc-I in Ovarian Cancer
15. Diagnostic Influence of ST6GalNAc-I in Pancreatic Cancer
16. ST6GalNAc-I Dependent Mucin Glycosylation in Gastric Cancer
17. ST6GalNAc-I Based Immune Therapy in Breast Cancer
18. Mechanistic Insights of ST6GalNAc-I in Different Bladder Tumor
19. Clinical Implications of ST6GalNAc-I in Colon Carcinoma
20. MUC1 Regulation by ST6GalNAc-I in Ovarian Carcinoma
21. In Vitro and in Vivo Role of ST6GalNAc-I in Bladder Tumor
22. Identifying Splice Variant of ST6GalNAc-I in Prostate Cancer
23. Tumor Suppressor Role of C2GnT/GCNT in Pancreatic Cancer
24. Implications of GCNTs in Colorectal Cancer
25. Mechanistic Insights of C2GnT1/GCNT1 in Prostate Cancer
26. Immune Evasion by C2GnT in Bladder Tumor
27. Conclusions
Funding
Conflicts of Interest
References
- Corfield, A. Eukaryotic protein glycosylation: A primer for histochemists and cell biologists. Histochem. Cell Biol. 2017, 147, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Lannoo, N.; Van Damme, E.J. Review/N-glycans: The making of a varied toolbox. Plant Sci. Int. J. Exp. Plant Biol. 2015, 239, 67–83. [Google Scholar] [CrossRef] [PubMed]
- de Las Rivas, M.; Lira-Navarrete, E.; Gerken, T.A.; Hurtado-Guerrero, R. Polypeptide GalNAc-Ts: From redundancy to specificity. Curr. Opin. Struct. Biol. 2019, 56, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Nasirikenari, M.; Manhardt, C.T.; Ashline, D.J.; Hanneman, A.J.; Reinhold, V.N.; Lau, J.T. Platelets support extracellular sialylation by supplying the sugar donor substrate. J. Biol. Chem. 2014, 289, 8742–8748. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Matta, K.L.; Neelamegham, S. A systematic analysis of acceptor specificity and reaction kinetics of five human alpha(2,3)sialyltransferases: Product inhibition studies illustrate reaction mechanism for ST3Gal-I. Biochem. Biophys. Res. Commun. 2016, 469, 606–612. [Google Scholar] [CrossRef]
- Umana, P.; Bailey, J.E. A mathematical model of N-linked glycoform biosynthesis. Biotechnol. Bioeng. 1997, 55, 890–908. [Google Scholar] [CrossRef]
- Liu, G.; Marathe, D.D.; Matta, K.L.; Neelamegham, S. Systems-level modeling of cellular glycosylation reaction networks: O-linked glycan formation on natural selectin ligands. Bioinform. (Oxf. Engl.) 2008, 24, 2740–2747. [Google Scholar] [CrossRef]
- Bousfield, G.R.; Butnev, V.Y.; Rueda-Santos, M.A.; Brown, A.; Hall, A.S.; Harvey, D.J. Macro- and Micro-heterogeneity in Pituitary and Urinary Follicle-Stimulating Hormone Glycosylation. J. Glycom. Lipidom. 2014, 4. [Google Scholar] [CrossRef]
- Gil, G.C.; Velander, W.H.; Van Cott, K.E. N-glycosylation microheterogeneity and site occupancy of an Asn-X-Cys sequon in plasma-derived and recombinant protein C. Proteomics 2009, 9, 2555–2567. [Google Scholar] [CrossRef]
- Stavenhagen, K.; Hinneburg, H.; Thaysen-Andersen, M.; Hartmann, L.; Varon Silva, D.; Fuchser, J.; Kaspar, S.; Rapp, E.; Seeberger, P.H.; Kolarich, D. Quantitative mapping of glycoprotein micro-heterogeneity and macro-heterogeneity: An evaluation of mass spectrometry signal strengths using synthetic peptides and glycopeptides. J. Mass Spectrom. 2013, 48, 627–639. [Google Scholar] [CrossRef]
- Lo, C.Y.; Antonopoulos, A.; Gupta, R.; Qu, J.; Dell, A.; Haslam, S.M.; Neelamegham, S. Competition between core-2 GlcNAc-transferase and ST6GalNAc-transferase regulates the synthesis of the leukocyte selectin ligand on human P-selectin glycoprotein ligand-1. J. Biol. Chem. 2013, 288, 13974–13987. [Google Scholar] [CrossRef] [PubMed]
- Stolfa, G.; Mondal, N.; Zhu, Y.; Yu, X.; Buffone, A., Jr.; Neelamegham, S. Using CRISPR-Cas9 to quantify the contributions of O-glycans, N-glycans and Glycosphingolipids to human leukocyte-endothelium adhesion. Sci. Rep. 2016, 6, 30392. [Google Scholar] [CrossRef] [PubMed]
- Buffone, A., Jr.; Mondal, N.; Gupta, R.; McHugh, K.P.; Lau, J.T.; Neelamegham, S. Silencing alpha1,3-fucosyltransferases in human leukocytes reveals a role for FUT9 enzyme during E-selectin-mediated cell adhesion. J. Biol. Chem. 2013, 288, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.; Gnanapragassam, V.S.; Jain, M.; Rachagani, S.; Ponnusamy, M.P.; Batra, S.K. Pathobiological implications of mucin glycans in cancer: Sweet poison and novel targets. Biochim. Biophys. Acta 2015, 1856, 211–225. [Google Scholar] [CrossRef]
- Huang, Y.W.; Yang, H.I.; Wu, Y.T.; Hsu, T.L.; Lin, T.W.; Kelly, J.W.; Wong, C.H. Residues Comprising the Enhanced Aromatic Sequon Influence Protein N-Glycosylation Efficiency. J. Am. Chem. Soc. 2017, 139, 12947–12955. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, S.; Momi, N.; Sasson, A.R.; Batra, S.K. Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 607–620. [Google Scholar] [CrossRef]
- Brockhausen, I.; Schachter, H.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: California, NY, USA, 2009. [Google Scholar]
- Tian, E.; Ten Hagen, K.G. Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj. J. 2009, 26, 325–334. [Google Scholar] [CrossRef]
- Ikehara, Y.; Kojima, N.; Kurosawa, N.; Kudo, T.; Kono, M.; Nishihara, S.; Issiki, S.; Morozumi, K.; Itzkowitz, S.; Tsuda, T.; et al. Cloning and expression of a human gene encoding an N-acetylgalactosamine-alpha2,6-sialyltransferase (ST6GalNAc I): A candidate for synthesis of cancer-associated sialyl-Tn antigens. Glycobiology 1999, 9, 1213–1224. [Google Scholar] [CrossRef]
- Heimburg-Molinaro, J.; Lum, M.; Vijay, G.; Jain, M.; Almogren, A.; Rittenhouse-Olson, K. Cancer vaccines and carbohydrate epitopes. Vaccine 2011, 29, 8802–8826. [Google Scholar] [CrossRef]
- Datta, A.K. Comparative sequence analysis in the sialyltransferase protein family: Analysis of motifs. Curr. Drug Targets 2009, 10, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Aryal, R.P.; Ju, T.; Cummings, R.D.; Gahlay, G.; Jarvis, D.L.; Matta, K.L.; Vlahakis, J.Z.; Szarek, W.A.; Brockhausen, I. Acceptor specificities and selective inhibition of recombinant human Gal- and GlcNAc-transferases that synthesize core structures 1, 2, 3 and 4 of O-glycans. Biochim. Biophys. Acta 2013, 1830, 4274–4281. [Google Scholar] [CrossRef] [PubMed]
- Bierhuizen, M.F.; Fukuda, M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal beta 1-3-GalNAc-R (GlcNAc to GalNAc) beta 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc. Natl. Acad. Sci. USA 1992, 89, 9326–9330. [Google Scholar] [CrossRef] [PubMed]
- Datti, A.; Dennis, J.W. Regulation of UDP-GlcNAc:Gal beta 1-3GalNAc-R beta 1-6-N-acetylglucosaminyltransferase (GlcNAc to GalNAc) in Chinese hamster ovary cells. J. Biol. Chem. 1993, 268, 5409–5416. [Google Scholar] [PubMed]
- Sangadala, S.; Sivakami, S.; Mendicino, J. UDP-GlcNAc: Gal beta 3GalNAc-mucin: (GlcNAc----GalNAc) beta 6-N-acetylglucosaminyltransferase and UDP-GlcNAc: Gal beta 3(GlcNAc beta 6) GalNAc-mucin (GlcNAc----Gal) beta 3-N-acetylglucosaminyltransferase from swine trachea epithelium. Mol. Cell. Biochem. 1991, 101, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, E.V.; Xue, J.; Neelamegham, S.; Matta, K.L. The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: A predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydr. Res. 2006, 341, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, M.; Whitehouse, C.; McFarlane, I.; Brockhausen, I.; Gschmeissner, S.; Schwientek, T.; Clausen, H.; Burchell, J.M.; Taylor-Papadimitriou, J. The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J. Biol. Chem. 2001, 276, 11007–11015. [Google Scholar] [CrossRef]
- Neelamegham, S.; Liu, G. Systems glycobiology: Biochemical reaction networks regulating glycan structure and function. Glycobiology 2011, 21, 1541–1553. [Google Scholar] [CrossRef]
- Xia, L.; Ju, T.; Westmuckett, A.; An, G.; Ivanciu, L.; McDaniel, J.M.; Lupu, F.; Cummings, R.D.; McEver, R.P. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J. Cell Biol. 2004, 164, 451–459. [Google Scholar] [CrossRef]
- Ju, T.; Lanneau, G.S.; Gautam, T.; Wang, Y.; Xia, B.; Stowell, S.R.; Willard, M.T.; Wang, W.; Xia, J.Y.; Zuna, R.E.; et al. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 2008, 68, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Wang, Y.; Aryal, R.P.; Lehoux, S.D.; Ding, X.; Kudelka, M.R.; Cutler, C.; Zeng, J.; Wang, J.; Sun, X.; et al. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteom. Clin. Appl. 2013, 7, 618–631. [Google Scholar] [CrossRef]
- Zeng, J.; Mi, R.; Wang, Y.; Li, Y.; Lin, L.; Yao, B.; Song, L.; van Die, I.; Chapman, A.B.; Cummings, R.D.; et al. Promoters of Human Cosmc and T-synthase Genes Are Similar in Structure, Yet Different in Epigenetic Regulation. J. Biol. Chem. 2015, 290, 19018–19033. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.; Barkeer, S.; Rachagani, S.; Nimmakayala, R.K.; Perumal, N.; Pothuraju, R.; Atri, P.; Mahapatra, S.; Thapa, I.; Talmon, G.A.; et al. Disruption of C1galt1 Gene Promotes Development and Metastasis of Pancreatic Adenocarcinomas in Mice. Gastroenterology 2018, 155, 1608–1624. [Google Scholar] [CrossRef]
- Radhakrishnan, P.; Dabelsteen, S.; Madsen, F.B.; Francavilla, C.; Kopp, K.L.; Steentoft, C.; Vakhrushev, S.Y.; Olsen, J.V.; Hansen, L.; Bennett, E.P.; et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. USA 2014, 111, E4066–E4075. [Google Scholar] [CrossRef]
- Brockhausen, I.; Yang, J.M.; Burchell, J.; Whitehouse, C.; Taylor-Papadimitriou, J. Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur. J. Biochem. 1995, 233, 607–617. [Google Scholar] [CrossRef]
- Solatycka, A.; Owczarek, T.; Piller, F.; Piller, V.; Pula, B.; Wojciech, L.; Podhorska-Okolow, M.; Dziegiel, P.; Ugorski, M. MUC1 in human and murine mammary carcinoma cells decreases the expression of core 2 beta1,6-N-acetylglucosaminyltransferase and beta-galactoside alpha2,3-sialyltransferase. Glycobiology 2012, 22, 1042–1054. [Google Scholar] [CrossRef]
- Chou, C.H.; Huang, M.J.; Chen, C.H.; Shyu, M.K.; Huang, J.; Hung, J.S.; Huang, C.S.; Huang, M.C. Up-regulation of C1GALT1 promotes breast cancer cell growth through MUC1-C signaling pathway. Oncotarget 2015, 6, 6123–6135. [Google Scholar] [CrossRef]
- Song, K.; Herzog, B.H.; Fu, J.; Sheng, M.; Bergstrom, K.; McDaniel, J.M.; Kondo, Y.; McGee, S.; Cai, X.; Li, P.; et al. Loss of Core 1-derived O-Glycans Decreases Breast Cancer Development in Mice. J. Biol. Chem. 2015, 290, 20159–20166. [Google Scholar] [CrossRef]
- Milde-Langosch, K.; Schutze, D.; Oliveira-Ferrer, L.; Wikman, H.; Muller, V.; Lebok, P.; Pantel, K.; Schroder, C.; Witzel, I.; Schumacher, U. Relevance of betaGal-betaGalNAc-containing glycans and the enzymes involved in their synthesis for invasion and survival in breast cancer patients. Breast Cancer Res. Treat. 2015, 151, 515–528. [Google Scholar] [CrossRef]
- Barrow, H.; Tam, B.; Duckworth, C.A.; Rhodes, J.M.; Yu, L.G. Suppression of core 1 Gal-transferase is associated with reduction of TF and reciprocal increase of Tn, sialyl-Tn and Core 3 glycans in human colon cancer cells. PLoS ONE 2013, 8, e59792. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.; Liu, X.; Zhao, Y.; Gao, N.; Wu, Q.; Song, K.; Cui, Y.; Li, Y.; McDaniel, J.M.; McGee, S.; et al. Defective Intestinal Mucin-Type O-Glycosylation Causes Spontaneous Colitis-Associated Cancer in Mice. Gastroenterology 2016, 151, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Bergstrom, K.; Fu, J.; Xie, B.; Chen, W.; Xia, L. Loss of intestinal O-glycans promotes spontaneous duodenal tumors. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G74–G83. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.S.; Huang, J.; Lin, Y.C.; Huang, M.J.; Lee, P.H.; Lai, H.S.; Liang, J.T.; Huang, M.C. C1GALT1 overexpression promotes the invasive behavior of colon cancer cells through modifying O-glycosylation of FGFR2. Oncotarget 2014, 5, 2096–2106. [Google Scholar] [CrossRef]
- Dong, X.; Jiang, Y.; Liu, J.; Liu, Z.; Gao, T.; An, G.; Wen, T. T-Synthase Deficiency Enhances Oncogenic Features in Human Colorectal Cancer Cells via Activation of Epithelial-Mesenchymal Transition. Biomed Res. Int. 2018, 2018, 9532389. [Google Scholar] [CrossRef]
- Huang, J.; Che, M.I.; Lin, N.Y.; Hung, J.S.; Huang, Y.T.; Lin, W.C.; Huang, H.C.; Lee, P.H.; Liang, J.T.; Huang, M.C. The molecular chaperone Cosmc enhances malignant behaviors of colon cancer cells via activation of Akt and ERK. Mol. Carcinog. 2014, 53, E62–E71. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Xu, F.; Dong, X.; Cheng, Y.; Hu, Y.; Gao, T.; Liu, J.; Yang, L.; Jia, X.; et al. Aberrant O-glycosylation contributes to tumorigenesis in human colorectal cancer. J. Cell. Mol. Med. 2018, 22, 4875–4885. [Google Scholar] [CrossRef]
- Wu, Y.M.; Liu, C.H.; Huang, M.J.; Lai, H.S.; Lee, P.H.; Hu, R.H.; Huang, M.C. C1GALT1 enhances proliferation of hepatocellular carcinoma cells via modulating MET glycosylation and dimerization. Cancer Res. 2013, 73, 5580–5590. [Google Scholar] [CrossRef]
- Liu, C.H.; Hu, R.H.; Huang, M.J.; Lai, I.R.; Chen, C.H.; Lai, H.S.; Wu, Y.M.; Huang, M.C. C1GALT1 promotes invasive phenotypes of hepatocellular carcinoma cells by modulating integrin beta1 glycosylation and activity. PLoS ONE 2014, 9, e94995. [Google Scholar] [CrossRef]
- Tsai, C.H.; Tzeng, S.F.; Chao, T.K.; Tsai, C.Y.; Yang, Y.C.; Lee, M.T.; Hwang, J.J.; Chou, Y.C.; Tsai, M.H.; Cha, T.L.; et al. Metastatic Progression of Prostate Cancer Is Mediated by Autonomous Binding of Galectin-4-O-Glycan to Cancer Cells. Cancer Res. 2016, 76, 5756–5767. [Google Scholar] [CrossRef]
- Tzeng, S.F.; Tsai, C.H.; Chao, T.K.; Chou, Y.C.; Yang, Y.C.; Tsai, M.H.; Cha, T.L.; Hsiao, P.W. O-Glycosylation-mediated signaling circuit drives metastatic castration-resistant prostate cancer. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Huang, M.J.; Liao, Y.Y.; Chen, C.H.; Huang, M.C. C1GALT1 Seems to Promote In Vitro Disease Progression in Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Chien, P.H.; Wu, H.Y.; Chen, S.T.; Juan, H.F.; Lou, P.J.; Huang, M.C. C1GALT1 predicts poor prognosis and is a potential therapeutic target in head and neck cancer. Oncogene 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, X.; Qiu, L.; Peng, F.; Geng, S.; Shen, L.; Luo, Z. Knockdown of C1GalT1 inhibits radioresistance of human esophageal cancer cells through modifying beta1-integrin glycosylation. J. Cancer 2018, 9, 2666–2677. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, P.; Grandgenett, P.M.; Mohr, A.M.; Bunt, S.K.; Yu, F.; Chowdhury, S.; Hollingsworth, M.A. Expression of core 3 synthase in human pancreatic cancer cells suppresses tumor growth and metastasis. Int. J. Cancer 2013, 133, 2824–2833. [Google Scholar] [CrossRef]
- Iwai, T.; Kudo, T.; Kawamoto, R.; Kubota, T.; Togayachi, A.; Hiruma, T.; Okada, T.; Kawamoto, T.; Morozumi, K.; Narimatsu, H. Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4572–4577. [Google Scholar] [CrossRef]
- Ye, J.; Wei, X.; Shang, Y.; Pan, Q.; Yang, M.; Tian, Y.; He, Y.; Peng, Z.; Chen, L.; Chen, W.; et al. Core 3 mucin-type O-glycan restoration in colorectal cancer cells promotes MUC1/p53/miR-200c-dependent epithelial identity. Oncogene 2017, 36, 6391–6407. [Google Scholar] [CrossRef]
- An, G.; Wei, B.; Xia, B.; McDaniel, J.M.; Ju, T.; Cummings, R.D.; Braun, J.; Xia, L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J. Exp. Med. 2007, 204, 1417–1429. [Google Scholar] [CrossRef]
- Robbe-Masselot, C.; Herrmann, A.; Maes, E.; Carlstedt, I.; Michalski, J.C.; Capon, C. Expression of a core 3 disialyl-Le(x) hexasaccharide in human colorectal cancers: A potential marker of malignant transformation in colon. J. Proteome Res. 2009, 8, 702–711. [Google Scholar] [CrossRef]
- Lee, S.H.; Hatakeyama, S.; Yu, S.Y.; Bao, X.; Ohyama, C.; Khoo, K.H.; Fukuda, M.N.; Fukuda, M. Core3 O-glycan synthase suppresses tumor formation and metastasis of prostate carcinoma PC3 and LNCaP cells through down-regulation of alpha2beta1 integrin complex. J. Biol. Chem. 2009, 284, 17157–17169. [Google Scholar] [CrossRef]
- Eavarone, D.A.; Al-Alem, L.; Lugovskoy, A.; Prendergast, J.M.; Nazer, R.I.; Stein, J.N.; Dransfield, D.T.; Behrens, J.; Rueda, B.R. Humanized anti-Sialyl-Tn antibodies for the treatment of ovarian carcinoma. PLoS ONE 2018, 13, e0201314. [Google Scholar] [CrossRef] [PubMed]
- Starbuck, K.; Al-Alem, L.; Eavarone, D.A.; Hernandez, S.F.; Bellio, C.; Prendergast, J.M.; Stein, J.; Dransfield, D.T.; Zarrella, B.; Growdon, W.B.; et al. Treatment of ovarian cancer by targeting the tumor stem cell-associated carbohydrate antigen, Sialyl-Thomsen-nouveau. Oncotarget 2018, 9, 23289–23305. [Google Scholar] [CrossRef] [PubMed]
- Remmers, N.; Anderson, J.M.; Linde, E.M.; DiMaio, D.J.; Lazenby, A.J.; Wandall, H.H.; Mandel, U.; Clausen, H.; Yu, F.; Hollingsworth, M.A. Aberrant expression of mucin core proteins and o-linked glycans associated with progression of pancreatic cancer. Clin. Cancer Res. 2013, 19, 1981–1993. [Google Scholar] [CrossRef] [PubMed]
- Marcos, N.T.; Cruz, A.; Silva, F.; Almeida, R.; David, L.; Mandel, U.; Clausen, H.; Von Mensdorff-Pouilly, S.; Reis, C.A. Polypeptide GalNAc-transferases, ST6GalNAc-transferase I, and ST3Gal-transferase I expression in gastric carcinoma cell lines. J. Histochem. Cytochem. 2003, 51, 761–771. [Google Scholar] [CrossRef]
- Tamura, F.; Sato, Y.; Hirakawa, M.; Yoshida, M.; Ono, M.; Osuga, T.; Okagawa, Y.; Uemura, N.; Arihara, Y.; Murase, K.; et al. RNAi-mediated gene silencing of ST6GalNAc I suppresses the metastatic potential in gastric cancer cells. Gastric Cancer 2016, 19, 85–97. [Google Scholar] [CrossRef]
- Pinto, R.; Barros, R.; Pereira-Castro, I.; Mesquita, P.; da Costa, L.T.; Bennett, E.P.; Almeida, R.; David, L. CDX2 homeoprotein is involved in the regulation of ST6GalNAc-I gene in intestinal metaplasia. Lab. Investig. J. Tech. Methods Pathol. 2015, 95, 718–727. [Google Scholar] [CrossRef]
- Julien, S.; Videira, P.A.; Delannoy, P. Sialyl-tn in cancer: (how) did we miss the target? Biomolecules 2012, 2, 435–466. [Google Scholar] [CrossRef]
- Conze, T.; Carvalho, A.S.; Landegren, U.; Almeida, R.; Reis, C.A.; David, L.; Soderberg, O. MUC2 mucin is a major carrier of the cancer-associated sialyl-Tn antigen in intestinal metaplasia and gastric carcinomas. Glycobiology 2010, 20, 199–206. [Google Scholar] [CrossRef]
- David, L.; Nesland, J.M.; Clausen, H.; Carneiro, F.; Sobrinho-Simoes, M. Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn and T) in gastric mucosa, carcinomas and metastases. APMIS Suppl. 1992, 27, 162–172. [Google Scholar]
- Marcos, N.T.; Pinho, S.; Grandela, C.; Cruz, A.; Samyn-Petit, B.; Harduin-Lepers, A.; Almeida, R.; Silva, F.; Morais, V.; Costa, J.; et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004, 64, 7050–7057. [Google Scholar] [CrossRef]
- Marcos, N.T.; Bennett, E.P.; Gomes, J.; Magalhaes, A.; Gomes, C.; David, L.; Dar, I.; Jeanneau, C.; DeFrees, S.; Krustrup, D.; et al. ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Front. Biosci. 2011, 3, 1443–1455. [Google Scholar] [CrossRef]
- Santos-Silva, F.; Fonseca, A.; Caffrey, T.; Carvalho, F.; Mesquita, P.; Reis, C.; Almeida, R.; David, L.; Hollingsworth, M.A. Thomsen-Friedenreich antigen expression in gastric carcinomas is associated with MUC1 mucin VNTR polymorphism. Glycobiology 2005, 15, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Marcos, N.T.; Ferreira, B.; Carvalho, A.S.; Oliveira, M.J.; Santos-Silva, F.; Harduin-Lepers, A.; Reis, C.A. Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007, 249, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Carvalho, A.S.; Conze, T.; Magalhaes, A.; Picco, G.; Burchell, J.M.; Taylor-Papadimitriou, J.; Reis, C.A.; Almeida, R.; Mandel, U.; et al. Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation. J. Cell. Mol. Med. 2012, 16, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Thor, A.; Ohuchi, N.; Szpak, C.A.; Johnston, W.W.; Schlom, J. Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B72.3. Cancer Res. 1986, 46, 3118–3124. [Google Scholar]
- Contegiacomo, A.; Alimandi, M.; Muraro, R.; Pizzi, C.; Calderopoli, R.; De Marchis, L.; Sgambato, A.; Pettinato, G.; Petrella, G.; De Filippo, M.R.; et al. Expression of epitopes of the tumour-associated glycoprotein 72 and clinicopathological correlations in mammary carcinomas. Eur. J. Cancer 1994, 30, 813–820. [Google Scholar] [CrossRef]
- Leivonen, M.; Nordling, S.; Lundin, J.; von Boguslawski, K.; Haglund, C. STn and prognosis in breast cancer. Oncology 2001, 61, 299–305. [Google Scholar] [CrossRef]
- Cho, S.H.; Sahin, A.; Hortobagyi, G.N.; Hittelman, W.N.; Dhingra, K. Sialyl-Tn antigen expression occurs early during human mammary carcinogenesis and is associated with high nuclear grade and aneuploidy. Cancer Res. 1994, 54, 6302–6305. [Google Scholar]
- Kinney, A.Y.; Sahin, A.; Vernon, S.W.; Frankowski, R.F.; Annegers, J.F.; Hortobagyi, G.N.; Buzdar, A.U.; Frye, D.K.; Dhingra, K. The prognostic significance of sialyl-Tn antigen in women treated with breast carcinoma treated with adjuvant chemotherapy. Cancer 1997, 80, 2240–2249. [Google Scholar] [CrossRef]
- Soares, R.; Marinho, A.; Schmitt, F. Expression of sialyl-Tn in breast cancer. Correlation with prognostic parameters. Pathol. Res. Pract. 1996, 192, 1181–1186. [Google Scholar] [CrossRef]
- Julien, S.; Krzewinski-Recchi, M.A.; Harduin-Lepers, A.; Gouyer, V.; Huet, G.; Le Bourhis, X.; Delannoy, P. Expression of sialyl-Tn antigen in breast cancer cells transfected with the human CMP-Neu5Ac: GalNAc alpha2,6-sialyltransferase (ST6GalNac I) cDNA. Glycoconj. J. 2001, 18, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Adriaenssens, E.; Ottenberg, K.; Furlan, A.; Courtand, G.; Vercoutter-Edouart, A.S.; Hanisch, F.G.; Delannoy, P.; Le Bourhis, X. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 2006, 16, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Lagadec, C.; Krzewinski-Recchi, M.A.; Courtand, G.; Le Bourhis, X.; Delannoy, P. Stable expression of sialyl-Tn antigen in T47-D cells induces a decrease of cell adhesion and an increase of cell migration. Breast Cancer Res. Treat. 2005, 90, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Sewell, R.; Backstrom, M.; Dalziel, M.; Gschmeissner, S.; Karlsson, H.; Noll, T.; Gatgens, J.; Clausen, H.; Hansson, G.C.; Burchell, J.; et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J. Biol. Chem. 2006, 281, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Picco, G.; Sewell, R.; Vercoutter-Edouart, A.S.; Tarp, M.; Miles, D.; Clausen, H.; Taylor-Papadimitriou, J.; Burchell, J.M. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br. J. Cancer 2009, 100, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K.; Murray, J.L.; Zhou, D.; Mittendorf, E.A.; Sample, D.; Tautchin, M.; Miles, D. Survival Advantage in Patients with Metastatic Breast Cancer Receiving Endocrine Therapy plus Sialyl Tn-KLH Vaccine: Post Hoc Analysis of a Large Randomized Trial. J. Cancer 2013, 4, 577–584. [Google Scholar] [CrossRef]
- Lima, L.; Severino, P.F.; Silva, M.; Miranda, A.; Tavares, A.; Pereira, S.; Fernandes, E.; Cruz, R.; Amaro, T.; Reis, C.A.; et al. Response of high-risk of recurrence/progression bladder tumours expressing sialyl-Tn and sialyl-6-T to BCG immunotherapy. Br. J. Cancer 2013, 109, 2106–2114. [Google Scholar] [CrossRef]
- Severino, P.F.; Silva, M.; Carrascal, M.; Malagolini, N.; Chiricolo, M.; Venturi, G.; Astolfi, A.; Catera, M.; Videira, P.A.; Dall’Olio, F. Expression of sialyl-Tn sugar antigen in bladder cancer cells affects response to Bacillus Calmette Guerin (BCG) and to oxidative damage. Oncotarget 2017, 8, 54506–54517. [Google Scholar] [CrossRef][Green Version]
- Cotton, S.; Azevedo, R.; Gaiteiro, C.; Ferreira, D.; Lima, L.; Peixoto, A.; Fernandes, E.; Neves, M.; Neves, D.; Amaro, T.; et al. Targeted O-glycoproteomics explored increased sialylation and identified MUC16 as a poor prognosis biomarker in advanced-stage bladder tumours. Mol. Oncol. 2017, 11, 895–912. [Google Scholar] [CrossRef]
- Peixoto, A.; Fernandes, E.; Gaiteiro, C.; Lima, L.; Azevedo, R.; Soares, J.; Cotton, S.; Parreira, B.; Neves, M.; Amaro, T.; et al. Hypoxia enhances the malignant nature of bladder cancer cells and concomitantly antagonizes protein O-glycosylation extension. Oncotarget 2016, 7, 63138–63157. [Google Scholar] [CrossRef]
- Costa, C.; Pereira, S.; Lima, L.; Peixoto, A.; Fernandes, E.; Neves, D.; Neves, M.; Gaiteiro, C.; Tavares, A.; Gil da Costa, R.M.; et al. Abnormal Protein Glycosylation and Activated PI3K/Akt/mTOR Pathway: Role in Bladder Cancer Prognosis and Targeted Therapeutics. PLoS ONE 2015, 10, e0141253. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Neves, M.; Oliveira, M.I.; Dieguez, L.; Freitas, R.; Azevedo, R.; Gaiteiro, C.; Soares, J.; Ferreira, D.; Peixoto, A.; et al. Sialyl-Tn identifies muscle-invasive bladder cancer basal and luminal subtypes facing decreased survival, being expressed by circulating tumor cells and metastases. Urol. Oncol. 2017, 35, 675. [Google Scholar] [CrossRef] [PubMed]
- Itzkowitz, S.H.; Bloom, E.J.; Kokal, W.A.; Modin, G.; Hakomori, S.; Kim, Y.S. Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer 1990, 66, 1960–1966. [Google Scholar] [CrossRef]
- Mihalache, A.; Delplanque, J.F.; Ringot-Destrez, B.; Wavelet, C.; Gosset, P.; Nunes, B.; Groux-Degroote, S.; Leonard, R.; Robbe-Masselot, C. Structural Characterization of Mucin O-Glycosylation May Provide Important Information to Help Prevent Colorectal Tumor Recurrence. Front. Oncol. 2015, 5, 217. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Fan, C.; Fan, S.; Liu, F.; Wen, T.; An, G.; Feng, G. Expression profile of mucin-associated sialyl-Tn antigen in Chinese patients with different colorectal lesions (adenomas, carcinomas). Int. J. Clin. Exp. Pathol. 2015, 8, 11549–11554. [Google Scholar] [PubMed]
- Nakagoe, T.; Sawai, T.; Tuji, T.; Jibiki, M.; Nanashima, A.; Yamaguchi, H.; Yasutake, T.; Ayabe, H.; Matuo, T.; Tagawa, Y. Prognostic value of expression of sialosyl-Tn antigen in colorectal carcinoma and transitional mucosa. Dig. Dis. Sci. 2002, 47, 322–330. [Google Scholar] [CrossRef]
- Marathe, D.D.; Chandrasekaran, E.V.; Lau, J.T.; Matta, K.L.; Neelamegham, S. Systems-level studies of glycosyltransferase gene expression and enzyme activity that are associated with the selectin binding function of human leukocytes. FASEB J. 2008, 22, 4154–4167. [Google Scholar] [CrossRef]
- Vazquez-Martin, C.; Cuevas, E.; Gil-Martin, E.; Fernandez-Briera, A. Correlation analysis between tumor-associated antigen sialyl-Tn expression and ST6GalNAc I activity in human colon adenocarcinoma. Oncology 2004, 67, 159–165. [Google Scholar] [CrossRef]
- An, Y.; Han, W.; Chen, X.; Zhao, X.; Lu, D.; Feng, J.; Yang, D.; Song, L.; Yan, X. A novel anti-sTn monoclonal antibody 3P9 Inhibits human xenografted colorectal carcinomas. J. Immunother. 1997, 36, 20–28. [Google Scholar] [CrossRef]
- Neves, M.; Azevedo, R.; Lima, L.; Oliveira, M.I.; Peixoto, A.; Ferreira, D.; Soares, J.; Fernandes, E.; Gaiteiro, C.; Palmeira, C.; et al. Exploring sialyl-Tn expression in microfluidic-isolated circulating tumour cells: A novel biomarker and an analytical tool for precision oncology applications. New Biotechnol. 2019, 49, 77–87. [Google Scholar] [CrossRef]
- Ogawa, T.; Hirohashi, Y.; Murai, A.; Nishidate, T.; Okita, K.; Wang, L.; Ikehara, Y.; Satoyoshi, T.; Usui, A.; Kubo, T.; et al. ST6GALNAC1 plays important roles in enhancing cancer stem phenotypes of colorectal cancer via the Akt pathway. Oncotarget 2017, 8, 112550–112564. [Google Scholar] [CrossRef] [PubMed]
- Akita, K.; Yoshida, S.; Ikehara, Y.; Shirakawa, S.; Toda, M.; Inoue, M.; Kitawaki, J.; Nakanishi, H.; Narimatsu, H.; Nakada, H. Different levels of sialyl-Tn antigen expressed on MUC16 in patients with endometriosis and ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Van Elssen, C.H.; Frings, P.W.; Bot, F.J.; Van de Vijver, K.K.; Huls, M.B.; Meek, B.; Hupperets, P.; Germeraad, W.T.; Bos, G.M. Expression of aberrantly glycosylated Mucin-1 in ovarian cancer. Histopathology 2010, 57, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Akamine, S.; Nakagoe, T.; Sawai, T.; Tsuji, T.; Tanaka, K.; Hidaka, S.; Shibasaki, S.; Nanashima, A.; Yamaguchi, H.; Nagayasu, T.; et al. Differences in prognosis of colorectal cancer patients based on the expression of sialyl Lewisa, sialyl Lewisx and sialyl Tn antigens in serum and tumor tissue. Anticancer Res. 2004, 24, 2541–2546. [Google Scholar] [PubMed]
- Ferreira, J.A.; Videira, P.A.; Lima, L.; Pereira, S.; Silva, M.; Carrascal, M.; Severino, P.F.; Fernandes, E.; Almeida, A.; Costa, C.; et al. Overexpression of tumour-associated carbohydrate antigen sialyl-Tn in advanced bladder tumours. Mol. Oncol. 2013, 7, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, C.; Costa, C.; Amaro, T.; Goncalves, M.; Lopes, P.; Freitas, R.; Gartner, F.; Amado, F.; Ferreira, J.A.; Santos, L. Patient-derived sialyl-Tn-positive invasive bladder cancer xenografts in nude mice: An exploratory model study. Anticancer Res. 2014, 34, 735–744. [Google Scholar]
- Carrascal, M.A.; Severino, P.F.; Guadalupe Cabral, M.; Silva, M.; Ferreira, J.A.; Calais, F.; Quinto, H.; Pen, C.; Ligeiro, D.; Santos, L.L.; et al. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Mol. Oncol. 2014, 8, 753–765. [Google Scholar] [CrossRef]
- Munkley, J.; Vodak, D.; Livermore, K.E.; James, K.; Wilson, B.T.; Knight, B.; McCullagh, P.; McGrath, J.; Crundwell, M.; Harries, L.W.; et al. Glycosylation is an Androgen-Regulated Process Essential for Prostate Cancer Cell Viability. EBioMedicine 2016, 8, 103–116. [Google Scholar] [CrossRef]
- Munkley, J.; Oltean, S.; Vodak, D.; Wilson, B.T.; Livermore, K.E.; Zhou, Y.; Star, E.; Floros, V.I.; Johannessen, B.; Knight, B.; et al. The androgen receptor controls expression of the cancer-associated sTn antigen and cell adhesion through induction of ST6GalNAc1 in prostate cancer. Oncotarget 2015, 6, 34358–34374. [Google Scholar] [CrossRef]
- Beum, P.V.; Singh, J.; Burdick, M.; Hollingsworth, M.A.; Cheng, P.W. Expression of core 2 beta-1,6-N-acetylglucosaminyltransferase in a human pancreatic cancer cell line results in altered expression of MUC1 tumor-associated epitopes. J. Biol. Chem. 1999, 274, 24641–24648. [Google Scholar] [CrossRef]
- Rao, C.V.; Janakiram, N.B.; Madka, V.; Kumar, G.; Scott, E.J.; Pathuri, G.; Bryant, T.; Kutche, H.; Zhang, Y.; Biddick, L.; et al. Small-Molecule Inhibition of GCNT3 Disrupts Mucin Biosynthesis and Malignant Cellular Behaviors in Pancreatic Cancer. Cancer Res. 2016, 76, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vallinas, M.; Molina, S.; Vicente, G.; Zarza, V.; Martin-Hernandez, R.; Garcia-Risco, M.R.; Fornari, T.; Reglero, G.; Ramirez de Molina, A. Expression of microRNA-15b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of Rosemary diterpenes in colon and pancreatic cancer. PLoS ONE 2014, 9, e98556. [Google Scholar] [CrossRef] [PubMed]
- St Hill, C.A.; Farooqui, M.; Mitcheltree, G.; Gulbahce, H.E.; Jessurun, J.; Cao, Q.; Walcheck, B. The high affinity selectin glycan ligand C2-O-sLex and mRNA transcripts of the core 2 beta-1,6-N-acetylglucosaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas. BMC Cancer 2009, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, K.; Nakayama, J.; Nakamura, N.; Hasebe, O.; Katsuyama, T.; Fukuda, M. Carcinoma-associated expression of core 2 beta-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: Role of O-glycans in tumor progression. Cancer Res. 1997, 57, 5201–5206. [Google Scholar] [PubMed]
- Huang, M.C.; Chen, H.Y.; Huang, H.C.; Huang, J.; Liang, J.T.; Shen, T.L.; Lin, N.Y.; Ho, C.C.; Cho, I.M.; Hsu, S.M. C2GnT-M is downregulated in colorectal cancer and its re-expression causes growth inhibition of colon cancer cells. Oncogene 2006, 25, 3267–3276. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vallinas, M.; Vargas, T.; Moreno-Rubio, J.; Molina, S.; Herranz, J.; Cejas, P.; Burgos, E.; Aguayo, C.; Custodio, A.; Reglero, G.; et al. Clinical relevance of the differential expression of the glycosyltransferase gene GCNT3 in colon cancer. Eur. J. Cancer 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Chao, C.C.; Wu, P.H.; Huang, H.C.; Chung, H.Y.; Chou, Y.C.; Cai, B.H.; Kannagi, R. Downregulation of miR-199a/b-5p is associated with GCNT2 induction upon epithelial-mesenchymal transition in colon cancer. FEBS Lett. 2017, 591, 1902–1917. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamashita, K.; Sawaki, H.; Waraya, M.; Katoh, H.; Nakayama, N.; Kawamata, H.; Nishimiya, H.; Ema, A.; Narimatsu, H.; et al. Aberrant methylation of GCNT2 is tightly related to lymph node metastasis of primary CRC. Anticancer Res. 2015, 35, 1411–1421. [Google Scholar]
- Wang, L.; Mitoma, J.; Tsuchiya, N.; Narita, S.; Horikawa, Y.; Habuchi, T.; Imai, A.; Ishimura, H.; Ohyama, C.; Fukuda, M. An A/G polymorphism of core 2 branching enzyme gene is associated with prostate cancer. Biochem. Biophys. Res. Commun. 2005, 331, 958–963. [Google Scholar] [CrossRef]
- Radhakrishnan, P.; Chachadi, V.; Lin, M.F.; Singh, R.; Kannagi, R.; Cheng, P.W. TNFalpha enhances the motility and invasiveness of prostatic cancer cells by stimulating the expression of selective glycosyl- and sulfotransferase genes involved in the synthesis of selectin ligands. Biochem. Biophys. Res. Commun. 2011, 409, 436–441. [Google Scholar] [CrossRef]
- Okamoto, T.; Yoneyama, M.S.; Hatakeyama, S.; Mori, K.; Yamamoto, H.; Koie, T.; Saitoh, H.; Yamaya, K.; Funyu, T.; Fukuda, M.; et al. Core2 O-glycan-expressing prostate cancer cells are resistant to NK cell immunity. Mol. Med. Rep. 2013, 7, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yoneyama, T.; Tobisawa, Y.; Hatakeyama, S.; Yamamoto, H.; Kojima, Y.; Mikami, J.; Mori, K.; Hashimoto, Y.; Koie, T.; et al. Core 2 beta-1, 6-N-acetylglucosaminyltransferase-1 expression in prostate biopsy specimen is an indicator of prostate cancer aggressiveness. Biochem. Biophys. Res. Commun. 2016, 470, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gulzar, Z.G.; St Hill, C.A.; Walcheck, B.; Brooks, J.D. Increased expression of GCNT1 is associated with altered O-glycosylation of PSA, PAP, and MUC1 in human prostate cancers. Prostate 2014, 74, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sutoh, M.; Hatakeyama, S.; Mori, K.; Yamamoto, H.; Koie, T.; Saitoh, H.; Yamaya, K.; Funyu, T.; Habuchi, T.; et al. MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int. J. Oncol. 2012, 40, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, S.; Sutoh, M.; Hatakeyama, S.; Hiraoka, N.; Habuchi, T.; Horikawa, Y.; Hashimoto, Y.; Yoneyama, T.; Mori, K.; Koie, T.; et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011, 30, 3173–3185. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, R.; Leon, F.; Rauth, S.; Batra, S.K.; Ponnusamy, M.P. A Systematic Review on the Implications of O-linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis. Cells 2020, 9, 446. https://doi.org/10.3390/cells9020446
Gupta R, Leon F, Rauth S, Batra SK, Ponnusamy MP. A Systematic Review on the Implications of O-linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis. Cells. 2020; 9(2):446. https://doi.org/10.3390/cells9020446
Chicago/Turabian StyleGupta, Rohitesh, Frank Leon, Sanchita Rauth, Surinder K. Batra, and Moorthy P. Ponnusamy. 2020. "A Systematic Review on the Implications of O-linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis" Cells 9, no. 2: 446. https://doi.org/10.3390/cells9020446
APA StyleGupta, R., Leon, F., Rauth, S., Batra, S. K., & Ponnusamy, M. P. (2020). A Systematic Review on the Implications of O-linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis. Cells, 9(2), 446. https://doi.org/10.3390/cells9020446





