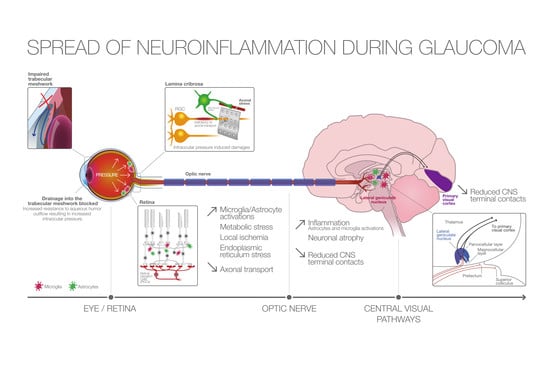
Glaucoma: A Degenerative Optic Neuropathy Related to Neuroinflammation?
Abstract
:1. Introduction
2. Glial Reactivity and Infiltration of Immune Cells in Glaucomatous Retina
2.1. Macroglial Cell Activation
2.2. Microglial Activation
2.3. Transendothelial Migration of Monocytes
3. Pro-Inflammatory Signaling Pathways in Glaucoma
3.1. Toll-Like Receptor Pathway
3.2. P2X7 Receptor
3.3. TNFα Pathway
4. Is Glaucoma a Neurodegenerative Disease?
5. Neuropathologic Study of Neuronal Degeneration in the Visual Pathways
6. Neuroimaging Cerebral Anomalies in Glaucoma
6.1. Magnetic Resonance Imaging
6.2. Emission Tomography Imaging of Glial Activation
7. Conclusions
Funding
Conflicts of Interest
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Soto, I.; Howell, G.R. The complex role of neuroinflammation in glaucoma. Cold Spring Harb. Perspect. Med. 2014, 4, a017269. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wax, M.B.; Tezel, G.; Yang, J.; Peng, G.; Patil, R.V.; Agarwal, N.; Sappington, R.M.; Calkins, D.J. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J. Neurosci. 2008, 28, 12085–12096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Yucel, Y.H. Brain changes in glaucoma. Eur. J. Ophthalmol. 2003, 13 (Suppl. 3), S32–S35. [Google Scholar] [CrossRef]
- Gupta, N.; Yucel, Y.H. Should we treat the brain in glaucoma? Can. J. Ophthalmol. 2007, 42, 409–413. [Google Scholar] [CrossRef]
- Gupta, N.; Yucel, Y.H. Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol. 2007, 18, 110–114. [Google Scholar] [CrossRef]
- Gupta, N.; Yucel, Y.H. What changes can we expect in the brain of glaucoma patients? Surv. Ophthalmol. 2007, 52 (Suppl. 2), S122–S126. [Google Scholar] [CrossRef]
- Yucel, Y.; Gupta, N. Glaucoma of the brain: A disease model for the study of transsynaptic neural degeneration. Prog. Brain Res. 2008, 173, 465–478. [Google Scholar]
- Weber, A.J.; Chen, H.; Hubbard, W.C.; Kaufman, P.L. Experimental glaucoma and cell size, density, and number in the primate lateral geniculate nucleus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1370–1379. [Google Scholar]
- Yucel, Y.H.; Zhang, Q.; Gupta, N.; Kaufman, P.L.; Weinreb, R.N. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch. Ophthalmol. 2000, 118, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.L.; Harwerth, R.S.; Smith, E.L.; Shen, F.; Carter-Dawson, L. Glaucoma in primates: Cytochrome oxidase reactivity in parvo- and magnocellular pathways. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1791–1802. [Google Scholar]
- Imamura, K.; Onoe, H.; Shimazawa, M.; Nozaki, S.; Wada, Y.; Kato, K.; Nakajima, H.; Mizuma, H.; Onoe, K.; Taniguchi, T.; et al. Molecular imaging reveals unique degenerative changes in experimental glaucoma. Neuroreport 2009, 20, 139–144. [Google Scholar] [CrossRef]
- Lam, D.Y.; Kaufman, P.L.; Gabelt, B.T.; To, E.C.; Matsubara, J.A. Neurochemical correlates of cortical plasticity after unilateral elevated intraocular pressure in a primate model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2573–2581. [Google Scholar] [CrossRef] [Green Version]
- Howell, G.R.; Soto, I.; Zhu, X.; Ryan, M.; Macalinao, D.G.; Sousa, G.L.; Caddle, L.B.; MacNicoll, K.H.; Barbay, J.M.; Porciatti, V.; et al. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J. Clin. Investig. 2012, 122, 1246–1261. [Google Scholar] [CrossRef] [Green Version]
- Nickells, R.W.; Howell, G.R.; Soto, I.; John, S.W. Under pressure: Cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu. Rev. Neurosci. 2012, 35, 153–179. [Google Scholar] [CrossRef] [Green Version]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur. J. Pharmacol. 2016, 787, 134–142. [Google Scholar] [CrossRef]
- Ridet, J.L.; Malhotra, S.K.; Privat, A.; Gage, F.H. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997, 20, 570–577. [Google Scholar] [CrossRef]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef]
- Tuccari, G.; Trombetta, C.; Giardinelli, M.M.; Arena, F.; Barresi, G. Distribution of glial fibrillary acidic protein in normal and gliotic human retina. Basic Appl. Histochem. 1986, 30, 425–432. [Google Scholar] [PubMed]
- Wang, L.; Cioffi, G.A.; Cull, G.; Dong, J.; Fortune, B. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1088–1094. [Google Scholar]
- Kanamori, A.; Nakamura, M.; Nakanishi, Y.; Yamada, Y.; Negi, A. Long-term glial reactivity in rat retinas ipsilateral and contralateral to experimental glaucoma. Exp. Eye Res. 2005, 81, 48–56. [Google Scholar] [CrossRef]
- Woldemussie, E.; Wijono, M.; Ruiz, G. Muller cell response to laser-induced increase in intraocular pressure in rats. Glia 2004, 47, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.P.; Lu, J.; Cao, Q.; Hu, S.; Ding, P.; Ling, E.A. Muller glial cells express nestin coupled with glial fibrillary acidic protein in experimentally induced glaucoma in the rat retina. Neuroscience 2006, 139, 723–732. [Google Scholar] [CrossRef]
- Inman, D.M.; Horner, P.J. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia 2007, 55, 942–953. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Lu, Q.; Qing, G.; Wang, N.; Wang, Y.; Li, S.; Yang, D.; Yan, F. Detection of early neuron degeneration and accompanying glial responses in the visual pathway in a rat model of acute intraocular hypertension. Brain Res. 2009, 1303, 131–143. [Google Scholar] [CrossRef]
- Newman, E.; Reichenbach, A. The Muller cell: A functional element of the retina. Trends Neurosci. 1996, 19, 307–312. [Google Scholar] [CrossRef]
- Derouiche, A.; Rauen, T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: Evidence for coupling of GLAST and GS in transmitter clearance. J. Neurosci. Res. 1995, 42, 131–143. [Google Scholar] [CrossRef]
- Otori, Y.; Shimada, S.; Tanaka, K.; Ishimoto, I.; Tano, Y.; Tohyama, M. Marked increase in glutamate-aspartate transporter (GLAST/GluT-1) mRNA following transient retinal ischemia. Mol. Brain Res. 1994, 27, 310–314. [Google Scholar] [CrossRef]
- Hu, B.; Yip, H.K.; So, K.F. Localization of p75 neurotrophin receptor in the retina of the adult SD rat: An immunocytochemical study at light and electron microscopic levels. Glia 1998, 24, 187–197. [Google Scholar] [CrossRef]
- Lebrun-Julien, F.; Morquette, B.; Douillette, A.; Saragovi, H.U.; Di Polo, A. Inhibition of p75(NTR) in glia potentiates TrkA-mediated survival of injured retinal ganglion cells. Mol. Cell. Neurosci. 2009, 40, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wei, Y.; Lu, Q.; Zheng, D.; Zhang, F.; Gao, E.; Wang, N. Immunohistochemical localization of sortilin and p75(NTR) in normal and ischemic rat retina. Neurosci. Lett. 2009, 454, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular signaling and factors involved in Müller cell gliosis: Neuroprotective and detrimental effects. Prog. Retin. Eye Res. 2009, 28, 423–451. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Garcia, T.B.; Pannicke, T.; Vogler, S.; Berk, B.A.; Grosche, A.; Wiedemann, P.; Seeger, J.; Reichenbach, A.; Herculano, A.M.; Bringmann, A. Nerve growth factor inhibits osmotic swelling of rat retinal glial (Müller) and bipolar cells by inducing glial cytokine release. J. Neurochem. 2014, 131, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Bringmann, A.; Uckermann, O.; Pannicke, T.; Iandiev, I.; Reichenbach, A.; Wiedemann, P. Neuronal versus glial cell swelling in the ischaemic retina. Acta Ophthalmol. Scand. 2005, 83, 528–538. [Google Scholar] [CrossRef]
- Iandiev, I.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Grimm, C.; Reme, C.E.; Reichenbach, A.; Pannicke, T.; Bringmann, A. Muller cell response to blue light injury of the rat retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3559–3567. [Google Scholar] [CrossRef] [Green Version]
- Rose, C.R.; Ransom, B.R. Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia 1997, 20, 299–307. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Miao, H.; Lukas, T. Astrocytes in glaucomatous optic neuropathy. Prog. Brain Res. 2008, 173, 353–373. [Google Scholar]
- Kerr, N.M.; Johnson, C.S.; Green, C.R.; Danesh-Meyer, H.V. Gap junction protein connexin43 (GJA1) in the human glaucomatous optic nerve head and retina. J. Clin. Neurosci. 2011, 18, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.C.; Morrison, J.C. Friend or foe? Resolving the impact of glial responses in glaucoma. J. Glaucoma 2009, 18, 341–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, A.; Raveu, A.L.; Reboussin, E.; Roubeix, C.; Boucher, C.; Degardin, J.; Godefroy, D.; Rostene, W.; Reaux-Le Goazigo, A.; Baudouin, C.; et al. Bilateral neuroinflammatory processes in visual pathways induced by unilateral ocular hypertension in the rat. J. Neuroinflamm. 2016, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Hong, J.S. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem. Soc. Trans. 2007, 35, 1127–1132. [Google Scholar] [CrossRef] [Green Version]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Streit, W.J.; Conde, J.R.; Fendrick, S.E.; Flanary, B.E.; Mariani, C.L. Role of microglia in the central nervous system’s immune response. Neurol. Res. 2005, 27, 685–691. [Google Scholar]
- Walter, L.; Neumann, H. Role of microglia in neuronal degeneration and regeneration. Semin. Immunopathol. 2009, 31, 513–525. [Google Scholar] [CrossRef]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef]
- Neufeld, A.H. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch. Ophthalmol. 1999, 117, 1050–1056. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Neufeld, A.H. Activated microglia in the human glaucomatous optic nerve head. J. Neurosci. Res. 2001, 64, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Steele, M.R.; Vetter, M.L. Early microglia activation in a mouse model of chronic glaucoma. J. Comp. Neurol. 2011, 519, 599–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Tay, S.S.; Ng, Y.K. An immunohistochemical study of neuronal and glial cell reactions in retinae of rats with experimental glaucoma. Exp. Brain Res. 2000, 132, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Ebneter, A.; Casson, R.J.; Wood, J.P.; Chidlow, G. Microglial activation in the visual pathway in experimental glaucoma: Spatiotemporal characterization and correlation with axonal injury. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6448–6460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, E.C.; Jia, L.; Cepurna, W.O.; Doser, T.A.; Morrison, J.C. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3161–3177. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Inman, D.M.; Steele, M.R.; Wu, G.; Soto, I.; Marsh-Armstrong, N.; Hubbard, W.C.; Calkins, D.J.; Horner, P.J.; Vetter, M.L. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Varano, G.P.; Parisi, V.; Adornetto, A.; Cavaliere, F.; Amantea, D.; Nucci, C.; Corasaniti, M.T.; Morrone, L.A.; Bagetta, G.; Russo, R. Post-ischemic treatment with azithromycin protects ganglion cells against retinal ischemia/reperfusion injury in the rat. Mol. Vis. 2017, 23, 911–921. [Google Scholar]
- Nakazawa, T.; Nakazawa, C.; Matsubara, A.; Noda, K.; Hisatomi, T.; She, H.; Michaud, N.; Hafezi-Moghadam, A.; Miller, J.W.; Benowitz, L.I. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J. Neurosci. 2006, 26, 12633–12641. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Peng, B.; Lin, B. Fractalkine receptor regulates microglial neurotoxicity in an experimental mouse glaucoma model. Glia 2014, 62, 1943–1954. [Google Scholar] [CrossRef]
- Tsou, C.L.; Peters, W.; Si, Y.; Slaymaker, S.; Aslanian, A.M.; Weisberg, S.P.; Mack, M.; Charo, I.F. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Investig. 2007, 117, 902–909. [Google Scholar] [CrossRef] [Green Version]
- Roh, M.; Zhang, Y.; Murakami, Y.; Thanos, A.; Lee, S.C.; Vavvas, D.G.; Benowitz, L.I.; Miller, J.W. Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS ONE 2012, 7, e40065. [Google Scholar] [CrossRef] [PubMed]
- Nikolskaya, T.; Nikolsky, Y.; Serebryiskaya, T.; Zvereva, S.; Sviridov, E.; Dezso, Z.; Rahkmatulin, E.; Brennan, R.J.; Yankovsky, N.; Bhattacharya, S.K.; et al. Network analysis of human glaucomatous optic nerve head astrocytes. BMC Med. Genom. 2009, 2, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Yang, X.; Kain, A.D.; Powell, D.W.; Kuehn, M.H.; Tezel, G. Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5697–5707. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Yang, X.; Luo, C.; Cai, J.; Powell, D.W. An astrocyte-specific proteomic approach to inflammatory responses in experimental rat glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4220–4233. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, C.; Cai, J.; Powell, D.W.; Yu, D.; Kuehn, M.H.; Tezel, G. Neurodegenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8442–8454. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Barakat, D.; Brambilla, R.; Agudelo, C.; Hernandez, E.; Bethea, J.R.; Shestopalov, V.I.; Ivanov, D. Inactivation of astroglial NF-kappa B promotes survival of retinal neurons following ischemic injury. Eur. J. Neurosci. 2009, 30, 175–185. [Google Scholar] [CrossRef]
- North, R.A. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Sakamoto, K.; Endo, K.; Suzuki, T.; Fujimura, K.; Kurauchi, Y.; Mori, A.; Nakahara, T.; Ishii, K. P2X7 receptor antagonists protect against N-methyl-d-aspartic acid-induced neuronal injury in the rat retina. Eur. J. Pharmacol. 2015, 756, 52–58. [Google Scholar] [CrossRef]
- Ishii, K.; Kaneda, M.; Li, H.; Rockland, K.S.; Hashikawa, T. Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina. J. Comp. Neurol. 2003, 459, 267–277. [Google Scholar] [CrossRef]
- Puthussery, T.; Fletcher, E.L. Synaptic Localization of P2X7 Receptors in the Rat Retina. J. Comp. Neurol. 2004, 472, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Puthussery, T.; Yee, P.; Vingrys, A.J.; Fletcher, E.L. Evidence for the involvement of purinergic P2X7 receptors in outer retinal processing. Eur. J. Neurosci. 2006, 24, 7–19. [Google Scholar] [CrossRef]
- Vessey, K.A.; Fletcher, E.L. Rod and Cone Pathway Signalling Is Altered in the P2X7 Receptor Knock Out Mouse. PLoS ONE 2012, 7, e29990. [Google Scholar] [CrossRef] [PubMed]
- Wheeler-Schilling, T.H.; Marquordt, K.; Kohler, K.; Guenther, E.; Jabs, R. Identification of purinergic receptors in retinal ganglion cells. Mol. Brain Res. 2001, 92, 177–180. [Google Scholar] [CrossRef]
- Franke, H.; Klimke, K.; Brinckmann, U.; Grosche, J.; Francke, M.; Sperlagh, B.; Reichenbach, A.; Liebert, U.G.; Illes, P. P2X7 receptor-mRNA and -protein in the mouse retina; changes during retinal degeneration in BALBCrds mice. Neurochem. Int. 2005, 47, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lu, W.; Zhang, M.; Zhang, X.; Argall, A.J.; Patel, S.; Lee, G.E.; Kim, Y.-C.; Jacobson, K.A.; Laties, A.M.; et al. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp. Eye Res. 2010, 91, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reigada, D.; Lu, W.; Zhang, M.; Mitchell, C.H. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience 2008, 157, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Resta, V.; Novelli, E.; Di Virgilio, F.; Galli-Resta, L. Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development 2005, 132, 2873–2882. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Lim, J.C.; Lu, W.; Beckel, J.M.; Macarak, E.J.; Laties, A.M.; Mitchell, C.H. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J. Physiol. 2012, 590, 2285–2304. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Zhang, X.; Zheng, D.; Ge, J.; Laties, A.M.; Mitchell, C.H. Sustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucoma. Exp. Eye Res. 2011, 93, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, T.; Lee, S.Y.; Horie, T.; Oku, H.; Takai, S.; Tanioka, H.; Kuriki, Y.; Kojima, S.; Ikeda, T. P2X7 receptor activation may be involved in neuronal loss in the retinal ganglion cell layer after acute elevation of intraocular pressure in rats. Mol. Vis. 2013, 19, 2080–2091. [Google Scholar] [PubMed]
- Zhang, X.; Li, A.; Ge, J.; Reigada, D.; Laties, A.M.; Mitchell, C.H. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp. Eye Res. 2007, 85, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Niyadurupola, N.; Sidaway, P.; Ma, N.; Rhodes, J.D.; Broadway, D.C.; Sanderson, J. P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2163–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez de Lara, M.J.; Avilés-Trigueros, M.; Guzmán-Aránguez, A.; Valiente-Soriano, F.J.; de la Villa, P.; Vidal-Sanz, M.; Pintor, J. Potential role of P2X7 receptor in neurodegenerative processes in a murine model of glaucoma. Brain Res. Bull. 2019, 150, 61–74. [Google Scholar] [CrossRef]
- Resta, V.; Novelli, E.; Vozzi, G.; Scarpa, C.; Caleo, M.; Ahluwalia, A.; Solini, A.; Santini, E.; Parisi, V.; Di Virgilio, F.; et al. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur. J. Neurosci. 2007, 25, 2741–2754. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Fukuchi, T.; Tanaka, T.; Abe, H. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 903–906. [Google Scholar] [CrossRef]
- Huang, P.; Qi, Y.; Xu, Y.S.; Liu, J.; Liao, D.; Zhang, S.S.; Zhang, C. Serum cytokine alteration is associated with optic neuropathy in human primary open angle glaucoma. J. Glaucoma 2010, 19, 324–330. [Google Scholar] [CrossRef]
- Tezel, G.; Li, L.Y.; Patil, R.V.; Wax, M.B. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1787–1794. [Google Scholar]
- Yan, X.; Tezel, G.; Wax, M.B.; Edward, D.P. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch. Ophthalmol. 2000, 118, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Neufeld, A.H. Tumor necrosis factor-alpha: A potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia 2000, 32, 42–50. [Google Scholar] [CrossRef]
- Madigan, M.C.; Sadun, A.A.; Rao, N.S.; Dugel, P.U.; Tenhula, W.N.; Gill, P.S. Tumor necrosis factor-alpha (TNF-alpha)-induced optic neuropathy in rabbits. Neurol. Res. 1996, 18, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Yang, X.; Yang, J.; Wax, M.B. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004, 996, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Mesci, L.; Irkec, M.; Ozdag, B.B.; Sanal, O.; Arslan, U.; Ersoy, F.; Tezcan, I. Association of tumour necrosis factor-alpha -308 G/A polymorphism with primary open-angle glaucoma. Clin. Exp. Ophthalmol. 2012, 40, e156–e162. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.J.; Liu, K.; Wang, D.Y.; Tham, C.C.; Tam, P.O.; Lam, D.S.; Pang, C.P. Association of polymorphisms of tumor necrosis factor and tumor protein p53 with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4110–4116. [Google Scholar] [CrossRef] [Green Version]
- Dengler-Crish, C.M.; Smith, M.A.; Inman, D.M.; Wilson, G.N.; Young, J.W.; Crish, S.D. Anterograde transport blockade precedes deficits in retrograde transport in the visual projection of the DBA/2J mouse model of glaucoma. Front. Neurosci. 2014, 8, 290. [Google Scholar] [CrossRef] [Green Version]
- Dekeyster, E.; Aerts, J.; Valiente-Soriano, F.J.; De Groef, L.; Vreysen, S.; Salinas-Navarro, M.; Vidal-Sanz, M.; Arckens, L.; Moons, L. Ocular hypertension results in retinotopic alterations in the visual cortex of adult mice. Curr. Eye Res. 2015, 40, 1269–1283. [Google Scholar] [CrossRef]
- Lam, D.; Jim, J.; To, E.; Rasmussen, C.; Kaufman, P.L.; Matsubara, J. Astrocyte and microglial activation in the lateral geniculate nucleus and visual cortex of glaucomatous and optic nerve transected primates. Mol. Vis. 2009, 15, 2217–2229. [Google Scholar]
- Sasaoka, M.; Nakamura, K.; Shimazawa, M.; Ito, Y.; Araie, M.; Hara, H. Changes in visual fields and lateral geniculate nucleus in monkey laser-induced high intraocular pressure model. Exp. Eye Res. 2008, 86, 770–782. [Google Scholar] [CrossRef]
- Mirzaei, M.; Gupta, V.B.; Chick, J.M.; Greco, T.M.; Wu, Y.; Chitranshi, N.; Wall, R.V.; Hone, E.; Deng, L.; Dheer, Y.; et al. Age-related neurodegenerative disease associated pathways identified in retinal and vitreous proteome from human glaucoma eyes. Sci. Rep. 2017, 7, 12685. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; Lopez-Cuenca, I.; Rojas, P.; Trivino, A.; Ramirez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harwerth, R.S.; Quigley, H.A. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch. Ophthalmol. 2006, 124, 853–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, N.; Hedley-Whyte, E.T.; Dreyer, E.B. Lateral geniculate nucleus in glaucoma. Am. J. Ophthalmol. 1993, 116, 182–188. [Google Scholar] [CrossRef]
- Gupta, N.; Ang, L.C.; Noel de Tilly, L.; Bidaisee, L.; Yucel, Y.H. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br. J. Ophthalmol. 2006, 90, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Vickers, J.C.; Hof, P.R.; Schumer, R.A.; Wang, R.F.; Podos, S.M.; Morrison, J.H. Magnocellular and parvocellular visual pathways are both affected in a macaque monkey model of glaucoma. Aust. N. Z. J. Ophthalmol. 1997, 25, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Yucel, Y.H.; Zhang, Q.; Weinreb, R.N.; Kaufman, P.L.; Gupta, N. Atrophy of relay neurons in magno- and parvocellular layers in the lateral geniculate nucleus in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3216–3222. [Google Scholar]
- Liu, M.; Guo, L.; Salt, T.E.; Cordeiro, M.F. Dendritic changes in rat visual pathway associated with experimental ocular hypertension. Curr. Eye Res. 2014, 39, 953–963. [Google Scholar] [CrossRef]
- Iba-Zizen, M.T.; Istoc, A.; Cabanis, E.A. The results of MRI exploration of glaucoma patients: What are the benefits? J. Fr. Ophtalmol. 2008, 31, 2S24–2S28. [Google Scholar]
- Dai, H.; Mu, K.T.; Qi, J.P.; Wang, C.Y.; Zhu, W.Z.; Xia, L.M.; Chen, Z.Q.; Zhang, H.; Ai, F.; Morelli, J.N. Assessment of lateral geniculate nucleus atrophy with 3T MR imaging and correlation with clinical stage of glaucoma. AJNR Am. J. Neuroradiol. 2011, 32, 1347–1353. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Greenberg, G.; de Tilly, L.N.; Gray, B.; Polemidiotis, M.; Yucel, Y.H. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br. J. Ophthalmol. 2009, 93, 56–60. [Google Scholar] [CrossRef]
- Hernowo, A.T.; Boucard, C.C.; Jansonius, N.M.; Hooymans, J.M.; Cornelissen, F.W. Automated morphometry of the visual pathway in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2758–2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Xie, L.; Dai, C.; Xie, B.; Liang, M.; Zhao, L.; Yin, X.; Wang, J. Progressive thinning of visual cortex in primary open-angle glaucoma of varying severity. PLoS ONE 2015, 10, e0121960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelhorn, T.; Michelson, G.; Waerntges, S.; Struffert, T.; Haider, S.; Doerfler, A. Diffusion tensor imaging detects rarefaction of optic radiation in glaucoma patients. Acad. Radiol. 2011, 18, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Garaci, F.G.; Bolacchi, F.; Cerulli, A.; Melis, M.; Spano, A.; Cedrone, C.; Floris, R.; Simonetti, G.; Nucci, C. Optic nerve and optic radiation neurodegeneration in patients with glaucoma: In vivo analysis with 3-T diffusion-tensor MR imaging. Radiology 2009, 252, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zikou, A.K.; Kitsos, G.; Tzarouchi, L.C.; Astrakas, L.; Alexiou, G.A.; Argyropoulou, M.I. Voxel-based morphometry and diffusion tensor imaging of the optic pathway in primary open-angle glaucoma: A preliminary study. AJNR Am. J. Neuroradiol. 2012, 33, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Boucard, C.C.; Hernowo, A.T.; Maguire, R.P.; Jansonius, N.M.; Roerdink, J.B.; Hooymans, J.M.; Cornelissen, F.W. Changes in cortical grey matter density associated with long-standing retinal visual field defects. Brain 2009, 132, 1898–1906. [Google Scholar] [CrossRef]
- Li, C.; Cai, P.; Shi, L.; Lin, Y.; Zhang, J.; Liu, S.; Xie, B.; Shi, Y.; Yang, H.; Li, S.; et al. Voxel-based morphometry of the visual-related cortex in primary open angle glaucoma. Curr. Eye Res. 2012, 37, 794–802. [Google Scholar] [CrossRef]
- Dai, H.; Morelli, J.N.; Ai, F.; Yin, D.; Hu, C.; Xu, D.; Li, Y. Resting-state functional MRI: Functional connectivity analysis of the visual cortex in primary open-angle glaucoma patients. Hum. Brain Mapp. 2013, 34, 2455–2463. [Google Scholar] [CrossRef]
- Shimazawa, M.; Ito, Y.; Inokuchi, Y.; Yamanaka, H.; Nakanishi, T.; Hayashi, T.; Ji, B.; Higuchi, M.; Suhara, T.; Imamura, K.; et al. An alteration in the lateral geniculate nucleus of experimental glaucoma monkeys: In vivo positron emission tomography imaging of glial activation. PLoS ONE 2012, 7, e30526. [Google Scholar] [CrossRef] [Green Version]
- Wilson, G.N.; Inman, D.M.; Dengler Crish, C.M.; Smith, M.A.; Crish, S.D. Early pro-inflammatory cytokine elevations in the DBA/2J mouse model of glaucoma. J. Neuroinflamm. 2015, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Rapino, C.; Tortolani, D.; Scipioni, L.; Maccarrone, M. Neuroprotection by (endo)Cannabinoids in Glaucoma and Retinal Neurodegenerative Diseases. Curr. Neuropharmacol. 2018, 16, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Fellabaum, C.; Arsenijevic, A.; Markovic, B.S.; Djonov, V.; Volarevic, V. Therapeutic Potential of Mesenchymal Stem Cells and Their Secretome in the Treatment of Glaucoma. Stem Cells Int. 2019, 2019, 7869130. [Google Scholar] [CrossRef] [PubMed]
- Adornetto, A.; Russo, R.; Parisi, V. Neuroinflammation as a target for glaucoma therapy. Neural Regen. Res. 2019, 14, 391–394. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mélik Parsadaniantz, S.; Réaux-le Goazigo, A.; Sapienza, A.; Habas, C.; Baudouin, C. Glaucoma: A Degenerative Optic Neuropathy Related to Neuroinflammation? Cells 2020, 9, 535. https://doi.org/10.3390/cells9030535
Mélik Parsadaniantz S, Réaux-le Goazigo A, Sapienza A, Habas C, Baudouin C. Glaucoma: A Degenerative Optic Neuropathy Related to Neuroinflammation? Cells. 2020; 9(3):535. https://doi.org/10.3390/cells9030535
Chicago/Turabian StyleMélik Parsadaniantz, Stéphane, Annabelle Réaux-le Goazigo, Anaïs Sapienza, Christophe Habas, and Christophe Baudouin. 2020. "Glaucoma: A Degenerative Optic Neuropathy Related to Neuroinflammation?" Cells 9, no. 3: 535. https://doi.org/10.3390/cells9030535
APA StyleMélik Parsadaniantz, S., Réaux-le Goazigo, A., Sapienza, A., Habas, C., & Baudouin, C. (2020). Glaucoma: A Degenerative Optic Neuropathy Related to Neuroinflammation? Cells, 9(3), 535. https://doi.org/10.3390/cells9030535