Valproic Acid Promotes Early Neural Differentiation in Adult Mesenchymal Stem Cells Through Protein Signalling Pathways
Abstract
:1. Introduction
2. Methods
2.1. Cell Culture
2.2. Chemical Induction for Differentiation
2.3. Cell Harvesting Sample Preparation
2.4. Alkaline Phosphatase Activity Assay
2.5. Cytotoxicity Assay
2.6. Cytokine and Chemokine Bioplex Analysis
2.7. Cell Lysate Protein Extraction Sample Preparation
2.8. Liquid Chromatography-Tandem Mass Spectrometry
2.9. Mass Spectrometry, Protein Identification and Data Analysis
3. Results
3.1. Live Cell Temporal Microscopy during Neurogenic Induction Differentiation of Human ADSCs
3.2. Cytokine and Chemokine Bioplex Analysis
3.3. Proteome Comparisons of VPA Treated Cells
3.4. Pathway Analysis of Gene Ontology Clustered Proteins
4. Discussion
4.1. Cellular Morphology
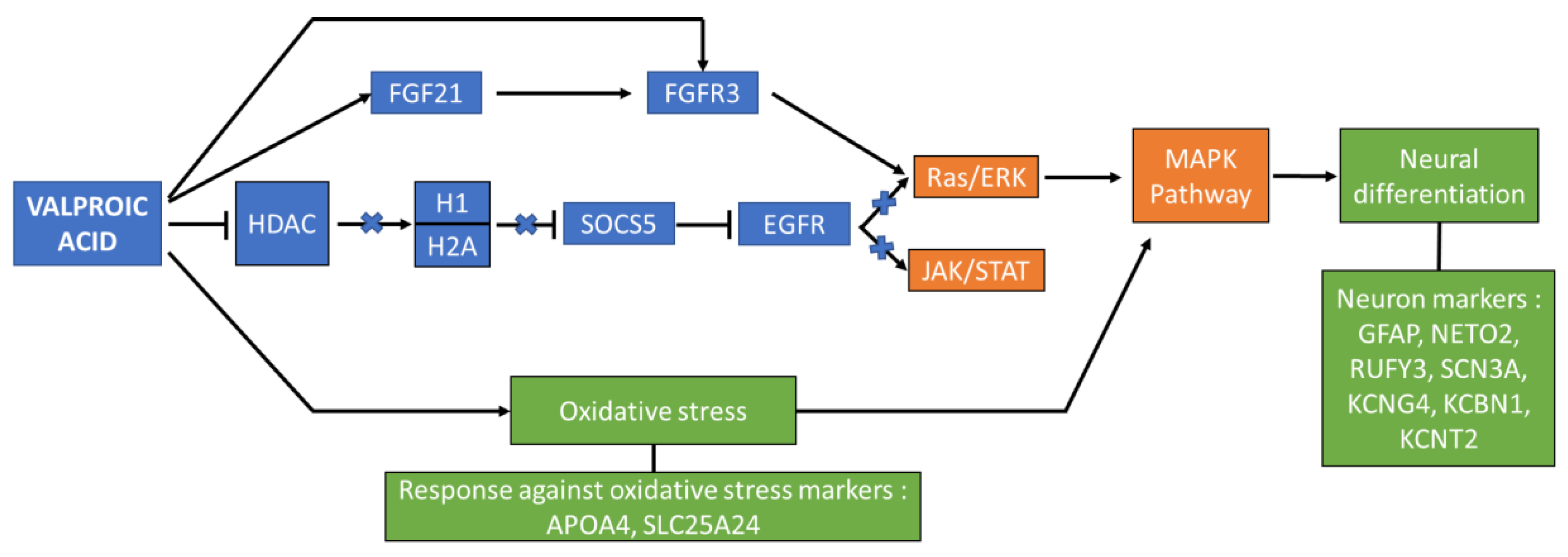
4.2. Secreted Molecules Role in Signalling Pathways Controlling Neural Differentiation
4.3. Protein Expression and Interaction Pathways Affected by VPA Treatment
4.4. The Oxidative Stress Role in Neural Differentiation Activation
4.5. Functional Roles of Identified Neural Proteins in VPA Treated MSCs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | Adipose Derived Stem Cells |
| ALP | Alkaline phosphatase |
| AVIL | Advillin |
| BHA | butylated hydroxyanisole |
| BME | beta-mercaptoethanol |
| DMEM | Dulbecco’s Modified Eagle’s Medium: Nutrient Mixture F-12 |
| DMSO | dimethylsulfoxide |
| ERK | Extracellular Receptor Kinase |
| FGF21 | Fibroblast growth factor 21 |
| FGFR3 | Fibroblast growth factor receptor 3 |
| GFAP | Glial fibrillary acidic protein |
| hADSCs | human Adipose Derived Stem Cells |
| HDAC | histone deacetylase |
| JAK | Janus kinase |
| JAK3 | Tyrosine-protein kinase JAK3 |
| KCNB1 | Potassium voltage-gated channel subfamily B member 1 |
| KCNG4 | Potassium voltage-gated channel subfamily G member 4 |
| KCNT2 | Potassium channel subfamily T member 2 |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| MAPK1 | Mitogen-activated protein kinase |
| NETO2 | Neuropilin and tolloid-like protein 2 |
| PBS | Phosphate-buffered saline |
| RA | Retinoic acid |
| SLC25A24 | Calcium-binding mitochondrial carrier protein SCaMC-1 |
| SLC8A3 | Sodium/calcium exchanger 3 |
| STAT1 | Signal transducer and activator of transcription 1-alpha/beta |
| STAT6 | Signal transducer and activator of transcription 6 |
| VPA | Valproic Acid |
References
- Franco Lambert, A.P.; Fraga Zandonai, A.; Bonatto, D.; Cantarelli Machado, D.; Pegas Henriques, J.A. Differentiation of human adipose-derived adult stem cells into neuronal tissue: Does it work? Differ. Res. Biol. Divers. 2009, 77, 221–228. [Google Scholar] [CrossRef]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef]
- Santos, J.; Milthorpe, B.K.; Herbert, B.R.; Padula, M.P. Proteomic Analysis of Human Adipose Derived Stem Cells during Small Molecule Chemical Stimulated Pre-neuronal Differentiation. Int. J. Stem Cells 2017. [Google Scholar] [CrossRef] [Green Version]
- Kondo, T.; Johnson, S.A.; Yoder, M.C.; Romand, R.; Hashino, E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4789–4794. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.M.; Bunnell, B.A.; Kang, S.K. Neural differentiation of human adipose tissue-derived stem cells. Methods Mol. Biol. (Clifton, N.J.) 2011, 702, 219–231. [Google Scholar] [CrossRef]
- Mu, M.W.; Zhao, Z.Y.; Li, C.G. Comparative study of neural differentiation of bone marrow mesenchymal stem cells by different induction methods. Genet. Mol. Res. GMR 2015, 14, 14169–14176. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Milthorpe, B.K.; Padula, M.P. Proteomic Analysis of Cyclic Ketamine Compounds Ability to Induce Neural Differentiation in Human Adult Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talwadekar, M.; Fernandes, S.; Kale, V.; Limaye, L. Valproic acid enhances the neural differentiation of human placenta derived-mesenchymal stem cells in vitro. J. Tissue Eng. Regen. Med. 2017, 11, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, V.; Qin, N.; Balyura, M.; Eisenhofer, G.; Wong, M.L.; Licinio, J.; Bornstein, S.R.; Ehrhart-Bornstein, M. Valproic acid enhances neuronal differentiation of sympathoadrenal progenitor cells. Mol. Psychiatry 2015, 20, 941–950. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef]
- Gottlicher, M.; Minucci, S.; Zhu, P.; Kramer, O.H.; Schimpf, A.; Giavara, S.; Sleeman, J.P.; Lo Coco, F.; Nervi, C.; Pelicci, P.G.; et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001, 20, 6969–6978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, I.T.; Park, J.-Y.; Kim, S.H.; Lee, J.-S.; Kim, Y.-S.; Son, H. Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation. Neuropharmacology 2009, 56, 473–480. [Google Scholar] [CrossRef]
- Hsieh, J.; Nakashima, K.; Kuwabara, T.; Mejia, E.; Gage, F.H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16659–16664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.S.; Chopp, M.; Kassis, H.; Jia, L.F.; Hozeska-Solgot, A.; Zhang, R.L.; Chen, C.; Cui, Y.S.; Zhang, Z.G. Valproic acid increases white matter repair and neurogenesis after stroke. Neuroscience 2012, 220, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, F.; Tiraihi, T.; Abdanipour, A.; Hassoun, H.K.; Taheri, T. Immunocytochemical analysis of valproic acid induced histone H3 and H4 acetylation during differentiation of rat adipose derived stem cells into neuron-like cells. Biotech. Histochem. 2018, 93, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Olszewski, M.; Huang, W.; Kletzel, M. Neural cell differentiation in vitro from adult human bone marrow mesenchymal stem cells. Stem Cells Dev. 2005, 14, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Mori, T.; Maruyama, K.; Zahir, T.; Ward, M.; Umezawa, A.; Young, M.J. A comparison of neural differentiation and retinal transplantation with bone marrow-derived cells and retinal progenitor cells. Stem Cells (Dayton, Ohio) 2006, 24, 2270–2278. [Google Scholar] [CrossRef]
- Galindo, L.T.; Filippo, T.R.M.; Semedo, P.; Ariza, C.B.; Moreira, C.M.; Camara, N.O.S.; Porcionatto, M.A. Mesenchymal Stem Cell Therapy Modulates the Inflammatory Response in Experimental Traumatic Brain Injury. Neurol. Res. Int. 2011, 2011, 564089. [Google Scholar] [CrossRef] [Green Version]
- Štefková, K.; Procházková, J.; Pacherník, J. Alkaline phosphatase in stem cells. Stem Int. 2015, 2015, 628368. [Google Scholar] [CrossRef] [Green Version]
- Hanna, H.; Mir, L.M.; Andre, F.M. In vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res. Ther. 2018, 9, 203. [Google Scholar] [CrossRef]
- Taverner, T.; Karpievitch, Y.V.; Polpitiya, A.D.; Brown, J.N.; Dabney, A.R.; Anderson, G.A.; Smith, R.D. DanteR: An extensible R-based tool for quantitative analysis of -omics data. Bioinformatics 2012, 28, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Lu, P.; Blesch, A.; Tuszynski, M.H. Induction of bone marrow stromal cells to neurons: Differentiation, transdifferentiation, or artifact? J. Neurosci. Res. 2004, 77, 174–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tan, J.; Xing, Y.; Kan, N.; Ling, J.; Dong, G.; Liu, G.; Chen, H. p43 induces IP-10 expression through the JAK-STAT signaling pathway in HMEC-1 cells. Int. J. Mol. Med. 2016, 38, 1217–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moshapa, F.T.; Riches-Suman, K.; Palmer, T.M. Therapeutic Targeting of the Proinflammatory IL-6-JAK/STAT Signalling Pathways Responsible for Vascular Restenosis in Type 2 Diabetes Mellitus. Cardiol. Res. Pract. 2019, 2019, 9846312. [Google Scholar] [CrossRef] [Green Version]
- Jatiani, S.S.; Baker, S.J.; Silverman, L.R.; Reddy, E.P. JAK/STAT Pathways in Cytokine Signaling and Myeloproliferative Disorders: Approaches for Targeted Therapies. Genes Cancer 2010, 1, 979–993. [Google Scholar] [CrossRef] [Green Version]
- Rezaie, P.; Trillo-Pazos, G.; Everall, I.P.; Male, D.K. Expression of beta-chemokines and chemokine receptors in human fetal astrocyte and microglial co-cultures: Potential role of chemokines in the developing CNS. Glia 2002, 37, 64–75. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.-I.; Kim, S.U. Human Astrocytes: Secretome Profiles of Cytokines and Chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Yuan, W.; Liu, Y.; Zhang, Y.; Wang, Z.; Zhou, X.; Ning, G.; Zhang, L.; Yao, L.; Feng, S.; et al. The role of the JAK-STAT pathway in neural stem cells, neural progenitor cells and reactive astrocytes after spinal cord injury. Biomed. Rep. 2015, 3, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Boehme, M.; Guenther, M.; Stahr, A.; Liebmann, M.; Jaenisch, N.; Witte, O.W.; Frahm, C. Impact of indomethacin on neuroinflammation and hippocampal neurogenesis in aged mice. Neurosci. Lett. 2014, 572, 7–12. [Google Scholar] [CrossRef]
- Koo, J.W.; Duman, R.S. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. USA 2008, 105, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Price, J.; Modo, M. Effect of Inflammatory Cytokines on Major Histocompatibility Complex Expression and Differentiation of Human Neural Stem/Progenitor Cells. Stem Cells (Dayton, Ohio) 2008, 26, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Xu, D.; Lan, X.; Jia, B.; Sun, L.; Zheng, J.C.; Peng, H. A Novel Role of the STAT3 Pathway in Brain Inflammation-induced Human Neural Progenitor Cell Differentiation. Curr. Mol. Med. 2013, 13, 1474–1484. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.; Zhang, T.; Fong, S.L.; Lim, C.P.; Cao, X. Repression of Stat3 activity by activation of mitogen-activated protein kinase (MAPK). Oncogene 1998, 17, 3157–3167. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Kim, M.-S.; Kim, W.; Kang, M.A.; Cacalano, N.A.; Kang, S.-B.; Shin, Y.-J.; Jeong, J.-H. Suppressor of Cytokine Signaling (SOCS) Genes Are Silenced by DNA Hypermethylation and Histone Deacetylation and Regulate Response to Radiotherapy in Cervical Cancer Cells. PLoS ONE 2015, 10, e0123133. [Google Scholar] [CrossRef]
- Cooney, R.N. Suppressors of cytokine signaling (SOCS): Inhibitors of the JAK/STAT pathway. Shock (Augusta, Ga.) 2002, 17, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Nickl, C.; Kang, H.; Winter, S.S.; Cannon, J.; Matlawska-Wasowska, K. SOCS5 Regulates JAK-STAT Signaling and T-ALL Development. Blood 2017, 130, 370. [Google Scholar]
- Henson, E.S.; Gibson, S.B. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: Implications for cancer therapy. Cell. Signal. 2006, 18, 2089–2097. [Google Scholar] [CrossRef]
- Bonni, A.; Sun, Y.; Nadal-Vicens, M.; Bhatt, A.; Frank, D.A.; Rozovsky, I.; Stahl, N.; Yancopoulos, G.D.; Greenberg, M.E. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 1997, 278, 477–483. [Google Scholar] [CrossRef]
- Kario, E.; Marmor, M.D.; Adamsky, K.; Citri, A.; Amit, I.; Amariglio, N.; Rechavi, G.; Yarden, Y. Suppressors of cytokine signaling 4 and 5 regulate epidermal growth factor receptor signaling. J. Biol. Chem. 2005, 280, 7038–7048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, E.W.; Winn, L.M. Valproic acid increases formation of reactive oxygen species and induces apoptosis in postimplantation embryos: A role for oxidative stress in valproic acid-induced neural tube defects. Mol. Pharmacol. 2011, 80, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-F.; Gu, S.; Shan, C.; Marchado, S.; Arias-Carrión, O. Oxidative Stress and Adult Neurogenesis. Stem Cell Rev. Rep. 2015, 11, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Fujimoto, S.; Hayashi, D.; Suzuki, T.; Sakaue, M.; Miyazaki, Y.; Tanaka, K.; Usami, M.; Takizawa, T. Valproic acid promotes mature neuronal differentiation of adipose tissue-derived stem cells through iNOS–NO–sGC signaling pathway. Nitric Oxide 2019, 93, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Han, E.-S.; Muller, F.L.; Pérez, V.I.; Qi, W.; Liang, H.; Xi, L.; Fu, C.; Doyle, E.; Hickey, M.; Cornell, J.; et al. The in vivo gene expression signature of oxidative stress. Physiol. Genom. 2008, 34, 112–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traba, J.; Del Arco, A.; Duchen, M.R.; Szabadkai, G.; Satrustegui, J. SCaMC-1 promotes cancer cell survival by desensitizing mitochondrial permeability transition via ATP/ADP-mediated matrix Ca(2+) buffering. Cell Death Differ. 2012, 19, 650–660. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2018, 47, D419–D426. [Google Scholar] [CrossRef]
- Kurata, S.-I. Selective Activation of p38 MAPK Cascade and Mitotic Arrest Caused by Low Level Oxidative Stress. J. Biol. Chem. 2000, 275, 23413–23416. [Google Scholar] [CrossRef] [Green Version]
- Leng, Y.; Wang, J.; Wang, Z.; Liao, H.M.; Wei, M.; Leeds, P.; Chuang, D.M. Valproic Acid and Other HDAC Inhibitors Upregulate FGF21 Gene Expression and Promote Process Elongation in Glia by Inhibiting HDAC2 and 3. Int. J. Neuropsychopharmacol. 2016, 19. [Google Scholar] [CrossRef]
- Kuroda, M.; Muramatsu, R.; Maedera, N.; Koyama, Y.; Hamaguchi, M.; Fujimura, H.; Yoshida, M.; Konishi, M.; Itoh, N.; Mochizuki, H.; et al. Peripherally derived FGF21 promotes remyelination in the central nervous system. J. Clin. Investig. 2017, 127, 3496–3509. [Google Scholar] [CrossRef] [Green Version]
- Iwata, T.; Hevner, R.F. Fibroblast growth factor signaling in development of the cerebral cortex. Dev. Growth Differ. 2009, 51, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Shahror, R.A.; Linares, G.R.; Wang, Y.; Hsueh, S.C.; Wu, C.C.; Chuang, D.M.; Chiang, Y.H.; Chen, K.Y. Transplantation of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 Facilitates Cognitive Recovery and Enhances Neurogenesis in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2019. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.D.; Gauthier, A.S. Timing Is Everything: Making Neurons versus Glia in the Developing Cortex. Neuron 2007, 54, 357–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Sun, M.; Liu, X.; Zhang, J.; Jin, Y. Rufy3, a protein specifically expressed in neurons, interacts with actin-bundling protein Fascin to control the growth of axons. J. Neurochem. 2014, 130, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Vernon, C.G.; Swanson, G.T. Neto2 Assembles with Kainate Receptors in DRG Neurons during Development and Modulates Neurite Outgrowth in Adult Sensory Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 3352–3363. [Google Scholar] [CrossRef] [Green Version]
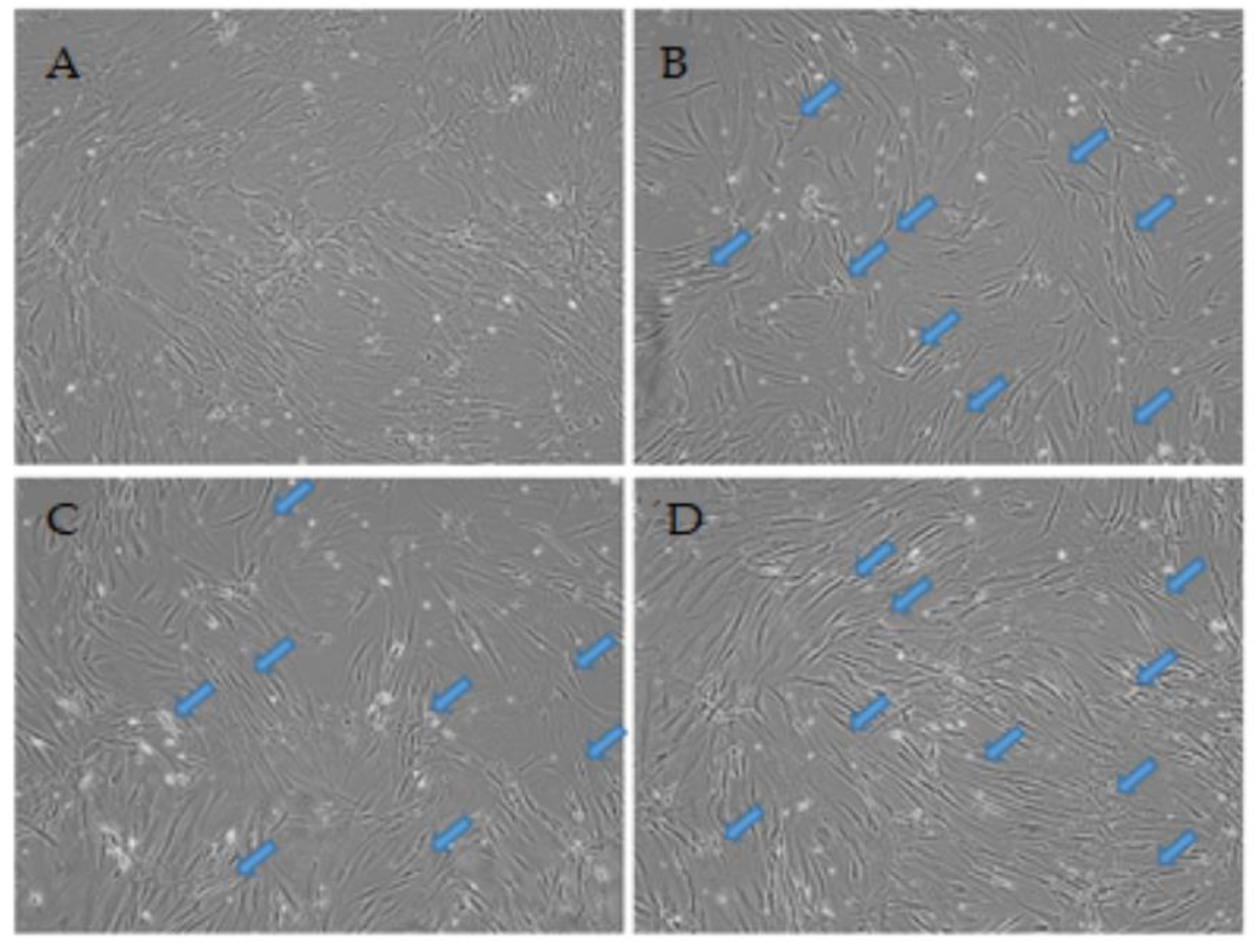
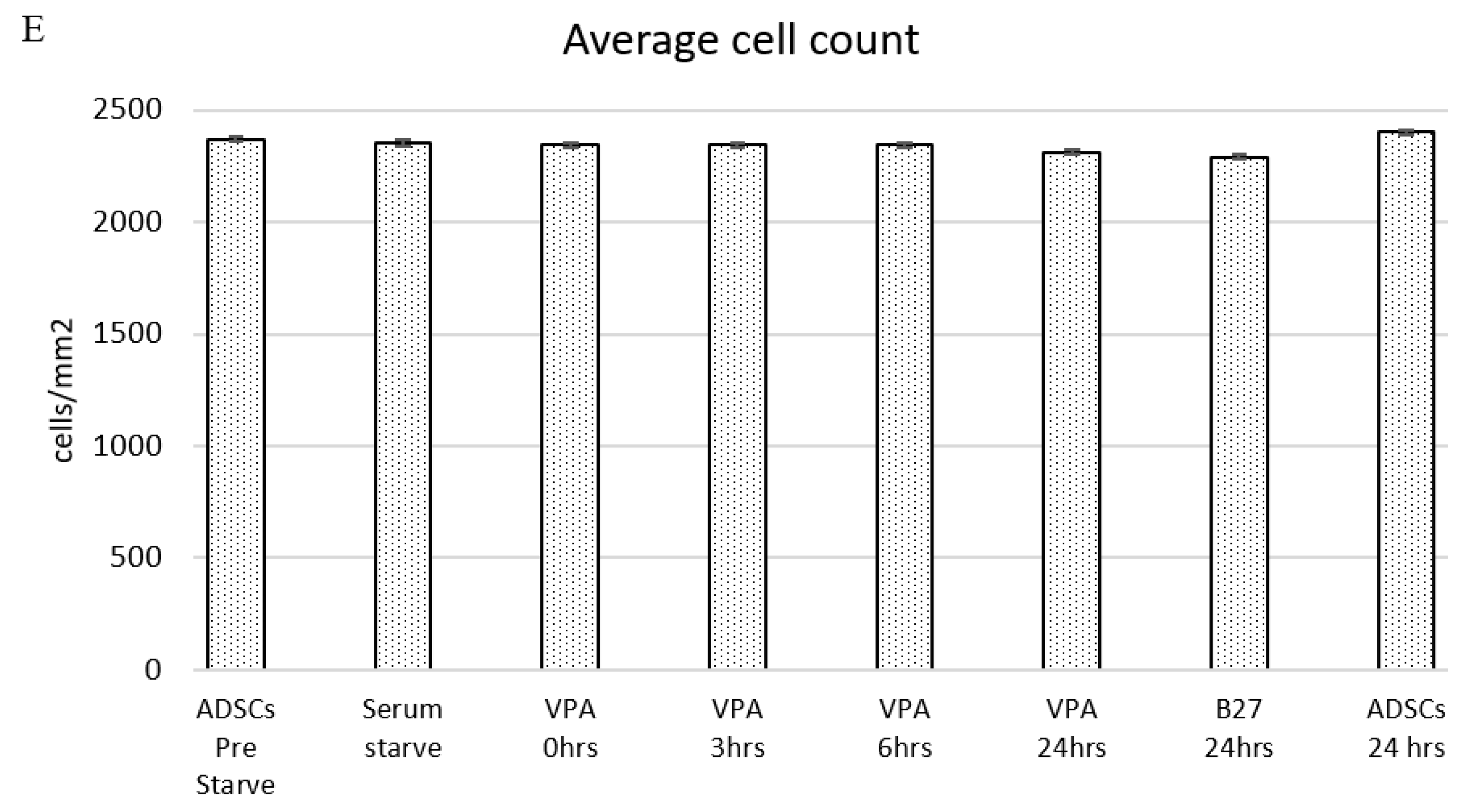

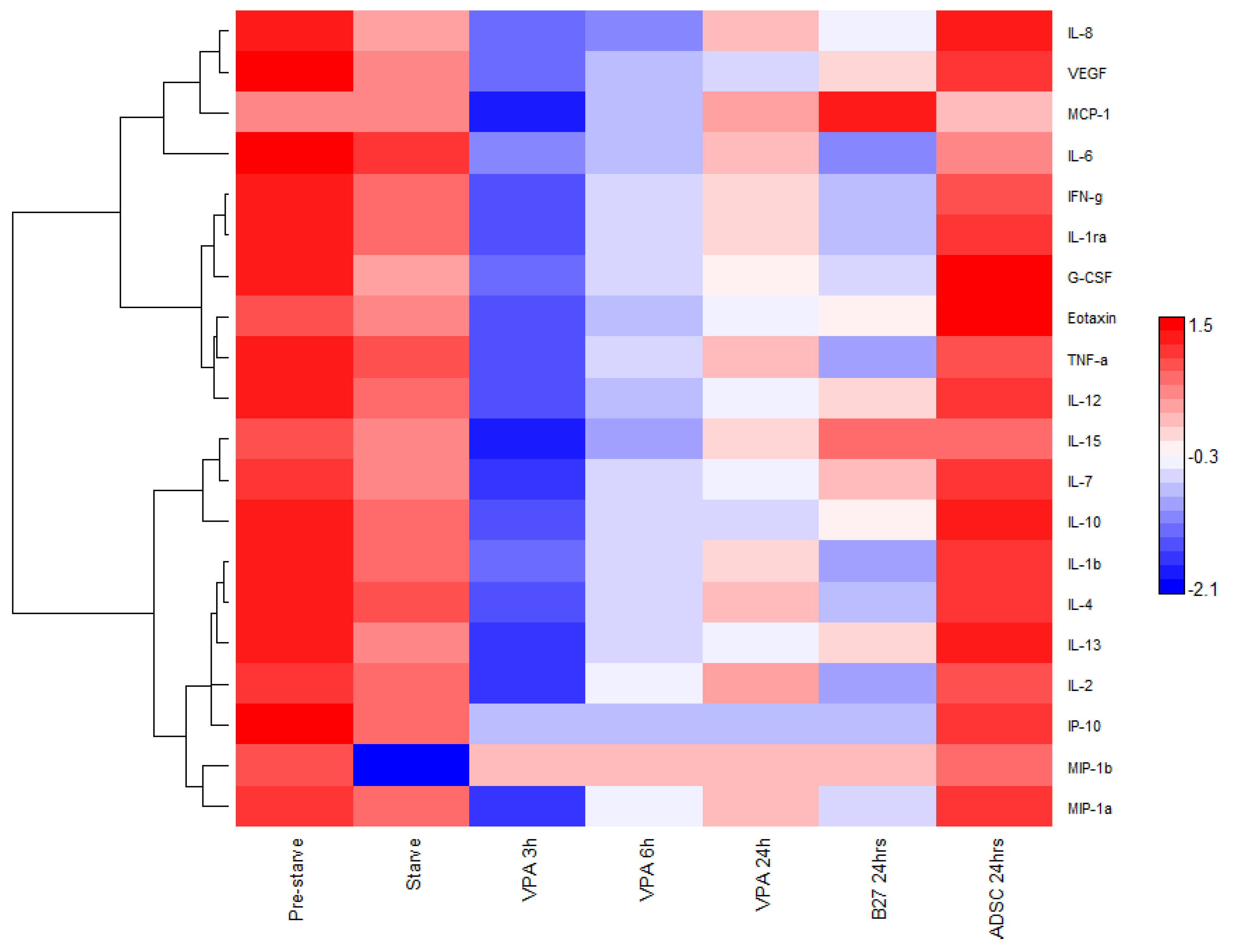

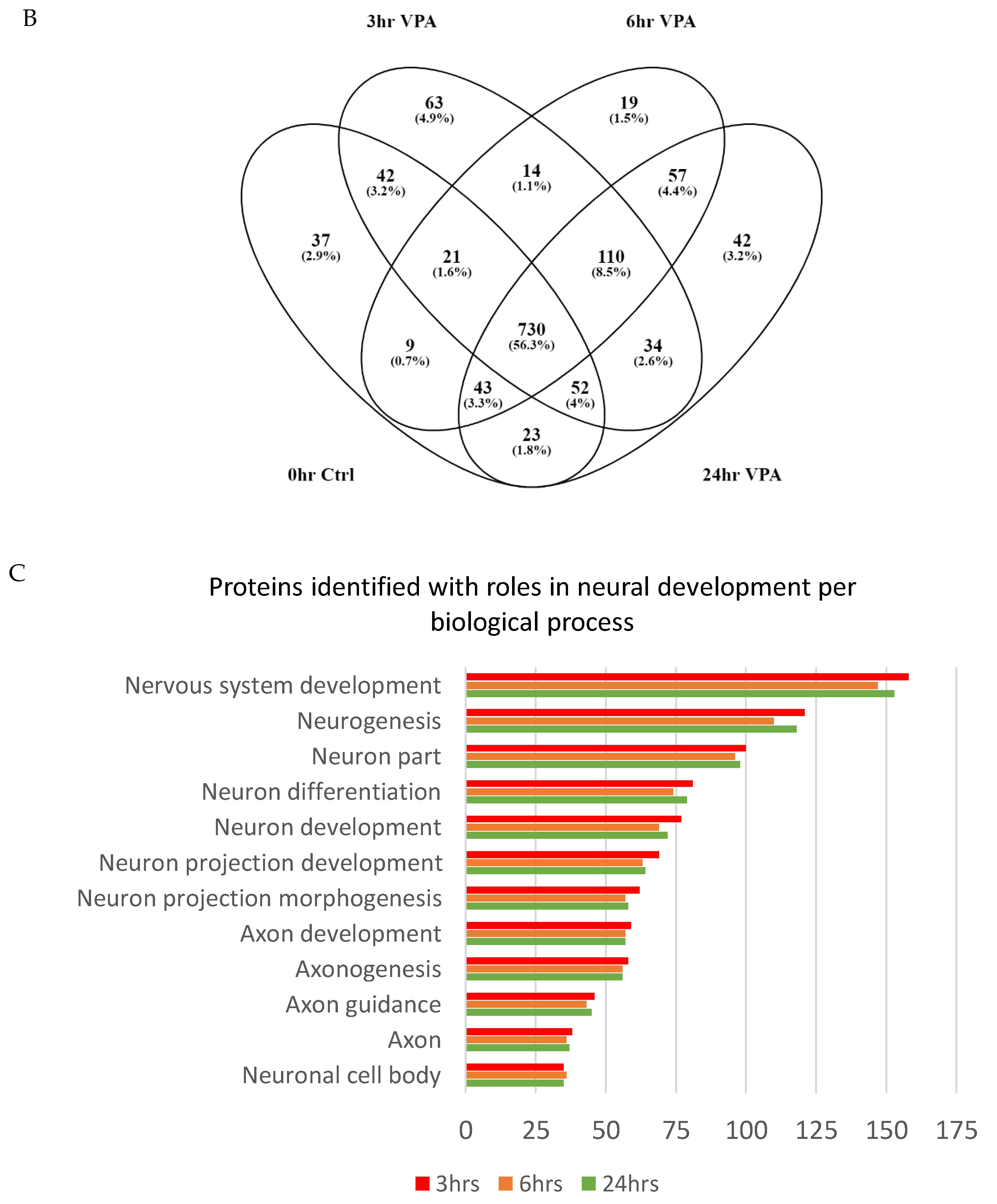

| Confidence Cut-off | Proteins Detected | Proteins Before Grouping | Distinct Peptides | Spectra Identified | %Total Spectra |
|---|---|---|---|---|---|
| >2.0 (99) | 622 | 765 | 8286 | 313,119 | 66.8 |
| >1.3 (95) | 2067 | 2269 | 20,011 | 344,510 | 67.7 |
| >0.47 (66) | 2334 | 2460 | 34,492 | 406,322 | 68.5 |
| GO Term | GO:ID | Number of Proteins | Associated Proteins Found | Percentage of Associated Proteins in GO | Term P-Value |
|---|---|---|---|---|---|
| organelle transport along microtubule | GO:0072384 | 5 | [CDC42, LAMP1, SUN1, SYNE2, UCHL1] | 4.72 | 9.03 × 10−3 |
| regulation of NMDA receptor activity | GO:2000310 | 3 | [DLG1, HSPA8, MAPK1] | 5.56 | 2.70 × 10−2 |
| response to fibroblast growth factor | GO:0071774 | 9 | [CCN2, FGF21, FGF3, FGFR3, MAPK1, POSTN, PTPN11, TFAP2C, THBS1] | 4.95 | 3.59 × 10−4 |
| membrane assembly | GO:0071709 | 6 | [ANXA2, CAV1, FLOT1, MAPK1, PDCD6IP, SPTBN1] | 11.54 | 3.57 × 10−5 |
| cell-substrate junction assembly | GO:0007044 | 14 | [BMX, CTTN, FN1, FYN, LAMB2, LRP1, PLEC, PPM1F, PTPRK, RAC1, RHOA, ROCK1, THBS1, THY1] | 10.22 | 1.08 × 10−9 |
| transforming growth factor beta production | GO:0071604 | 3 | [ITGAV, MTCO2P12, THBS1] | 5.88 | 2.32 × 10−2 |
| regulation of myelination | GO:0031641 | 3 | [DLG1, SOS1, TYMP] | 6.12 | 2.09 × 10−2 |
| positive regulation of cell projection organization | GO:0031346 | 24 | [AP2A1, ARPC2, BMX, CDC42, DDX21, FN1, FSCN1, FYN, HSPA5, HSPA8, ITGA3, KDM1A, LAP3, LRP1, NME2, PDCD6IP, PICALM, PSEN1, RAB21, RAC1, RHOA, SCARB2, SOS1, TMEM30A] | 4.60 | 1.90 × 10−8 |
| cellular response to nerve growth factor stimulus | GO:1990090 | 3 | [ARF6, HSPA5, PDCD6IP] | 4.05 | 5.94 × 10−2 |
| cellular response to epidermal growth factor stimulus | GO:0071364 | 5 | [EGFR, GSTP1, PDCD6IP, PTPN11, TFAP2C] | 6.33 | 2.60 × 10−3 |
| neuron projection regeneration | GO:0031102 | 5 | [GFAP, LAMB2, LRP1, SOS1, THY1] | 6.94 | 1.73 × 10−3 |
| action potential | GO:0001508 | 8 | [CACNB3, CAMK2D, CAV1, DLG1, KCNB1, MYH14, SCN2A, SCN3A] | 4.68 | 1.09 × 10−3 |
| regulation of receptor signalling pathway via STAT | GO:1904892 | 8 | [CAV1, FGFR3, FYN, HGS, IL3, JAK3, KLK3, TFRC] | 4.26 | 1.99 × 10−3 |
| neurotransmitter uptake | GO:0001504 | 3 | [FLOT1, GFAP, PSEN1] | 4.92 | 3.69 × 10−2 |
| cellular response to fibroblast growth factor stimulus | GO:0044344 | 9 | [CCN2, FGF21, FGF3, FGFR3, MAPK1, POSTN, PTPN11, TFAP2C, THBS1] | 5.26 | 2.26 × 10−4 |
| cellular response to amyloid-beta | GO:1904646 | 5 | [FYN, ICAM1, LRP1, PARP1, PSEN1] | 8.77 | 5.98 × 10−4 |
| midbrain development | GO:0030901 | 8 | [ACTB, CALM2, CDC42, HSPA5, PICALM, POTEF, RHOA, SOS1] | 6.15 | 1.75 × 10−4 |
| response to epidermal growth factor | GO:0070849 | 6 | [EGFR, GSTP1, MAPK1, PDCD6IP, PTPN11, TFAP2C] | 7.14 | 5.19 × 10−4 |
| regulation of neuron projection regeneration | GO:0070570 | 3 | [LRP1, SOS1, THY1] | 7.14 | 1.39 × 10−2 |
| regulation of axon extension | GO:0030516 | 5 | [CTTN, FN1, LRP1, RAB21, RTN4] | 4.46 | 1.13 × 10−2 |
| negative regulation of ERK1 and ERK2 cascade | GO:0070373 | 6 | [DLG1, EIF3A, GSTP1, MAPK1, PDCD6IP, TIMP3] | 6.19 | 1.11 × 10−3 |
| negative regulation of neuron apoptotic process | GO:0043524 | 8 | [BAX, FYN, LRP1, MSH2, PSEN1, RHOA, ROCK1, TFRC] | 4.04 | 2.74 × 10−3 |
| regulation of ERK1 and ERK2 cascade | GO:0070372 | 18 | [CCN2, DLG1, EGFR, EIF3A, FGF21, FGFR3, FN1, GNAI2, GSTP1, ICAM1, LRP1, MAPK1, MIF, PDCD6IP, PPP3CA, PTPN11, TFRC, TIMP3] | 4.46 | 1.94 × 10−6 |
| regulation of neuron apoptotic process | GO:0043523 | 11 | [BAX, FYN, KCNB1, LRP1, MSH2, PARP1, PCSK9, PSEN1, RHOA, ROCK1, TFRC] | 4.03 | 4.76 × 10−4 |
| ERK1 and ERK2 cascade | GO:0070371 | 20 | [CCN2, DLG1, EGFR, EIF3A, FGF21, FGFR3, FN1, GNAI2, GSTP1, ICAM1, ITGAV, LRP1, MAPK1, MIF, PDCD6IP, PPP3CA, PTPN11, TFAP2C, TFRC, TIMP3] | 4.56 | 3.60 × 10−7 |
| glial cell activation | GO:0061900 | 3 | [EGFR, LRP1, PSEN1] | 4.62 | 4.32 × 10−2 |
| telencephalon glial cell migration | GO:0022030 | 4 | [PDCD6IP, RTN4, SUN1, SYNE2] | 9.30 | 1.72 × 10−3 |
| telencephalon cell migration | GO:0022029 | 7 | [EGFR, PDCD6IP, PSEN1, RHOA, RTN4, SUN1, SYNE2] | 7.69 | 1.12 × 10−4 |
| cerebral cortex development | GO:0021987 | 11 | [ATIC, BAX, EGFR, KDM1A, LRP1, PDCD6IP, PSEN1, RHOA, RTN4, SUN1, SYNE2] | 6.63 | 5.33 × 10−6 |
| cerebral cortex neuron differentiation | GO:0021895 | 3 | [HPRT1, NCOA3, PSEN1] | 7.89 | 1.05 × 10−2 |
| forebrain cell migration | GO:0021885 | 7 | [EGFR, PDCD6IP, PSEN1, RHOA, RTN4, SUN1, SYNE2] | 7.45 | 1.37 × 10−4 |
| cell morphogenesis involved in differentiation | GO:0000904 | 37 | [ARPC2, BMX, CALM2, CAMK2A, CDC42, CTTN, FAM129B, FLOT1, FN1, FYN, GRB7, HPRT1, HSP90AA1, ITGAV, KRT7, LAMB2, LRP1, MAPK1, MYH9, NCOA3, PICALM, POSTN, PPP3CA, PSEN1, PTPN11, RAB21, RAC1, RB1, RHOA, ROCK1, RTN4, SOS1, SPTBN1, THY1, TUBB3, UCHL1, VAMP3] | 4.03 | 7.39 × 10−11 |
| cell motility involved in cerebral cortex radial glia guided migration | GO:0021814 | 3 | [PDCD6IP, SUN1, SYNE2] | 18.75 | 8.49 × 10−4 |
| cerebral cortex radial glia guided migration | GO:0021801 | 4 | [PDCD6IP, RTN4, SUN1, SYNE2] | 9.30 | 1.72 × 10−3 |
| cerebral cortex radially oriented cell migration | GO:0021799 | 4 | [PDCD6IP, RTN4, SUN1, SYNE2] | 7.84 | 3.24 × 10−3 |
| cerebral cortex cell migration | GO:0021795 | 7 | [EGFR, PDCD6IP, PSEN1, RHOA, RTN4, SUN1, SYNE2] | 9.46 | 2.95 × 10−5 |
| glial cell development | GO:0021782 | 7 | [EGFR, GFAP, GSTP1, LAMB2, LRP1, PSEN1, VTA1] | 4.67 | 2.25 × 10−3 |
| dendritic spine development | GO:0060996 | 6 | [ARF4, ARF6, CAMK2A, CDC42, MAPK1, PSEN1] | 4.58 | 5.02 × 10−3 |
| substantia nigra development | GO:0021762 | 7 | [ACTB, CALM2, CDC42, HSPA5, PICALM, POTEF, RHOA] | 10.45 | 1.53 × 10−5 |
| pallium development | GO:0021543 | 13 | [ALK, ATIC, ATP2B4, BAX, EGFR, KDM1A, LRP1, PDCD6IP, PSEN1, RHOA, RTN4, SUN1, SYNE2] | 5.31 | 8.59 × 10−6 |
| telencephalon development | GO:0021537 | 15 | [ALK, ATIC, ATP2B4, BAX, EGFR, HPRT1, KDM1A, LRP1, MAPK1, PDCD6IP, PSEN1, RHOA, RTN4, SUN1, SYNE2] | 4.34 | 1.97 × 10−5 |
| epidermal growth factor-activated receptor activity | GO:0005006 | 4 | [EFEMP1, EGFR, PSEN1, SOCS5] | 9.76 | 1.44 × 10−3 |
| NMDA glutamate receptor activity | GO:0004972 | 3 | [DLG1, HSPA8, MAPK1] | 5.26 | 3.10 × 10−2 |
| neuron maturation | GO:0042551 | 3 | [KDM1A, NCOA3, RB1] | 4.41 | 4.83 × 10−2 |
| ionotropic glutamate receptor activity | GO:0004970 | 4 | [DLG1, HSPA8, MAPK1, NETO2] | 4.44 | 2.31 × 10−2 |
| activation of MAPKK activity | GO:0000186 | 5 | [EGFR, MAPK1, PDCD6IP, PSEN1, TFRC] | 4.63 | 9.75 × 10−3 |
| MAP kinase kinase activity | GO:0004708 | 5 | [EGFR, MAPK1, PDCD6IP, PSEN1, TFRC] | 4.03 | 1.69 × 10−2 |
| Wnt signalling pathway, planar cell polarity pathway | GO:0060071 | 11 | [AP2A1, AP2A2, AP2B1, CDC42, PDCD6IP, PSMA1, PSMA3, PSMB9, PSMD4, RAC1, RHOA] | 8.09 | 7.54 × 10−7 |
| Bergmann glial cell differentiation | GO:0060020 | 3 | [GFAP, MAPK1, PTPN11] | 33.33 | 1.36 × 10−4 |
| dendrite development | GO:0016358 | 14 | [ARF4, ARF6, CALM2, CAMK2A, CDC42, FYN, HPRT1, MAPK1, PDCD6IP, PICALM, PPP3CA, PSEN1, RAB21, RHOA] | 4.52 | 2.39 × 10−5 |
| neural retina development | GO:0003407 | 3 | [ATP2B4, PSEN1, THY1] | 4.05 | 5.94 × 10−2 |
| neurotransmitter biosynthetic process | GO:0042136 | 6 | [ATP2B4, CAV1, HSP90AA1, ICAM1, MTCO2P12, RAC1] | 4.84 | 3.84 × 10−3 |
| Wnt signalling pathway | GO:0016055 | 25 | [AP2A1, AP2A2, AP2B1, CALM2, CAMK2A, CAV1, CDC42, CHD8, CTNND1, EGFR, G3BP1, ITGA3, LRP1, PDCD6IP, PICALM, PLCB3, PPP3CA, PRKAA1, PSEN1, PSMA1, PSMA3, PSMB9, PSMD4, RAC1, RHOA] | 4.05 | 1.10 × 10−7 |
| gliogenesis | GO:0042063 | 18 | [ANXA1, EGFR, GFAP, GSTP1, KRT7, LAMB2, LRP1, MAPK1, PDCD6IP, PSEN1, PSMD4, PTPN11, RELA, RHOA, RTN4, SUN1, SYNE2, VTA1] | 4.81 | 6.45 × 10−7 |
| regulation of epidermal growth factor receptor signalling pathway | GO:0042058 | 9 | [CDC42, EGFR, EPN1, HGS, PDCD6IP, PSEN1, RAB7A, SOCS5, SOS1] | 7.63 | 1.26 × 10−5 |
| neurotrophin signalling pathway | GO:0038179 | 4 | [CTNND1, PDCD6IP, PTPN11, SOS1] | 6.45 | 6.53 × 10−3 |
| ERBB2 signalling pathway | GO:0038128 | 4 | [EGFR, GRB7, HSP90AA1, SOS1] | 9.52 | 1.58 × 10−3 |
| regulation of neurotransmitter uptake | GO:0051580 | 3 | [FLOT1, GFAP, PSEN1] | 12.50 | 2.86 × 10−3 |
| p38MAPK cascade | GO:0038066 | 4 | [DLG1, PDCD6IP, SOS1, TFAP2C] | 6.56 | 6.16 × 10−3 |
| neuron apoptotic process | GO:0051402 | 14 | [BAX, FYN, HSPA5, KCNB1, LRP1, MSH2, PARP1, PCSK9, PSEN1, RB1, RHOA, ROCK1, SCN2A, TFRC] | 4.50 | 2.47 × 10−5 |
| positive regulation of neuron death | GO:1901216 | 7 | [BAX, CALM2, FYN, PARP1, PCSK9, PICALM, RHOA] | 5.98 | 5.27 × 10−4 |
| Schwann cell differentiation | GO:0014037 | 3 | [LAMB2, RELA, VTA1] | 6.00 | 2.21 × 10−2 |
| astrocyte development | GO:0014002 | 5 | [EGFR, GFAP, LAMB2, LRP1, PSEN1] | 8.33 | 7.58 × 10−4 |
| regulation of ERBB signalling pathway | GO:1901184 | 10 | [CDC42, EGFR, EPN1, HGS, PDCD6IP, PSEN1, RAB7A, RTN4, SOCS5, SOS1] | 7.87 | 3.09 × 10−6 |
| negative regulation of neuron projection development | GO:0010977 | 9 | [ARF6, GFAP, LRP1, MAPK1, PPP3CA, PSEN1, RHOA, RTN4, THY1] | 4.59 | 6.15 × 10−4 |
| positive regulation of neuron projection development | GO:0010976 | 18 | [AP2A1, BMX, DDX21, FN1, FYN, HSPA5, HSPA8, ITGA3, KDM1A, LRP1, NME2, PDCD6IP, PSEN1, RAB21, RHOA, SCARB2, SOS1, TMEM30A] | 4.75 | 7.81 × 10−7 |
| regulation of p38MAPK cascade | GO:1900744 | 3 | [DLG1, PDCD6IP, SOS1] | 6.00 | 2.21 × 10−2 |
| regulation of glutamate receptor signalling pathway | GO:1900449 | 5 | [DLG1, FYN, HSPA8, MAPK1, NETO2] | 5.49 | 4.79 × 10−3 |
| non-canonical Wnt signalling pathway | GO:0035567 | 16 | [AP2A1, AP2A2, AP2B1, CALM2, CAMK2A, CDC42, PDCD6IP, PICALM, PLCB3, PPP3CA, PSMA1, PSMA3, PSMB9, PSMD4, RAC1, RHOA] | 8.56 | 9.57 × 10−10 |
| negative regulation of cell morphogenesis involved in differentiation | GO:0010771 | 7 | [MAPK1, POSTN, PPP3CA, PSEN1, RHOA, RTN4, THY1] | 5.69 | 7.11 × 10−4 |
| positive regulation of cell morphogenesis involved in differentiation | GO:0010770 | 8 | [ARPC2, CDC42, FN1, LRP1, NCOA3, RAB21, RAC1, RHOA] | 4.12 | 2.41 × 10−3 |
| negative regulation of axonogenesis | GO:0050771 | 5 | [MAPK1, PSEN1, RHOA, RTN4, THY1] | 5.32 | 5.49 × 10−3 |
| positive regulation of plasma membrane bounded cell projection assembly | GO:0120034 | 7 | [ARPC2, CDC42, FN1, FSCN1, LAP3, PICALM, RAC1] | 4.64 | 2.33 × 10−3 |
| neuron projection fasciculation | GO:0106030 | 3 | [BMX, MAPK1, RTN4] | 8.57 | 8.40 × 10−3 |
| neuron projection organization | GO:0106027 | 6 | [CDC42, CTTN, FYN, ITGA3, MAPK1, PSEN1] | 4.62 | 4.84 × 10−3 |
| neural nucleus development | GO:0048857 | 7 | [ACTB, CALM2, CDC42, HSPA5, PICALM, POTEF, RHOA] | 7.69 | 1.12 × 10−4 |
| regulation of neurotransmitter receptor activity | GO:0099601 | 5 | [ATXN2, DLG1, HSPA8, MAPK1, NETO2] | 4.72 | 9.03 × 10−3 |
| dendrite morphogenesis | GO:0048813 | 9 | [CALM2, CAMK2A, CDC42, FYN, HPRT1, MAPK1, PICALM, PPP3CA, RAB21] | 4.48 | 7.36 × 10−4 |
| postsynapse organization | GO:0099173 | 12 | [ACTB, ARF4, ARF6, CDC42, CTTN, DLG1, FYN, ITGA3, MAPK1, PDCD6IP, POTEF, VTA1] | 5.22 | 2.26 × 10−5 |
| astrocyte differentiation | GO:0048708 | 8 | [EGFR, GFAP, KRT7, LAMB2, LRP1, MAPK1, PSEN1, PTPN11] | 7.27 | 5.41 × 10−5 |
| regulation of postsynaptic membrane neurotransmitter receptor levels | GO:0099072 | 5 | [AP2B1, CTNND1, DLG1, HSP90AA1, HSPA8] | 5.56 | 4.57 × 10−3 |
| response to axon injury | GO:0048678 | 5 | [ARF4, BAX, GNAI2, LAMB2, LRP1] | 5.15 | 9.03 × 10−3 |
| axon extension | GO:0048675 | 7 | [CTTN, FN1, HSP90AA1, LAMB2, LRP1, RAB21, RTN4] | 4.70 | 2.70 × 10−2 |
| regulation of action potential | GO:0098900 | 5 | [CACNB3, CAMK2D, CAV1, DLG1, KCNB1] | 7.04 | 3.59 × 10−4 |
| thymus development | GO:0048538 | 3 | [MAPK1, PRKDC, PSEN1] | 4.92 | 3.57 × 10−5 |
| synaptic vesicle endocytosis | GO:0048488 | 6 | [ACTB, ARF6, CALM2, PICALM, POTEF, ROCK1] | 8.33 | 1.08 × 10−9 |
| glial cell differentiation | GO:0010001 | 13 | [EGFR, GFAP, GSTP1, KRT7, LAMB2, LRP1, MAPK1, PSEN1, PSMD4, PTPN11, RELA, RHOA, VTA1] | 4.74 | 2.32 × 10−2 |
| regulation of fibroblast proliferation | GO:0048145 | 10 | [ANXA2, BAX, EGFR, FN1, GSTP1, MIF, PDCD6IP, PML, PPP3CA, PRKDC] | 8.06 | 2.09 × 10−2 |
| receptor signalling pathway via STAT | GO:0097696 | 10 | [CAV1, FGFR3, FYN, HGS, IL3, JAK3, KLK3, SOCS5, STAT1, TFRC] | 4.67 | 1.90 × 10−8 |
| astrocyte activation | GO:0048143 | 3 | [EGFR, LRP1, PSEN1] | 9.38 | 5.94 × 10−2 |
| calcium ion transmembrane import into cytosol | GO:0097553 | 9 | [ATP2B4, BAX, CALM2, CAMK2D, FYN, PICALM, PLCB3, TFRC, THY1] | 4.46 | 2.60 × 10−3 |
| neurotrophin TRK receptor signalling pathway | GO:0048011 | 3 | [CTNND1, PTPN11, SOS1] | 6.38 | 1.73 × 10−3 |
| dendritic spine organization | GO:0097061 | 5 | [CDC42, CTTN, FYN, ITGA3, MAPK1] | 4.39 | 1.09 × 10−3 |
| fibroblast growth factor receptor signalling pathway | GO:0008543 | 8 | [CCN2, FGF21, FGF3, FGFR3, MAPK1, POSTN, PTPN11, THBS1] | 6.15 | 1.99 × 10−3 |
| glial cell migration | GO:0008347 | 5 | [LRP1, PDCD6IP, RTN4, SUN1, SYNE2] | 6.58 | 3.69 × 10−2 |
| regulation of receptor signalling pathway via JAK-STAT | GO:0046425 | 8 | [CAV1, FGFR3, FYN, HGS, IL3, JAK3, KLK3, TFRC] | 4.37 | 2.26 × 10−4 |
| neuron recognition | GO:0008038 | 3 | [BMX, MAPK1, RTN4] | 4.55 | 5.98 × 10−4 |
| neuron migration | GO:0001764 | 9 | [BAX, CAMK2A, ELP3, FYN, ITGA3, NAV1, PDCD6IP, PSEN1, RAC1] | 4.39 | 1.75 × 10−4 |
| endoderm development | GO:0007492 | 5 | [BPTF, FN1, ITGAV, LAMB2, TFAP2C] | 5.62 | 5.19 × 10−4 |
| axonal fasciculation | GO:0007413 | 3 | [BMX, MAPK1, RTN4] | 8.57 | 1.39 × 10−2 |
| axonogenesis | GO:0007409 | 24 | [BMX, CALM2, CTTN, FAM129B, FLOT1, FN1, FYN, GRB7, HSP90AA1, LAMB2, LRP1, MAPK1, PICALM, PSEN1, PTPN11, RAB21, RAC1, RHOA, RTN4, SOS1, SPTBN1, THY1, TUBB3, UCHL1] | 4.05 | 1.13 × 10−2 |
| tyrosine phosphorylation of STAT protein | GO:0007260 | 5 | [CAV1, FGFR3, FYN, IL3, JAK3] | 4.72 | 1.11 × 10−3 |
| receptor signalling pathway via JAK-STAT | GO:0007259 | 10 | [CAV1, FGFR3, FYN, HGS, IL3, JAK3, KLK3, SOCS5, STAT1, TFRC] | 4.81 | 2.74 × 10−3 |
| Wnt signalling pathway, calcium modulating pathway | GO:0007223 | 5 | [CALM2, CAMK2A, PICALM, PLCB3, PPP3CA] | 9.80 | 1.94 × 10−6 |
| glutamate receptor signalling pathway | GO:0007215 | 6 | [DLG1, FYN, HSPA8, KCNB1, MAPK1, NETO2] | 4.62 | 4.76 × 10−4 |
| epidermal growth factor receptor signalling pathway | GO:0007173 | 15 | [ARF4, CDC42, CTNND1, EFEMP1, EGFR, EPN1, GRB7, HGS, HSP90AA1, PDCD6IP, PSEN1, PTPN11, RAB7A, SOCS5, SOS1] | 9.20 | 3.60 × 10−7 |
| Protein Name | Gene | Accession Numbers | Molecular Weight (Da) | Identification Probability | Gene Ontology |
|---|---|---|---|---|---|
| Signal transducer and activator of transcription 1-alpha/beta | STAT1 | P42224 | 87,336.90 | 100% | GO:0007259 |
| Calcium-binding mitochondrial carrier protein SCaMC-1 | SLC25A24 | Q6NUK1-2 | 53,356.60 | 92.60% | GO:0034599 |
| Fibroblast growth factor 21 | FGF21 | Q9NSA1 | 22,300.60 | 92.50% | GO:0090080 |
| Advillin | AVIL | O75366-2 | 92,029.10 | 92.20% | GO:0007399 |
| Potassium channel subfamily T member 2 | KCNT2 | Q6UVM3-2, Q6UVM3-3 | 130,506.30 | 89.50% | GO:0005249 |
| Mitogen-activated protein kinase 1 | MAPK1 | P28482-2 | 41,391.90 | 86.90% | GO:0000165 |
| Neuropilin and tolloid-like protein 2 | NETO2 | Q8NC67-3 | 59,393.90 | 85.80% | GO:2000312 |
| Tyrosine-protein kinase JAK3 | JAK3 | P52333-2 | 125,101.70 | 81.90% | GO:0046425 |
| Sodium/calcium exchanger 3 | SLC8A3 | P57103-2, P57103-6, P57103-7 | 103,011.70 | 77.90% | GO:0060291 |
| Signal transducer and activator of transcription 6 | STAT6 | P42226 | 94,136.90 | 59.90% | GO:0019221 |
| Glial fibrillary acidic protein | GFAP | P14136-2, P14136-3 | 49,881.40 | 35.40% | GO:0031102 |
| Potassium voltage-gated channel subfamily B member 1 | KCNB1 | Q14721 | 95,881.40 | 14.40% | GO:1900454 |
| Potassium voltage-gated channel subfamily G member 4 | KCNG4 | Q8TDN1 | 58,981.00 | 11.80% | GO:0005251 |
| Fibroblast growth factor receptor 3 | FGFR3 | P22607-2 | 877,100.00 | 6.20% | GO:0043410 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.; Hubert, T.; Milthorpe, B.K. Valproic Acid Promotes Early Neural Differentiation in Adult Mesenchymal Stem Cells Through Protein Signalling Pathways. Cells 2020, 9, 619. https://doi.org/10.3390/cells9030619
Santos J, Hubert T, Milthorpe BK. Valproic Acid Promotes Early Neural Differentiation in Adult Mesenchymal Stem Cells Through Protein Signalling Pathways. Cells. 2020; 9(3):619. https://doi.org/10.3390/cells9030619
Chicago/Turabian StyleSantos, Jerran, Thibaut Hubert, and Bruce K Milthorpe. 2020. "Valproic Acid Promotes Early Neural Differentiation in Adult Mesenchymal Stem Cells Through Protein Signalling Pathways" Cells 9, no. 3: 619. https://doi.org/10.3390/cells9030619





