Human Tonsil-Derived Mesenchymal Stromal Cells Maintain Proliferating and ROS-Regulatory Properties via Stanniocalcin-1
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of MSCs
2.2. Microarray Analysis
2.3. RNA Interference
2.4. Cell Proliferation Assessment
2.5. Quantitative Real-time PCR (qRT-PCR)
2.6. Apoptosis Assay
2.7. Live/Dead Staining
2.8. β-galactosidase Staining
2.9. TMSC Differentiation
2.9.1. Osteogenic Differentiation
2.9.2. Adipogenic Differentiation
2.10. ROS Measurement
2.11. Induction of Macrophage-like Cells and NLRP3 inflammasome Activation
2.12. Western Blotting Analysis
2.13. Statistical Analysis
3. Results
3.1. TMSCs Highly Express STC1 and Cell Proliferation is Decreased after STC1 Inhibition
3.2. STC1 Expression is not Altered in Chemically Induced Senescent TMSCs
3.3. STC1 is not Involved in Differentiation Potential of TMSCs
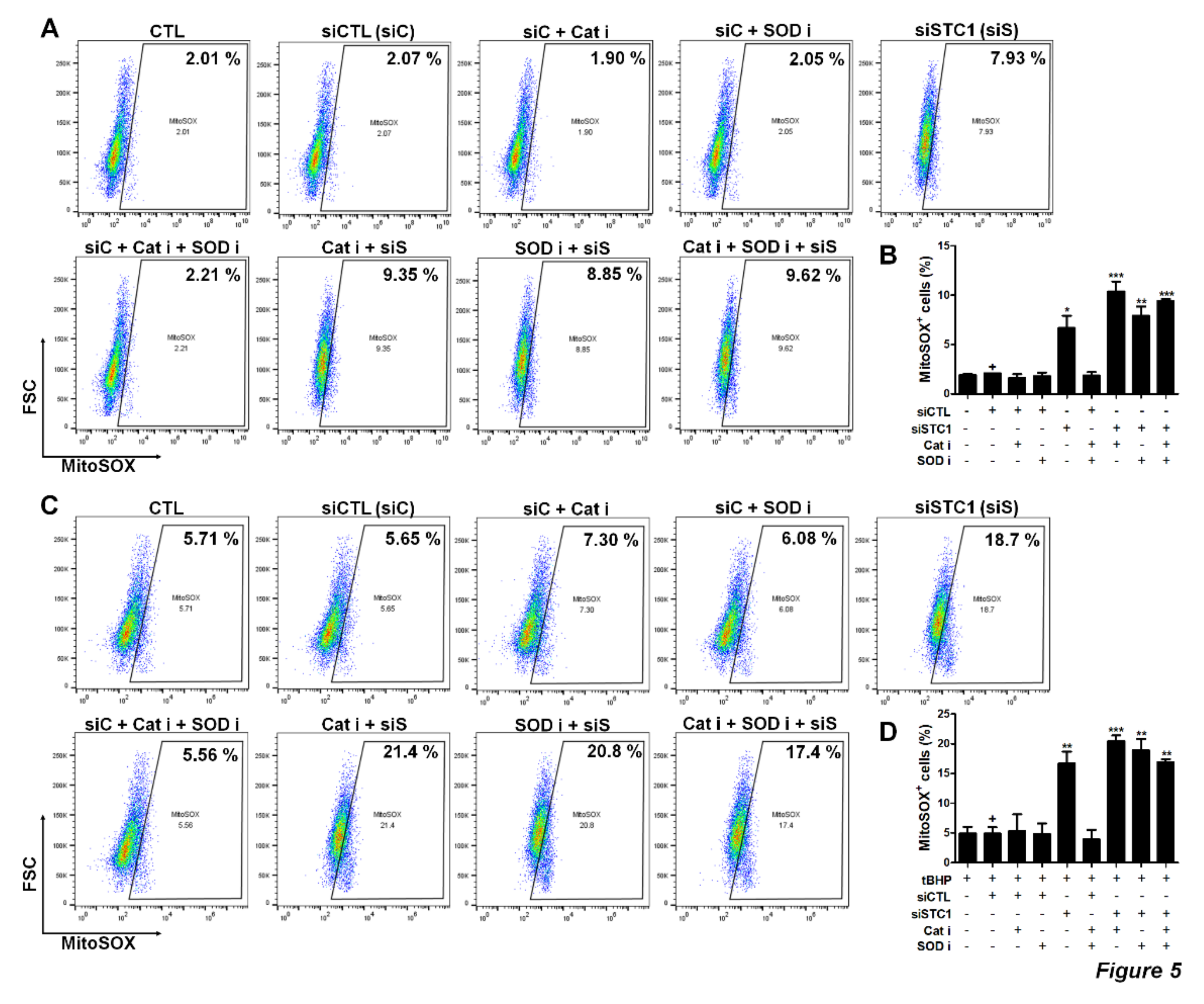
3.4. STC1 is Pivotal for the Maintenance of ROS Homeostasis, as Well as the Regulation of tBHP-Induced ROS Production in TMSCs
3.5. TMSCs Can Suppress ROS-Mediated Activation of NLRP3 Inflammasome in Macrophages
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.H.; Roh, K.H.; Jun, H.J.; Kang, K.S.; Kim, T.Y. Clinical Trial of Human Umbilical Cord Blood-Derived Stem Cells for the Treatment of Moderate-to-Severe Atopic Dermatitis: Phase I/IIa Studies. Stem Cells 2017, 35, 248–255. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, T.H.; Lee, B.C.; Yu, K.R.; Seo, Y.; Lee, S.; Seo, M.S.; Hong, I.S.; Choi, S.W.; Seo, K.W.; et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology 2013, 145, e1391–e1398. [Google Scholar] [CrossRef]
- Kim, H.S.; Yun, J.W.; Shin, T.H.; Lee, S.H.; Lee, B.C.; Yu, K.R.; Seo, Y.; Lee, S.; Kang, T.W.; Choi, S.W.; et al. Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-beta1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells 2015, 33, 1254–1266. [Google Scholar] [CrossRef]
- Shin, T.H.; Kim, H.S.; Choi, S.W.; Kang, K.S. Mesenchymal Stem Cell Therapy for Inflammatory Skin Diseases: Clinical Potential and Mode of Action. Int. J. Mol. Sci. 2017. [Google Scholar] [CrossRef]
- Shin, T.H.; Kim, H.S.; Kang, T.W.; Lee, B.C.; Lee, H.Y.; Kim, Y.J.; Shin, J.H.; Seo, Y.; Won Choi, S.; Lee, S.; et al. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 2016. [Google Scholar] [CrossRef]
- Shin, T.H.; Lee, B.C.; Choi, S.W.; Shin, J.H.; Kang, I.; Lee, J.Y.; Kim, J.J.; Lee, H.K.; Jung, J.E.; Choi, Y.W.; et al. Human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis via regulation of B lymphocyte maturation. Oncotarget 2017, 8, 512–522. [Google Scholar] [CrossRef]
- Donders, R.; Bogie, J.F.J.; Ravanidis, S.; Gervois, P.; Vanheusden, M.; Maree, R.; Schrynemackers, M.; Smeets, H.J.M.; Pinxteren, J.; Gijbels, K.; et al. Human Wharton’s Jelly-Derived Stem Cells Display a Distinct Immunomodulatory and Proregenerative Transcriptional Signature Compared to Bone Marrow-Derived Stem Cells. Stem Cells Dev. 2018, 27, 65–84. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J. Stem Cells 2019, 11, 347–374. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Montemurro, T.; Montelatici, E.; Lavazza, C.; Vigano, M.; Rebulla, P.; Giordano, R.; Lazzari, L. Differential microRNA signature of human mesenchymal stem cells from different sources reveals an “environmental-niche memory” for bone marrow stem cells. Exp. Cell Res. 2013, 319, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Janjanin, S.; Djouad, F.; Shanti, R.M.; Baksh, D.; Gollapudi, K.; Prgomet, D.; Rackwitz, L.; Joshi, A.S.; Tuan, R.S. Human palatine tonsil: A new potential tissue source of multipotent mesenchymal progenitor cells. Arthritis Res. Ther. 2008. [Google Scholar] [CrossRef]
- Ryu, K.H.; Cho, K.A.; Park, H.S.; Kim, J.Y.; Woo, S.Y.; Jo, I.; Choi, Y.H.; Park, Y.M.; Jung, S.C.; Chung, S.M.; et al. Tonsil-derived mesenchymal stromal cells: Evaluation of biologic, immunologic and genetic factors for successful banking. Cytotherapy 2012, 14, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, B.J.; Park, H.Y.; Song, J.S.; Shin, S.C.; Lee, J.C.; Wang, S.G.; Jung, J.S. Effects of donor age, long-term passage culture, and cryopreservation on tonsil-derived mesenchymal stem cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 85–99. [Google Scholar] [CrossRef]
- Park, G.C.; Song, J.S.; Park, H.Y.; Shin, S.C.; Jang, J.Y.; Lee, J.C.; Wang, S.G.; Lee, B.J.; Jung, J.S. Role of Fibroblast Growth Factor-5 on the Proliferation of Human Tonsil-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2016, 25, 1149–1160. [Google Scholar] [CrossRef]
- Lee, B.J.; Kang, D.W.; Park, H.Y.; Song, J.S.; Kim, J.M.; Jang, J.Y.; Lee, J.C.; Wang, S.G.; Jung, J.S.; Shin, S.C. Isolation and Localization of Mesenchymal Stem Cells in Human Palatine Tonsil by W5C5 (SUSD2). Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 38, 83–93. [Google Scholar] [CrossRef]
- Jung, N.; Park, S.; Choi, Y.; Park, J.W.; Hong, Y.B.; Park, H.H.; Yu, Y.; Kwak, G.; Kim, H.S.; Ryu, K.H.; et al. Tonsil-Derived Mesenchymal Stem Cells Differentiate into a Schwann Cell Phenotype and Promote Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2016. [Google Scholar] [CrossRef]
- Park, M.; Kim, Y.H.; Woo, S.Y.; Lee, H.J.; Yu, Y.; Kim, H.S.; Park, Y.S.; Jo, I.; Park, J.W.; Jung, S.C.; et al. Tonsil-derived mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in mice via autophagy activation. Sci. Rep. 2015. [Google Scholar] [CrossRef]
- Samivel, R.; Kim, E.H.; Chung, Y.J.; Mo, J.H. Immunomodulatory effect of tonsil-derived mesenchymal stem cells in a mouse model of allergic rhinitis. Am. J. Rhinol. Allergy 2015, 29, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C.; Seo, Y.; Park, H.Y.; Jung, D.W.; Shin, T.H.; Son, H.; Kim, Y.K.; Lee, J.C.; Sung, E.S.; Jang, J.Y.; et al. Regenerative potential of tonsil mesenchymal stem cells on surgical cutaneous defect. Cell Death Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.F.; Hampong, M.; Park, C.M.; Copp, D.H. Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen. Comp. Endocrinol. 1986, 63, 481–491. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, J.H.; Yun, J.H.; Kim, J.A.; Kim, T.E.; Lee, H.J.; Kim, S.H.; Park, K.H.; Oh, J.Y. Stanniocalcin-1 protects retinal ganglion cells by inhibiting apoptosis and oxidative damage. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Ohkouchi, S.; Block, G.J.; Katsha, A.M.; Kanehira, M.; Ebina, M.; Kikuchi, T.; Saijo, Y.; Nukiwa, T.; Prockop, D.J. Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1. Mol. Ther. 2012, 20, 417–423. [Google Scholar] [CrossRef]
- Roddy, G.W.; Rosa, R.H., Jr.; Oh, J.Y.; Ylostalo, J.H.; Bartosh, T.J., Jr.; Choi, H.; Lee, R.H.; Yasumura, D.; Ahern, K.; Nielsen, G.; et al. Stanniocalcin-1 rescued photoreceptor degeneration in two rat models of inherited retinal degeneration. Mol. Ther. 2012, 20, 788–797. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Abdelrahim, M.; Cai, Q.; Truong, A.; Bick, R.; Poindexter, B.; Sheikh-Hamad, D. Stanniocalcin-1 suppresses superoxide generation in macrophages through induction of mitochondrial UCP2. J. Leukoc. Biol. 2009, 86, 981–988. [Google Scholar] [CrossRef]
- Block, G.J.; Ohkouchi, S.; Fung, F.; Frenkel, J.; Gregory, C.; Pochampally, R.; DiMattia, G.; Sullivan, D.E.; Prockop, D.J. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells 2009, 27, 670–681. [Google Scholar] [CrossRef]
- Ono, M.; Ohkouchi, S.; Kanehira, M.; Tode, N.; Kobayashi, M.; Ebina, M.; Nukiwa, T.; Irokawa, T.; Ogawa, H.; Akaike, T.; et al. Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol. Ther. 2015, 23, 549–560. [Google Scholar] [CrossRef]
- Shi, M.; Yuan, Y.; Liu, J.; Chen, Y.; Li, L.; Liu, S.; An, X.; Luo, R.; Long, D.; Chen, B.; et al. MSCs protect endothelial cells from inflammatory injury partially by secreting STC1. Int. Immunopharmacol. 2018, 61, 109–118. [Google Scholar] [CrossRef]
- Oh, J.Y.; Ko, J.H.; Lee, H.J.; Yu, J.M.; Choi, H.; Kim, M.K.; Wee, W.R.; Prockop, D.J. Mesenchymal stem/stromal cells inhibit the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen species. Stem Cells 2014, 32, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Kim, B.; Choi, I.; Kim, H.; Choi, H.S.; Suh, K.; Bae, Y.C.; Jung, J.S. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2004, 14, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2007, 20, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yu, K.R.; Ryu, Y.S.; Oh, Y.S.; Hong, I.S.; Kim, H.S.; Lee, J.Y.; Kim, S.; Seo, K.W.; Kang, K.S. miR-543 and miR-590-3p regulate human mesenchymal stem cell aging via direct targeting of AIMP3/p18. Age 2014. [Google Scholar] [CrossRef]
- Bennecke, M.; Kriegl, L.; Bajbouj, M.; Retzlaff, K.; Robine, S.; Jung, A.; Arkan, M.C.; Kirchner, T.; Greten, F.R. Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell 2010, 18, 135–146. [Google Scholar] [CrossRef]
- Collado, M.; Blasco, M.A.; Serrano, M. Cellular senescence in cancer and aging. Cell 2007, 130, 223–233. [Google Scholar] [CrossRef]
- Lee, S.; Jung, J.W.; Park, S.B.; Roh, K.; Lee, S.Y.; Kim, J.H.; Kang, S.K.; Kang, K.S. Histone deacetylase regulates high mobility group A2-targeting microRNAs in human cord blood-derived multipotent stem cell aging. Cell. Mol. Life Sci. 2011, 68, 325–336. [Google Scholar] [CrossRef]
- Petrova, N.V.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Small molecule compounds that induce cellular senescence. Aging Cell 2016, 15, 999–1017. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hampton, M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008, 45, 549–561. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, T.; Dong, Q.; Nice, E.C.; Huang, C.; Wei, Y. Redox homeostasis: The linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013. [Google Scholar] [CrossRef]
- Valle-Prieto, A.; Conget, P.A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010, 19, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.M.; Yoon, J.H.; Lim, J.; Shin, J.W.; Cho, A.Y.; Heo, J.; Lee, K.B.; Lee, J.H.; Lee, W.J.; Kim, H.J.; et al. Real-Time Monitoring of Glutathione in Living Cells Reveals that High Glutathione Levels Are Required to Maintain Stem Cell Function. Stem Cell Rep. 2018, 10, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xiao, Y.; Dai, Y.; Chen, X.; Li, D.; Tan, X.; Zhang, X. Stanniocalcin 1 promotes cell proliferation via cyclin E1/cyclindependent kinase 2 in human prostate carcinoma. Oncol. Rep. 2017, 37, 2465–2471. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, Y.; Wang, J.; Li, Y.; Li, Y.; Li, G. Stanniocalcin1 (STC1) Inhibits Cell Proliferation and Invasion of Cervical Cancer Cells. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Yu, K.R.; Park, S.B.; Jung, J.W.; Seo, M.S.; Hong, I.S.; Kim, H.S.; Seo, Y.; Kang, T.W.; Lee, J.Y.; Kurtz, A.; et al. HMGA2 regulates the in vitro aging and proliferation of human umbilical cord blood-derived stromal cells through the mTOR/p70S6K signaling pathway. Stem Cell Res. 2013, 10, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; O’Brien, C.A.; Manolagas, S.C. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J. Biol. Chem. 2007, 282, 27298–27305. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Shih, Y.R.; Kuo, T.K.; Lee, O.K.; Wei, Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Byon, C.H.; Javed, A.; Dai, Q.; Kappes, J.C.; Clemens, T.L.; Darley-Usmar, V.M.; McDonald, J.M.; Chen, Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008, 283, 15319–15327. [Google Scholar] [CrossRef]
- Schroder, K.; Wandzioch, K.; Helmcke, I.; Brandes, R.P. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arter. Thromb. Vasc. Biol. 2009, 29, 239–245. [Google Scholar] [CrossRef]
- Reykdal, S.; Abboud, C.; Liesveld, J. Effect of nitric oxide production and oxygen tension on progenitor preservation in ex vivo culture. Exp. Hematol. 1999, 27, 441–450. [Google Scholar] [CrossRef]
- Higuchi, M.; Dusting, G.J.; Peshavariya, H.; Jiang, F.; Hsiao, S.T.; Chan, E.C.; Liu, G.S. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013, 22, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J. Mitochondria: Sovereign of inflammation? Eur. J. Immunol. 2011, 41, 1196–1202. [Google Scholar] [CrossRef]
- Kepp, O.; Galluzzi, L.; Kroemer, G. Mitochondrial control of the NLRP3 inflammasome. Nat. Immunol. 2011, 12, 199–200. [Google Scholar] [CrossRef]





| Target Name | Forward Primer | Reverse Primer |
|---|---|---|
| STC1 | GCAGGAAGAGTGCTACAGCAAG | CATTCCAGCAGGCTTCGGACAA |
| STC2 | GCATGACTTTTCTGCACAACGCT | GGCTTATGCAGCCGAACCTGTG |
| SOD1 | CTCACTCTCAGGAGACCATTGC | CCACAAGCCAAACGACTTCCAG |
| SOD2 | CTGGACAAACCTCAGCCCTAAC | AACCTGAGCCTTGGACACCAAC |
| PRDX1 | CTGCCAAGTGATTGGTGCTTCTG | AATGGTGCGCTTCGGGTCTGAT |
| GPX1 | GTGCTCGGCTTCCCGTGCAAC | CTCGAAGAGCATGAAGTTGGGC |
| P16 | CTCGTGCTGATGCTACTGAGGA | GGTCGGCGCAGTTGGGCTCC |
| P21 | AGGTGGACCTGGAGACTCTCAG | TCCTCTTGGAGAAGATCAGCCG |
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.; Shin, T.-H.; Ahn, J.-S.; Oh, S.-J.; Shin, Y.Y.; Yang, J.W.; Park, H.Y.; Shin, S.-C.; Kwon, H.-K.; Kim, J.M.; et al. Human Tonsil-Derived Mesenchymal Stromal Cells Maintain Proliferating and ROS-Regulatory Properties via Stanniocalcin-1. Cells 2020, 9, 636. https://doi.org/10.3390/cells9030636
Seo Y, Shin T-H, Ahn J-S, Oh S-J, Shin YY, Yang JW, Park HY, Shin S-C, Kwon H-K, Kim JM, et al. Human Tonsil-Derived Mesenchymal Stromal Cells Maintain Proliferating and ROS-Regulatory Properties via Stanniocalcin-1. Cells. 2020; 9(3):636. https://doi.org/10.3390/cells9030636
Chicago/Turabian StyleSeo, Yoojin, Tae-Hoon Shin, Ji-Su Ahn, Su-Jeong Oh, Ye Young Shin, Ji Won Yang, Hee Young Park, Sung-Chan Shin, Hyun-Keun Kwon, Ji Min Kim, and et al. 2020. "Human Tonsil-Derived Mesenchymal Stromal Cells Maintain Proliferating and ROS-Regulatory Properties via Stanniocalcin-1" Cells 9, no. 3: 636. https://doi.org/10.3390/cells9030636
APA StyleSeo, Y., Shin, T.-H., Ahn, J.-S., Oh, S.-J., Shin, Y. Y., Yang, J. W., Park, H. Y., Shin, S.-C., Kwon, H.-K., Kim, J. M., Sung, E.-S., Park, G. C., Lee, B.-J., & Kim, H.-S. (2020). Human Tonsil-Derived Mesenchymal Stromal Cells Maintain Proliferating and ROS-Regulatory Properties via Stanniocalcin-1. Cells, 9(3), 636. https://doi.org/10.3390/cells9030636





