Vascular Smooth Muscle Cell-Specific Progerin Expression Provokes Contractile Impairment in a Mouse Model of Hutchinson-Gilford Progeria Syndrome that Is Ameliorated by Nitrite Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Nitrite Treatment
2.3. Wire Myography
2.4. Statistical Analysis
3. Results
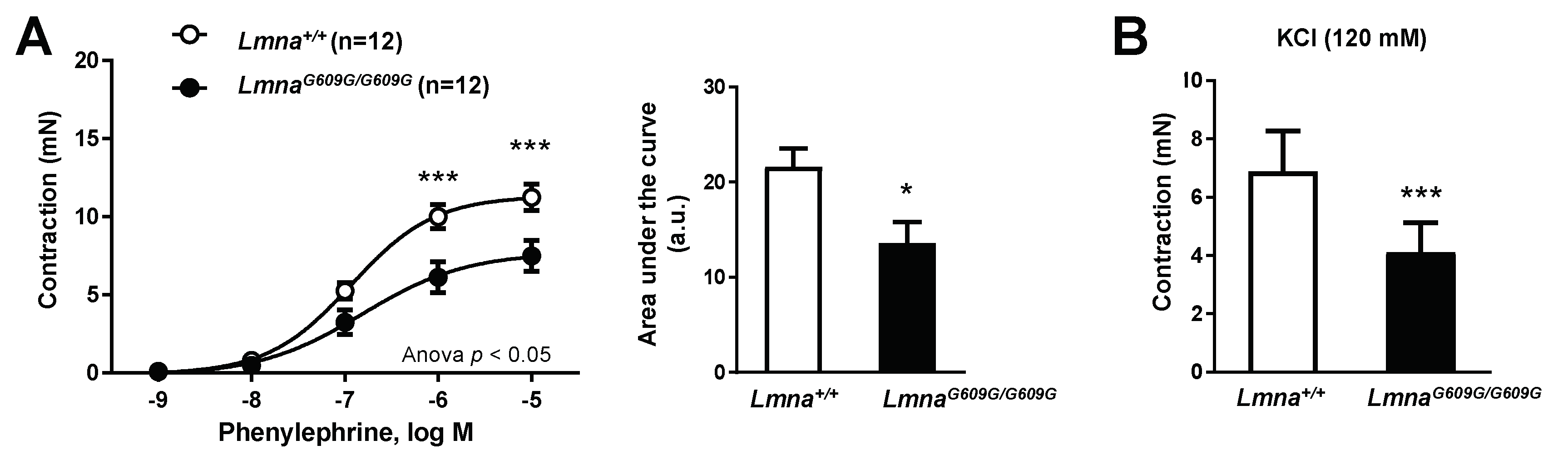
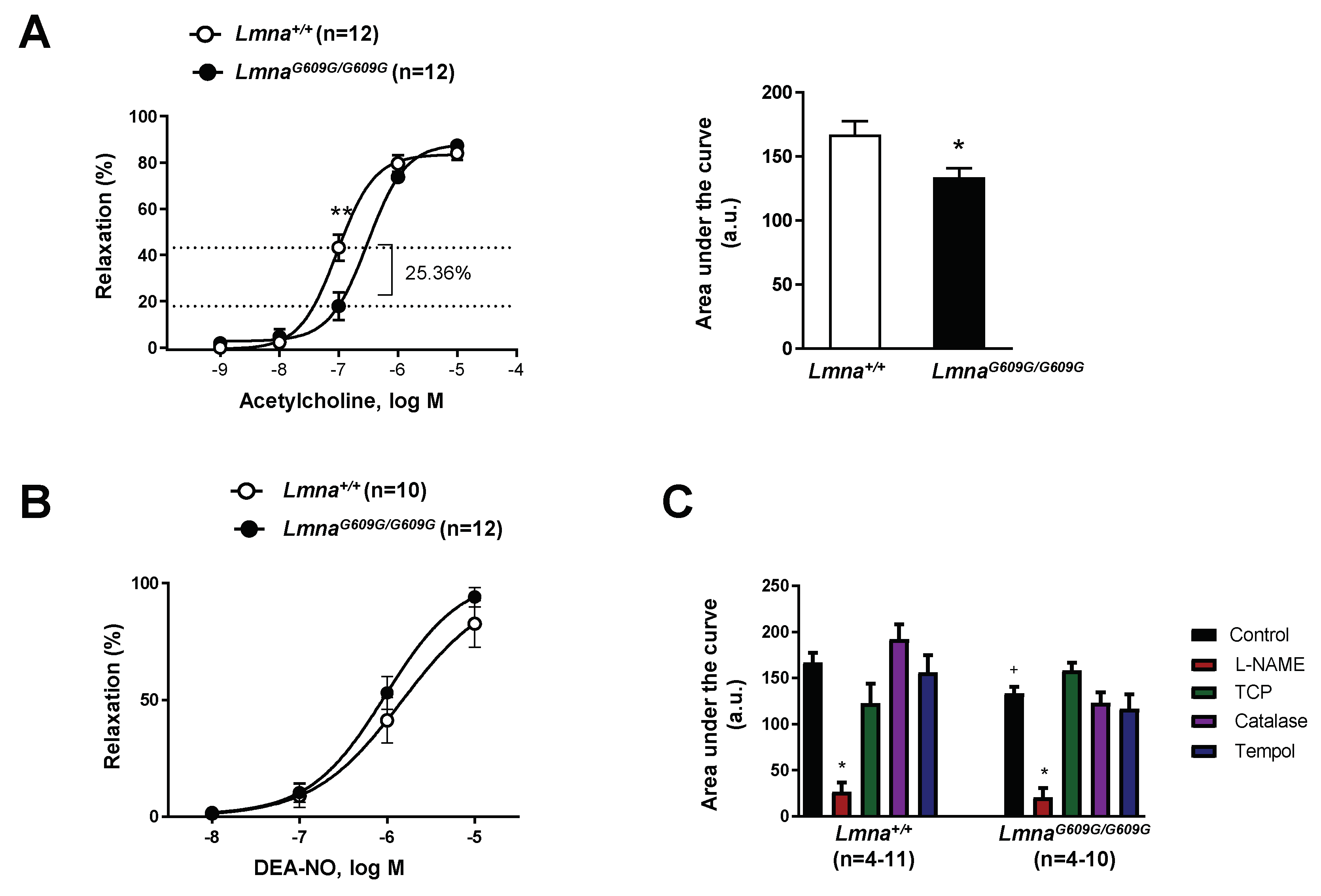
3.1. Impaired Vascular Function in Progeroid Mice with Ubiquitous Progerin Expression
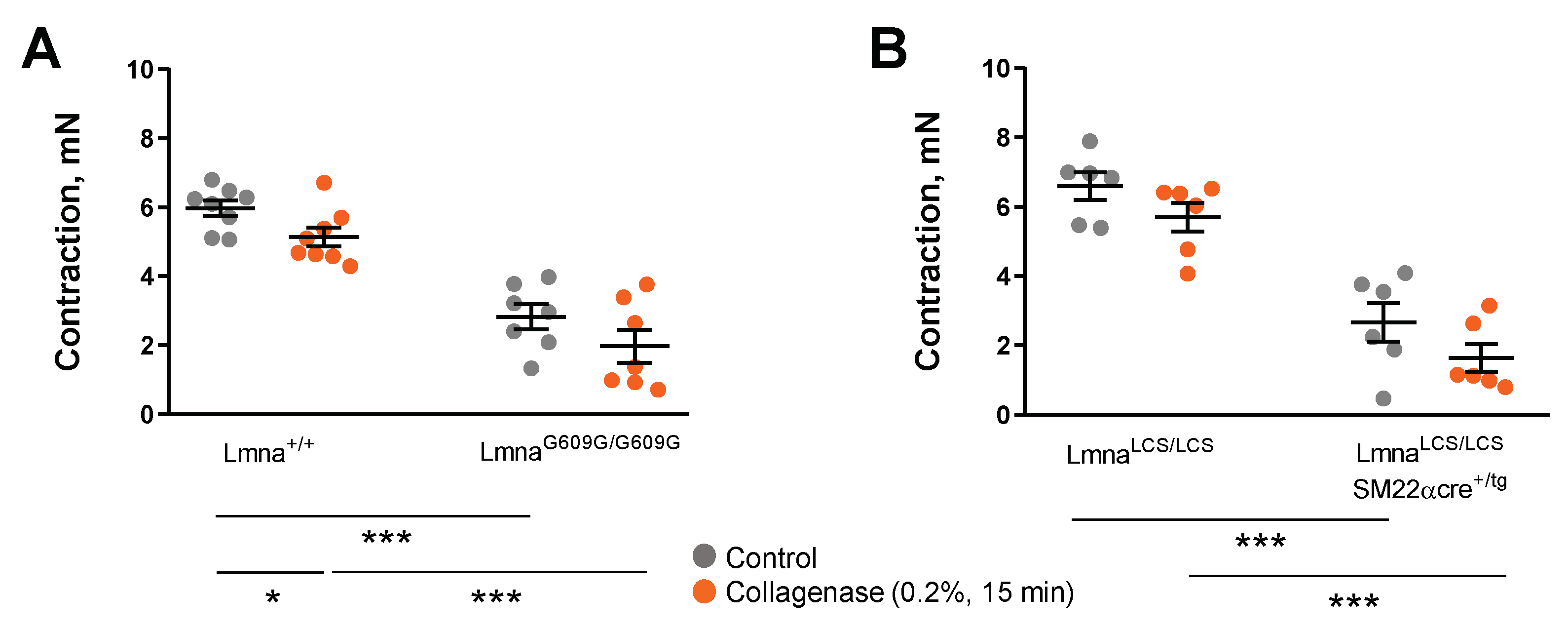
3.2. VSMC-Specific Progerin Expression Provokes Contractile Impairment, but Neither VSMC-Specific nor EC-Specific Expression Is Sufficient to Cause Endothelial Dysfunction
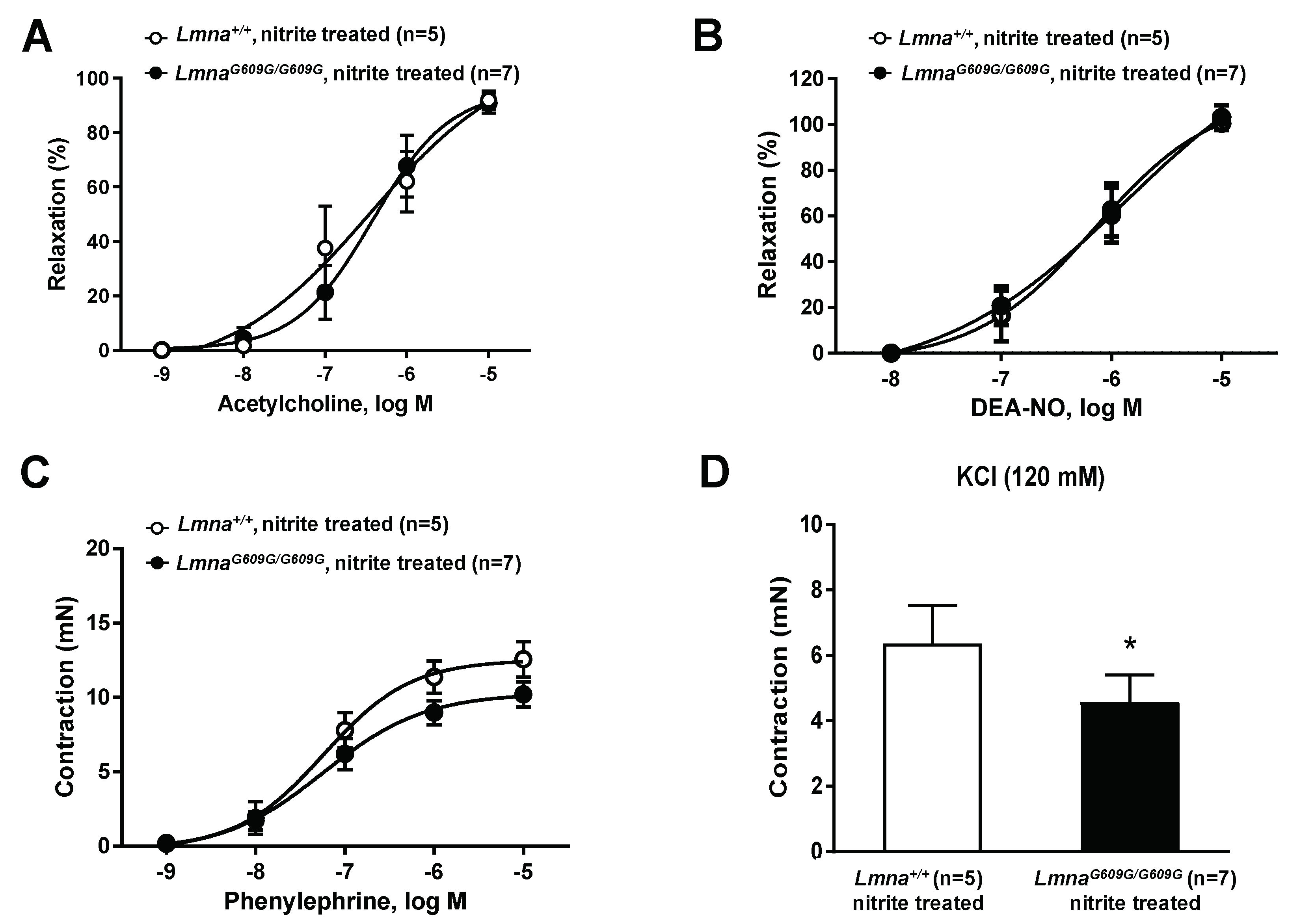
3.3. Treatment with Sodium Nitrite Improves Vascular Function in Progeroid Mice
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- North, B.J.; Sinclair, D.A. The Intersection between Aging and Cardiovascular Disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Strain, W.D.; Paldánius, P.M. Diabetes, Cardiovascular Disease and the Microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Pei, J.; Tang, L.; Hu, X. Traditional Cardiovascular Risk Factors and Coronary Collateral Circulation: Protocol for a Systematic Review and Meta-Analysis of Case-Control Studies. Medicine 2018, 97, e0417. [Google Scholar] [CrossRef]
- Gordon, L.B.; Rothman, F.G.; López-Otín, C.; Misteli, T. Progeria: A Paradigm for Translational Medicine. Cell 2014, 156, 400–407. [Google Scholar] [CrossRef]
- Hamczyk, M.R.; del Campo, L.; Andrés, V. Aging in the Cardiovascular System: Lessons from Hutchinson-Gilford Progeria Syndrome. Annu. Rev. Physiol. 2018, 80, 27–48. [Google Scholar] [CrossRef]
- Merideth, M.A.; Gordon, L.B.; Clauss, S.; Sachdev, V.; Smith, A.C.M.M.; Perry, M.B.; Brewer, C.C.; Zalewski, C.; Kim, H.J.; Solomon, B.; et al. Phenotype and Course of Hutchinson–Gilford Progeria Syndrome. N. Engl. J. Med. 2008, 358, 592–604. [Google Scholar] [CrossRef]
- Ullrich, N.J.; Gordon, L.B. Hutchinson–Gilford Progeria Syndrome. Handbook of Clin. Neurol. 2015, 132, 249–264. [Google Scholar]
- Vidak, S.; Foisner, R. Molecular Insights into the Premature Aging Disease Progeria. Histoch. Cell Biol. 2016, 145, 401–417. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.; Smoot, L.B.; Wake, N.; Kieran, M.W.; Kleinman, M.E.; Miller, D.T.; Schwartzman, A.; Giobbie-Hurder, A.; Neuberg, D.; Gordon, L.B. Mechanisms of Premature Vascular Aging in Children with Hutchinson-Gilford Progeria Syndrome. Hypertension 2012, 59, 92–97. [Google Scholar] [CrossRef]
- Olive, M.; Harten, I.; Mitchell, R.; Beers, J.K.; Djabali, K.; Cao, K.; Erdos, M.R.; Blair, C.D.; Funke, B.; Smoot, L.B.; et al. Cardiovascular Pathology in Hutchinson-Gilford Progeria: Correlation with the Vascular Pathology of Aging. Arter. Thromb. Vasc. Biol. 2010, 30, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- De Sandre-Giovannoli, A.; Bernard, R.; Cau, P.; Navarro, C.L.; Amiel, J.; Boccaccio, I.; Lyonnet, S.; Stewart, C.L.; Munnich, A.; Le Merrer, M.; et al. Lamin a Truncation in Hutchinson-Gilford Progeria. Science 2003, 300, 2055. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Brown, W.T.; Gordon, L.B.; Glynn, M.W.; Singer, J.; Scott, L.; Erdos, M.R.; Robbins, C.M.; Moses, T.Y.; Berglund, P.; et al. Recurrent de Novo Point Mutations in Lamin A Cause Hutchinson-Gilford Progeria Syndrome. Nature 2003, 423, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Dorado, B.; Andrés, V. A-Type Lamins and Cardiovascular Disease in Premature Aging Syndromes. Curr. Opin. Cell Biol. 2017, 46, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.M.; Pla, D.; Perez-Sala, D.; Andrés, V. A-Type Lamins and Hutchinson-Gilford Progeria Syndrome: Pathogenesis and Therapy. Front. Biosci. 2011, 3, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Ashapkin, V.V.; Kutueva, L.I.; Kurchashova, S.Y.; Kireev, I.I. Are There Common Mechanisms Between the Hutchinson–Gilford Progeria Syndrome and Natural Aging? Front. Genet. 2019, 10, 455. [Google Scholar] [CrossRef]
- McClintock, D.; Ratner, D.; Lokuge, M.; Owens, D.M.; Gordon, L.B.; Collins, F.S.; Djabali, K. The Mutant Form of Lamin A That Causes Hutchinson-Gilford Progeria Is a Biomarker of Cellular Aging in Human Skin. PLoS ONE 2007, 2, e1269. [Google Scholar] [CrossRef]
- Rodriguez, S.; Coppedè, F.; Sagelius, H.; Eriksson, M. Increased Expression of the Hutchinson-Gilford Progeria Syndrome Truncated Lamin A Transcript during Cell Aging. Eur. J. Hum. Genet. 2009, 17, 928–937. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T. Lamin A-Dependent Nuclear Defects in Human Aging. Science 2006, 312, 1059–1063. [Google Scholar] [CrossRef]
- Sullivan, T.; Escalante-Alcalde, D.; Bhatt, H.; Anver, M.; Bhat, N.; Nagashima, K.; Stewart, C.L.; Burke, B. Loss of A-Type Lamin Expression Compromises Nuclear Envelope Integrity Leading to Muscular Dystrophy. J. Cell Biol. 1999, 147, 913–920. [Google Scholar] [CrossRef]
- López-Mejía, I.C.; de Toledo, M.; Chavey, C.; Lapasset, L.; Cavelier, P.; Lopez-Herrera, C.; Chebli, K.; Fort, P.; Beranger, G.; Fajas, L.; et al. Antagonistic Functions of LMNA Isoforms in Energy Expenditure and Lifespan. EMBO Rep. 2014, 15, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kieckhaefer, J.E.; Cao, K. Mouse Models of Laminopathies. Aging Cell 2013, 12, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Osorio, F.G.; Navarro, C.L.; Cadiñanos, J.; López-Mejía, I.C.; Quirós, P.M.; Bartoli, C.; Rivera-Torres, J.; Tazi, J.; Guzmán, G.; Varela, I.; et al. Splicing-Directed Therapy in a New Mouse Model of Human Accelerated Aging. Sci. Transl. Med. 2011, 3, 106ra107. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R.; Rivera-Torres, J.; Osorio, F.G.; Acín-Pérez, R.; Enríquez, J.A.; López-Otín, C.; Andrés, V. Defective Extracellular Pyrophosphate Metabolism Promotes Vascular Calcification in a Mouse Model of Hutchinson-Gilford Progeria Syndrome That Is Ameliorated on Pyrophosphate Treatment. Circulation 2013, 127, 2442–2451. [Google Scholar] [CrossRef]
- del Campo, L.; Sánchez-López, A.; Salaices, M.; von Kleeck, R.A.; Expósito, E.; González-Gómez, C.; Cussó, L.; Guzmán-Martínez, G.; Ruiz-Cabello, J.; Desco, M.; et al. Vascular Smooth Muscle Cell Specific Progerin Expression in a Mouse Model of Hutchinson–Gilford Progeria Syndrome Promotes Arterial Stiffness: Therapeutic Effect of Dietary Nitrite. Aging Cell 2019, 18, e12936. [Google Scholar] [CrossRef]
- Hamczyk, M.R.; Villa-Bellosta, R.; Gonzalo, P.; Andrés-Manzano, M.J.; Nogales, P.; Bentzon, J.F.; López-Otín, C.; Andrés, V. Vascular Smooth Muscle-Specific Progerin Expression Accelerates Atherosclerosis and Death in a Mouse Model of Hutchinson-Gilford Progeria Syndrome. Circulation 2018, 138, 266–282. [Google Scholar] [CrossRef]
- Hamczyk, M.R.; Villa-Bellosta, R.; Quesada, V.; Gonzalo, P.; Vidak, S.; Nevado, R.M.; Andrés-Manzano, M.J.; Misteli, T.; López-Otín, C.; Andrés, V. Progerin Accelerates Atherosclerosis by Inducing Endoplasmic Reticulum Stress in Vascular Smooth Muscle Cells. EMBO Mol. Med. 2019, 11, e9736. [Google Scholar] [CrossRef]
- Levy, B.I.; Schiffrin, E.L.; Mourad, J.-J.; Agostini, D.; Vicaut, E.; Safar, M.E.; Struijker-Boudier, H.A.J. Impaired Tissue Perfusion: A Pathology Common to Hypertension, Obesity, and Diabetes Mellitus. Circulation 2008, 118, 968–976. [Google Scholar] [CrossRef]
- Park, K.H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Carter, S.E.; Green, D.J. Arterial Structure and Function in Vascular Ageing: Are You as Old as Your Arteries? J. Physiol. 2016, 594, 2275–2284. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhu, Y.; Sun, Z.; Trzeciakowski, J.P.; Gansner, M.; Depre, C.; Resuello, R.R.G.; Natividad, F.F.; Hunter, W.C.; Genin, G.M.; et al. Short Communication: Vascular Smooth Muscle Cell Stiffness as a Mechanism for Increased Aortic Stiffness with Aging. Circ. Res. 2010, 107, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Gordon, L.B.; Kleinman, M.E.; Gurary, E.B.; Massaro, J.; D’Agostino, R.B.; Kieran, M.W.; Gerhard-Herman, M.; Smoot, L.B. Cardiac Abnormalities in Patients with Hutchinson-Gilford Progeria Syndrome. JAMA Cardiol. 2018, 3, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitrite Reduction to Nitric Oxide in the Vasculature. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H477–H478. [Google Scholar] [CrossRef]
- Sindler, A.L.; Fleenor, B.S.; Calvert, J.W.; Marshall, K.D.; Zigler, M.L.; Lefer, D.J.; Seals, D.R. Nitrite Supplementation Reverses Vascular Endothelial Dysfunction and Large Elastic Artery Stiffness with Aging. Aging Cell 2011, 10, 429–437. [Google Scholar] [CrossRef]
- Kisanuki, Y.Y.; Hammer, R.E.; Miyazaki, J.; Williams, S.C.; Richardson, J.A.; Yanagisawa, M. Tie2-Cre Transgenic Mice: A New Model for Endothelial Cell-Lineage Analysis in Vivo. Dev. Biol. 2001, 230, 230–242. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Sodium Nitrite (CAS NO. 7632-00-0) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies). Natl. Toxicol. Program. Tech. Rep. Ser. 2001, 495, 7–273. [Google Scholar]
- del Campo, L.; Ferrer, M. Wire Myography to Study Vascular Tone and Vascular Structure of Isolated Mouse Arteries. In Methods in Mouse Atherosclerosis; Humana Press: New York, NY, USA, 2015; pp. 255–276. [Google Scholar]
- Wu, H.; van Thiel, B.S.; Bautista-Niño, P.K.; Reiling, E.; Durik, M.; Leijten, F.P.J.; Ridwan, Y.; Brandt, R.M.C.; van Steeg, H.; Dollé, M.E.T.; et al. Dietary Restriction but Not Angiotensin II Type 1 Receptor Blockade Improves DNA Damage-Related Vasodilator Dysfunction in Rapidly Aging Ercc1Δ/- Mice. Clin. Sci. 2017, 131, 1941–1953. [Google Scholar] [CrossRef]
- Durik, M.; Kavousi, M.; Van Der Pluijm, I.; Isaacs, A.; Cheng, C.; Verdonk, K.; Loot, A.E.; Oeseburg, H.; Bhaggoe, U.M.; Leijten, F.; et al. Nucleotide Excision DNA Repair Is Associated with Age-Related Vascular Dysfunction. Circulation 2012, 126, 468–478. [Google Scholar] [CrossRef]
- Osmanagic-Myers, S.; Kiss, A.; Manakanatas, C.; Hamza, O.; Sedlmayer, F.; Szabo, P.L.; Fischer, I.; Fichtinger, P.; Podesser, B.K.; Eriksson, M.; et al. Endothelial Progerin Expression Causes Cardiovascular Pathology through an Impaired Mechanoresponse. J. Clin. Investig. 2019, 129, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Sindler, A.L.; DeVan, A.E.; Fleenor, B.S.; Seals, D.R. Inorganic Nitrite Supplementation for Healthy Arterial Aging. J. Appl. Physiol. 2014, 116, 463–477. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Campo, L.; Sánchez-López, A.; González-Gómez, C.; Andrés-Manzano, M.J.; Dorado, B.; Andrés, V. Vascular Smooth Muscle Cell-Specific Progerin Expression Provokes Contractile Impairment in a Mouse Model of Hutchinson-Gilford Progeria Syndrome that Is Ameliorated by Nitrite Treatment. Cells 2020, 9, 656. https://doi.org/10.3390/cells9030656
del Campo L, Sánchez-López A, González-Gómez C, Andrés-Manzano MJ, Dorado B, Andrés V. Vascular Smooth Muscle Cell-Specific Progerin Expression Provokes Contractile Impairment in a Mouse Model of Hutchinson-Gilford Progeria Syndrome that Is Ameliorated by Nitrite Treatment. Cells. 2020; 9(3):656. https://doi.org/10.3390/cells9030656
Chicago/Turabian Styledel Campo, Lara, Amanda Sánchez-López, Cristina González-Gómez, María Jesús Andrés-Manzano, Beatriz Dorado, and Vicente Andrés. 2020. "Vascular Smooth Muscle Cell-Specific Progerin Expression Provokes Contractile Impairment in a Mouse Model of Hutchinson-Gilford Progeria Syndrome that Is Ameliorated by Nitrite Treatment" Cells 9, no. 3: 656. https://doi.org/10.3390/cells9030656
APA Styledel Campo, L., Sánchez-López, A., González-Gómez, C., Andrés-Manzano, M. J., Dorado, B., & Andrés, V. (2020). Vascular Smooth Muscle Cell-Specific Progerin Expression Provokes Contractile Impairment in a Mouse Model of Hutchinson-Gilford Progeria Syndrome that Is Ameliorated by Nitrite Treatment. Cells, 9(3), 656. https://doi.org/10.3390/cells9030656




