Electrical Stimulation Induces Retinal Müller Cell Proliferation and Their Progenitor Cell Potential
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Isolation and Culture of Müller Cells (MCs)
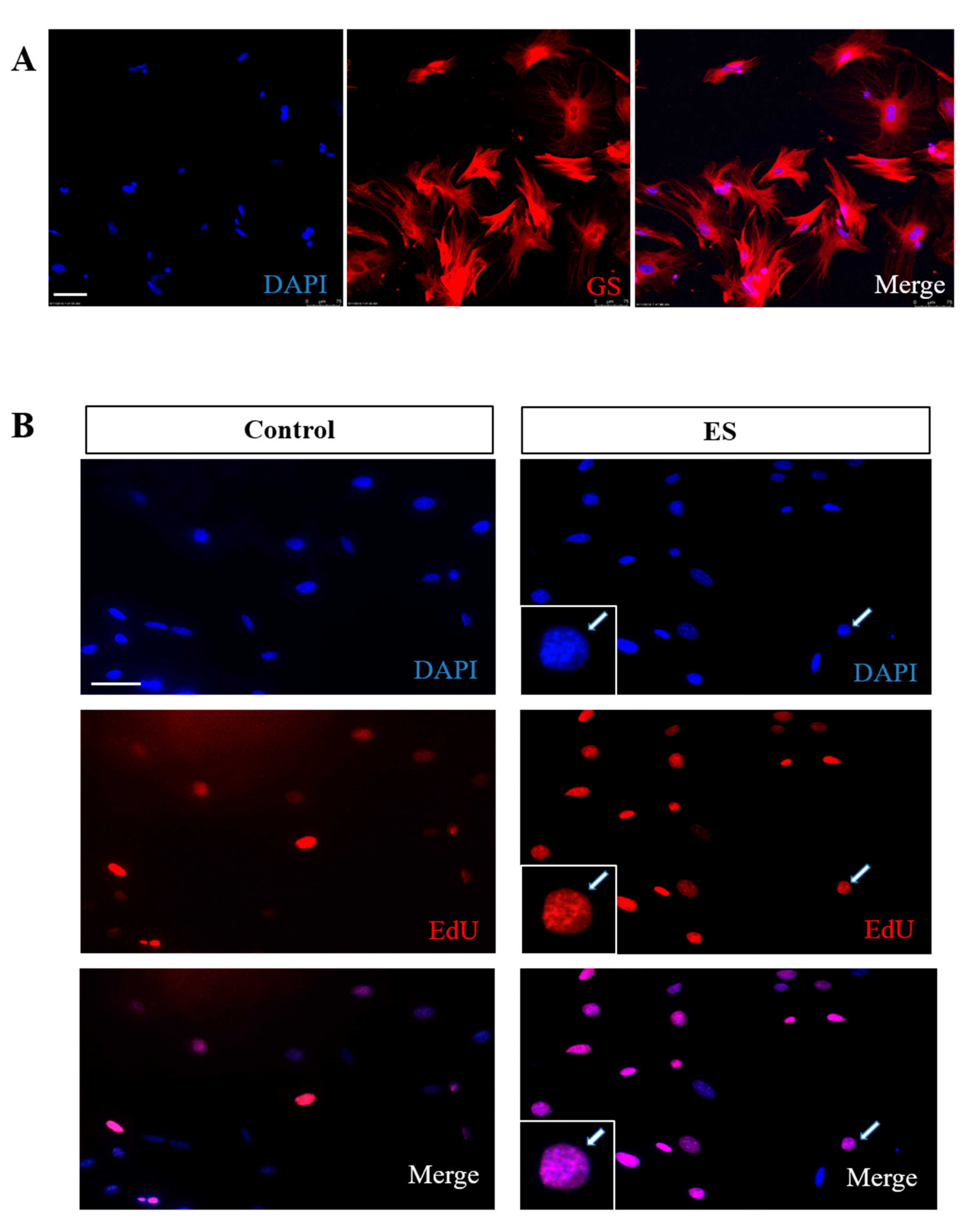
2.3. Labeling, Immunohistochemistry and Staining
2.4. Electrical Stimulation (ES) of MCs In Vitro
2.5. RNA Sequencing and Data Analysis
2.6. Calcium Channel Blocker and MC Proliferation
2.7. Real-Time PCR
2.8. Statistical Analysis
3. Results
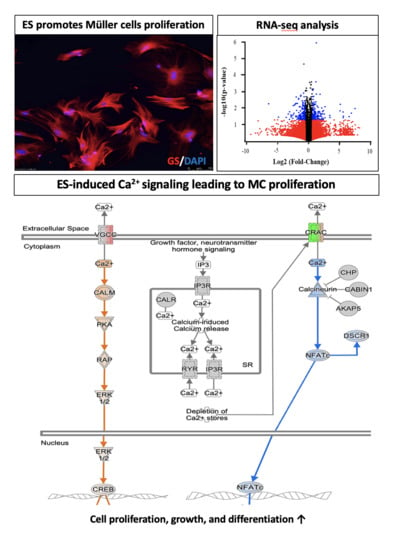
3.1. ES Promotes MC Proliferation
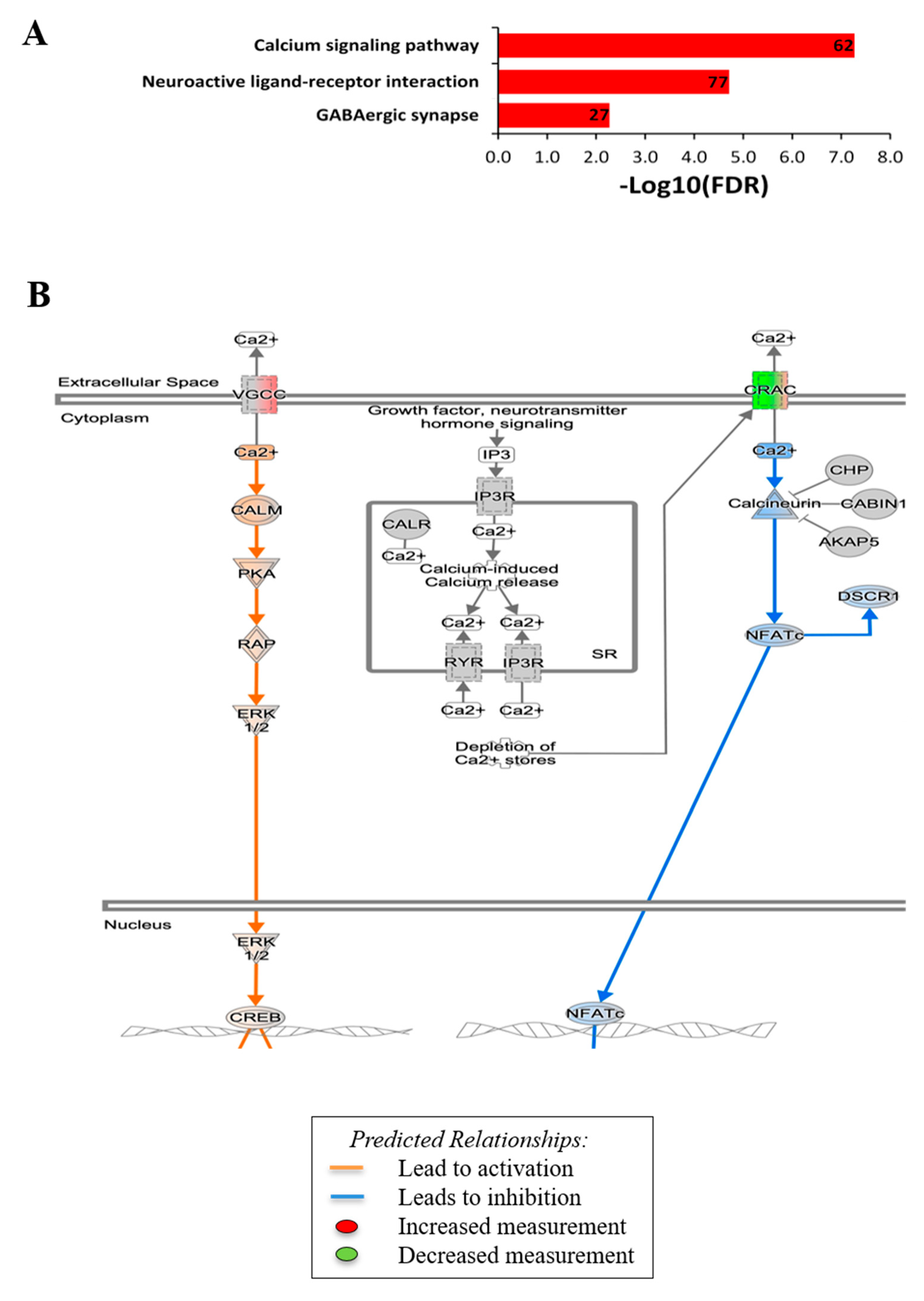
3.2. RNA Sequencing Gene Profiling and Analysis of MCs
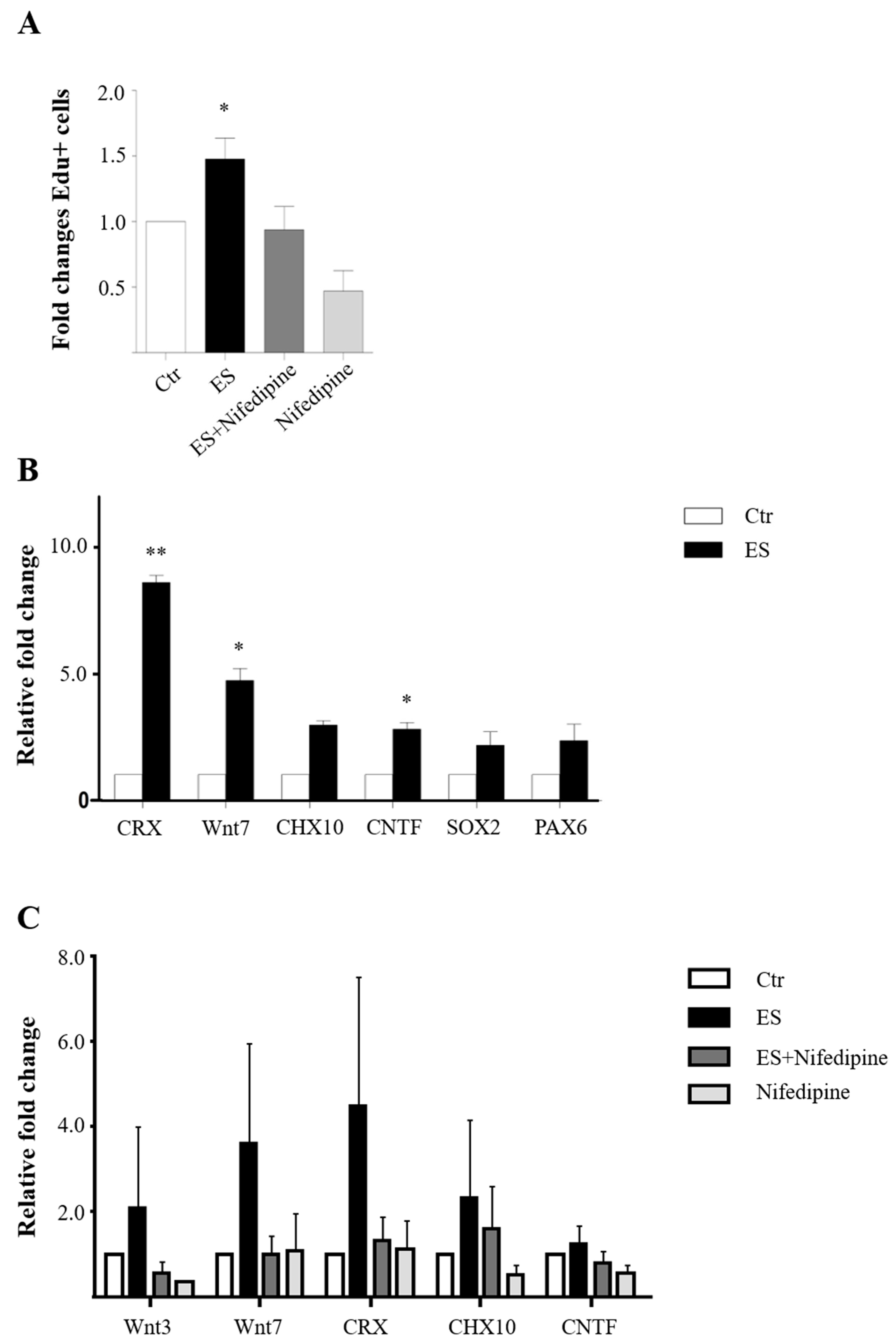
3.3. The L-Type Calcium Channel Mediates ES-Induced MC Proliferation and Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reichenbach, A.; Bringmann, A. New functions of müller cells. Glia 2013, 61, 651–678. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D. Müller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, R.; Sun, Z.; Li, C.; Ramakrishna, S.; Chiu, K.; He, L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp. Neurol. 2019, 319, 112963. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Enayati, S.; Chang, K.; Cho, K.; Lee, S.W.; Talib, M.; Zihlavnikova, K.; Xie, J.; Achour, H.; Fried, S.I.; et al. Noninvasive electrical stimulation improves retinal function and induces neural regeneration in mice with inherited photoreceptor degeneration. Iovs 2020, in press. [Google Scholar]
- Chow, A.Y.; Chow, V.Y.; Packo, K.H.; Pollack, J.S.; Peyman, G.A.; Schuchard, R. The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch. Ophthalmol. 2004, 122, 460–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehic, A.; Guo, S.; Cho, K.S.; Corraya, R.M.; Chen, D.F.; Utheim, T.P. Electrical Stimulation as a Means for Improving Vision. Am. J. Pathol. 2016, 186, 2783–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Watt, C.; Karystinou, A.; Roelofs, A.J.; Mccaig, C.D.; Gibson, I.R.; De Bari, C. Directed migration of human bone marrow mesenchymal stem cells in a physiological direct current electric field. Eur. Cell. Mater. 2011, 22, 344–358. [Google Scholar] [CrossRef]
- Thakral, G.; LaFontaine, J.; Najafi, B.; Talal, T.K.; Kim, P.; Lavery, L.A. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Obeso, I.; Oliviero, A.; Jahanshahi, M. Editorial: Non-invasive brain stimulation in neurology and psychiatry. Front. Neurosci. 2016, 10, 1–3. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Nitsche, M.S.; Klein, C.C.; Tergau, F.; Rothwell, J.C.; Paulus, W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin. Neurophysiol. 2003, 114, 600–604. [Google Scholar] [CrossRef]
- Tandon, N.; Cannizzaro, C.; Chao, P.P.-H.G.; Maidhof, R.; Marsano, A.; Au, H.T.H.; Radisic, M.; Vunjak-Novakovic, G. Electrical stimulation systems for cardiac tissue engineering. Nat. Protoc. 2009, 4, 155–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaikin, L.; Kashiwa, K.; Bennet, M.; Papastergiou, G.; Gregory, W. Microcurrent stimulation in the treatment of dry and wet macular degeneration. Clin. Ophthalmol. 2015, 9, 2345–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 2014, 25, 3835–3850. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Fujikado, T.; Morimoto, T.; Matsushita, K.; Shimojo, H.; Okawa, Y.; Tano, Y. Effect of transcorneal electrical stimulation in patients with nonarteritic ischemic optic neuropathy or traumatic optic neuropathy. Jpn. J. Ophthalmol. 2006, 50, 266–273. [Google Scholar] [CrossRef]
- Oono, S.; Kurimoto, T.; Kashimoto, R.; Tagami, Y.; Okamoto, N.; Mimura, O. Transcorneal electrical stimulation improves visual function in eyes with branch retinal artery occlusion. Clin. Ophthalmol. 2011, 5, 397–402. [Google Scholar]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A.; Miyake, K.; Yoshida, M.; Inoue, Y.; Hata, Y.; Press, D.; Schatz, A.; et al. The Artificial Silicon Retina Microchip for the Treatment of Vision Loss From Retinitis Pigmentosa. Am. J. Pathol. 2016, 186, 2783–2797. [Google Scholar]
- Morimoto, T.; Fukui, T.; Matsushita, K.; Okawa, Y.; Shimojyo, H.; Kusaka, S.; Tano, Y.; Fujikado, T. Evaluation of residual retinal function by pupillary constrictions and phosphenes using transcorneal electrical stimulation in patients with retinal degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 1283–1292. [Google Scholar] [CrossRef]
- Schatz, A.; Röck, T.; Naycheva, L.; Willmann, G.; Wilhelm, B.; Peters, T.; Bartz-Schmidt, K.U.; Zrenner, E.; Messias, A.; Gekeler, F. Transcorneal electrical stimulation for patients with retinitis pigmentosa: A prospective, randomized, sham-controlled exploratory study. Invest. Ophthalmol. Vis. Sci. 2011, 52, 4485–4496. [Google Scholar] [CrossRef]
- Seki, M.; Tanaka, T.; Sakai, Y.; Fukuchi, T.; Abe, H.; Nawa, H.; Takei, N. Müller cells as a source of brain-derived neurotrophic factor in the retina: Noradrenaline upregulates brain-derived neurotrophic factor levels in cultured rat Müller cells. Neurochem. Res. 2005, 30, 1163–1170. [Google Scholar] [CrossRef]
- Zhou, W.T.; Ni, Y.Q.; Jin, Z.B.; Zhang, M.; Wu, J.H.; Zhu, Y.; Xu, G.Z.; Gan, D.K. Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of Müller cells. Exp. Neurol. 2012, 238, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wen, R.; Li, F.; Cheng, T.; Steinberg, R.H. Induction of basic fibroblast growth factor mRNA by basic fibroblast growth factor in Muller cells. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1358–1366. [Google Scholar]
- Morimoto, T.; Miyoshi, T.; Matsuda, S.; Tano, Y.; Fujikado, T.; Fukuda, Y. Transcorneal electrical stimulation rescues axotomized retinal ganglion cells by activating endogenous retinal IGF-1 system. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2147–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecino, E.; David Rodriguez, F.; Ruzafa, N.; Pereiro, X.; Sharma, S.C.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zack, D.J. Neurotrophic Rescue of Photoreceptors. Neuron 2000, 26, 285–286. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, T.; Miyoshi, T.; Fujikado, T.; Tano, Y.; Fukuda, Y. Electrical stimulation enhances the survival of axotomized retinal ganglion cells in vivo. Neuroreport 2002, 13, 227–230. [Google Scholar] [CrossRef]
- Takeda, M.; Takamiya, A.; Jiao, J.W.; Cho, K.S.; Trevino, S.G.; Matsuda, T.; Chen, D.F. α-Aminoadipate induces progenitor cell properties of müller glia in adult mice. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1142–1150. [Google Scholar] [CrossRef]
- Sato, T.; Fujikado, T.; Morimoto, T.; Matsushita, K.; Harada, T.; Tano, Y. Effect of electrical stimulation on IGF-1 transcription by L-type calcium channels in cultured retinal Müller cells. Jpn. J. Ophthalmol. 2008, 52, 217–223. [Google Scholar] [CrossRef]
- Fang, Y.; Cho, K.-S.; Tchedre, K.; Lee, S.W.; Guo, C.; Kinouchi, H.; Fried, S.; Sun, X.; Chen, D.F. Ephrin-A3 Suppresses Wnt Signaling to Control Retinal Stem Cell Potency. Stem Cells 2013, 31, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.; Cheng, L.; Cho, K.; Malik, M.T.A.; Xiao, L.; Guo, C.; Yu, H.; Zhu, R.; Rao, R.C.; Chen, D.F. Postnatal onset of retinal degeneration by loss of embryonic Ezh2 repression of Six1. Sci. Rep. 2016, 6, 33887. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Wong, L.J.; Yan, N.; Han, R.C.; Yu, H.; Guo, C.; Batsuuri, K.; Zinzuwadia, A.; Guan, R.; Cho, K.-S.; et al. Ezh2 does not mediate retinal ganglion cell homeostasis or their susceptibility to injury. PLoS ONE 2018, 13, e0191853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Vasaikar, S.; Shi, Z.; Greer, M.; Zhang, B. WebGestalt 2017: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017, 45, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Cho, K.S.; Li, Y.; Tchedre, K.; Antolik, C.; Ma, J.; Chew, J.; Utheim, T.P.; Huang, X.A.; Yu, H.; et al. IGFBPL1 Regulates Axon Growth through IGF-1-mediated Signaling Cascades. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.; Yin, H.; Zhang, W.; Miao, Q.; Qin, Z.; Guo, S.; Fu, Q.; Ma, J.; Wu, F.; Yin, J.; et al. Transcorneal electrical stimulation promotes survival of retinal ganglion cells after optic nerve transection in rats accompanied by reduced microglial activation and TNF-α expression. Brain Res. 2016, 1650, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Gotea, V.; Ovcharenko, I. DiRE: Identifying distant regulatory elements of co-expressed genes. Nucleic Acids Res. 2008, 36, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattyn, A.; Vallstedt, A.; Dias, J.M.; Samad, O.A.; Krumlauf, R.; Rijli, F.M.; Brunet, J.-F.; Ericson, J. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 2003, 17, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dichmann, D.S.; Harland, R.M. Nkx6 genes pattern the frog neural plate and Nkx6.1 is necessary for motoneuron axon projection. Dev. Biol. 2011, 349, 378–386. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Wu, Y.; Feng, Y.; Lin, S.-C.; Lin, X. The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev. Cell 2009, 17, 470–481. [Google Scholar] [CrossRef] [Green Version]
- Takebayashi, K.; Sasai, Y.; Sakai, Y.; Watanabe, T.; Nakanishi, S.; Kageyama, R. Structure, chromosomal locus, and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES-1. Negative autoregulation through the multiple N box elements. J. Biol. Chem. 1994, 269, 5150–5156. [Google Scholar]
- Habener, J.F.; Kemp, D.M.; Thomas, M.K. Minireview: Transcriptional regulation in pancreatic development. Endocrinology 2005, 146, 1025–1034. [Google Scholar] [CrossRef]
- Muranishi, Y.; Terada, K.; Furukawa, T. An essential role for Rax in retina and neuroendocrine system development. Dev. Growth Differ. 2012, 54, 341–348. [Google Scholar] [CrossRef]
- Bourinet, E.; Mangoni, M.E.; Nargeot, J. Dissecting the functional role of different isoforms of the L-type Ca2+ channel. J. Clin. Invest. 2004, 113, 1382–1384. [Google Scholar] [CrossRef] [Green Version]
- Anastassiou, G.; Schneegans, A.L.; Selbach, M.; Kremmer, S. Transpalpebral electrotherapy for dry age-related macular degeneration (AMD): An exploratory trial. Restor. Neurol. Neurosci. 2013, 31, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-P.; Zhao, J.-W.; Yang, X.-L. Expression of voltage-dependent calcium channel subunits in the rat retina. Neurosci. Lett. 2002, 329, 297–300. [Google Scholar] [CrossRef]
- Shi, L.; Chang, J.Y.; Yu, F.; Ko, M.L.; Ko, G.Y. The Contribution of L-Type Cav1. 3 Channels to Retinal Light Responses. Front. Mol. Neurosci. 2017, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hunter, D.J.; Rooker, S.; Chan, A.; Paulus, Y.M.; Leucht, P.; Nusse, Y.; Nomoto, H.; Helms, J.A. Wnt Signaling Promotes Müller Cell Proliferation and Survival after Injury. Investig. Opthalmol. Vis. Sci. 2013, 54, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osakada, F.; Ooto, S.; Akagi, T.; Mandai, M.; Akaike, A.; Takahashi, M. Wnt Signaling Promotes Regeneration in the Retina of Adult Mammals. J. Neurosci. 2007, 27, 4210–4219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.; Chen, L.L.; Lei, X.X.; Yang, L.; Lin, H.; Carmichael, G.G.; Huang, Y. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells 2011, 29, 496–504. [Google Scholar] [CrossRef]
- Melton, C.; Judson, R.; Blelloch, R. NIH Public Access. Cell 2010, 463, 621–626. [Google Scholar]
- Yu, H.; Talib, M.; Khanh Vu, T.H.; Cho, K.S.; Guo, C.; Chen, D.F. Mobilizing Endogenous Stem Cells for Retinal Repair. Transl. Regen. Med. Clin. 2015, 163, 297–308. [Google Scholar]
- Rhee, K.D.; Nusinowitz, S.; Chao, K.; Yu, F.; Bok, D.; Yang, X.J. CNTF-mediated protection of photoreceptors requires initial activation of the cytokine receptor gp130 in Müller glial cells. Proc. Natl. Acad. Sci. USA 2013, 110, E4520–E4529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, M.; Werginz, P.; Fried, S.I. Electric stimulus duration alters network-mediated responses depending on retinal ganglion cell type. J. Neural Eng. 2018, 15, 036010. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Paydarfar, D. Optimizing stimulus waveforms for electroceuticals. Biol. Cybern. 2019, 113, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Morimoto, T.; Sawai, H. Parameters of optic nerve electrical stimulation affecting neuroprotection of axotomized retinal ganglion cells in adult rats. Neurosci. Res. 2008, 61, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Miyoshi, T.; Sawai, H.; Fujikado, T. Optimal parameters of transcorneal electrical stimulation (TES) to be neuroprotective of axotomized RGCs in adult rats. Exp. Eye Res. 2010, 90, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Fujikado, T.; Choi, J.S.; Kanda, H.; Miyoshi, T.; Fukuda, Y.; Tano, Y. Transcorneal electrical stimulation promotes the survival of photoreceptors and preserves retinal function in Royal College of Surgeons rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4725–4732. [Google Scholar] [CrossRef]
- Fu, L.; Fung, F.K.; Lo, A.C.-Y.; Chan, Y.-K.; So, K.-F.; Wong, I.Y.-H.; Shih, K.C.; Lai, J.S.-M. Transcorneal electrical stimulation inhibits retinal microglial activation and enhances retinal ganglion cell survival after acute ocular hypertensive injury. Transl. Vis. Sci. Technol. 2018, 7, 7. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enayati, S.; Chang, K.; Achour, H.; Cho, K.-S.; Xu, F.; Guo, S.; Z. Enayati, K.; Xie, J.; Zhao, E.; Turunen, T.; et al. Electrical Stimulation Induces Retinal Müller Cell Proliferation and Their Progenitor Cell Potential. Cells 2020, 9, 781. https://doi.org/10.3390/cells9030781
Enayati S, Chang K, Achour H, Cho K-S, Xu F, Guo S, Z. Enayati K, Xie J, Zhao E, Turunen T, et al. Electrical Stimulation Induces Retinal Müller Cell Proliferation and Their Progenitor Cell Potential. Cells. 2020; 9(3):781. https://doi.org/10.3390/cells9030781
Chicago/Turabian StyleEnayati, Sam, Karen Chang, Hamida Achour, Kin-Sang Cho, Fuyi Xu, Shuai Guo, Katarina Z. Enayati, Jia Xie, Eric Zhao, Tytteli Turunen, and et al. 2020. "Electrical Stimulation Induces Retinal Müller Cell Proliferation and Their Progenitor Cell Potential" Cells 9, no. 3: 781. https://doi.org/10.3390/cells9030781
APA StyleEnayati, S., Chang, K., Achour, H., Cho, K.-S., Xu, F., Guo, S., Z. Enayati, K., Xie, J., Zhao, E., Turunen, T., Sehic, A., Lu, L., Utheim, T. P., & Chen, D. F. (2020). Electrical Stimulation Induces Retinal Müller Cell Proliferation and Their Progenitor Cell Potential. Cells, 9(3), 781. https://doi.org/10.3390/cells9030781