The Brain–Skin Connection and the Pathogenesis of Psoriasis: A Review with a Focus on the Serotonergic System
Abstract
1. Introduction
2. The Brain–Skin Connection
2.1. The Skin as a Neuroendocrine Organ
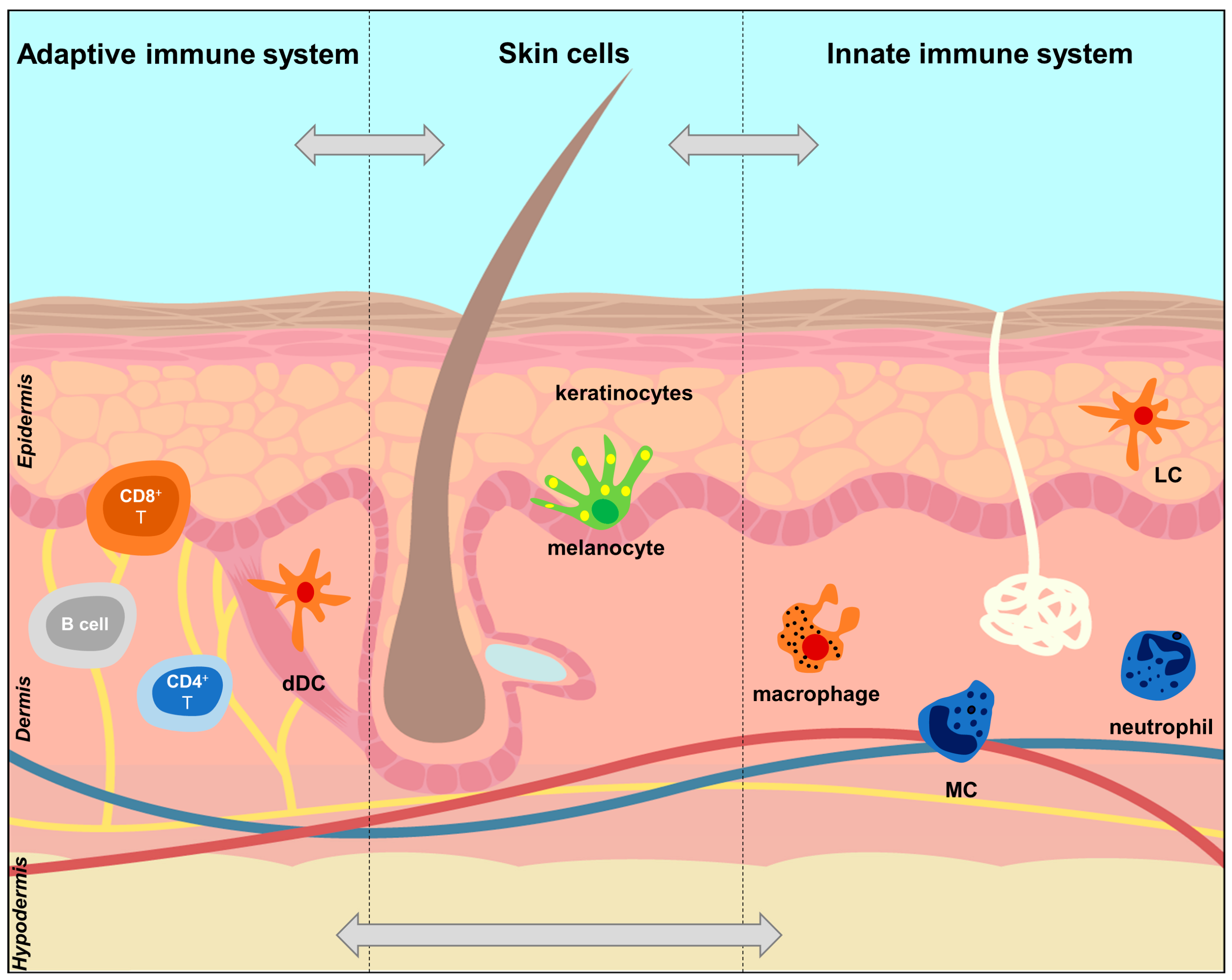
2.2. The Skin Immune System
2.3. The Serotonin System and Its Importance in Skin Neuroendocrine and Immune Systems
2.3.1. Serotonin Metabolism, Transport and Function
2.3.2. Serotonin and the Immune System
2.3.3. Serotonin in Skin
2.4. Effects of Psychological Stress on the Skin
3. Psoriasis
3.1. Introduction
3.2. The Pathogenesis of Psoriasis
3.2.1. Genetic Basis of Psoriasis
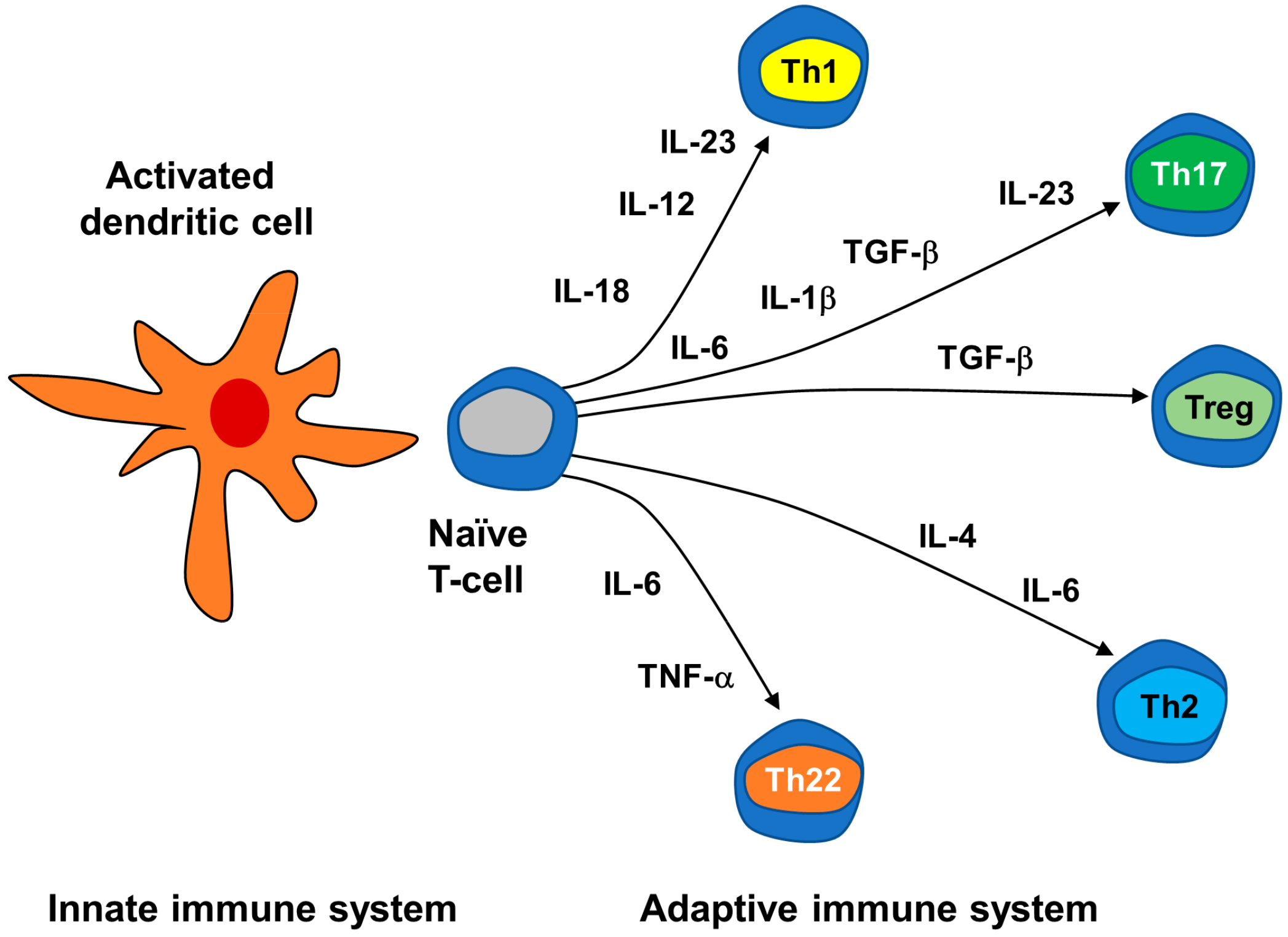
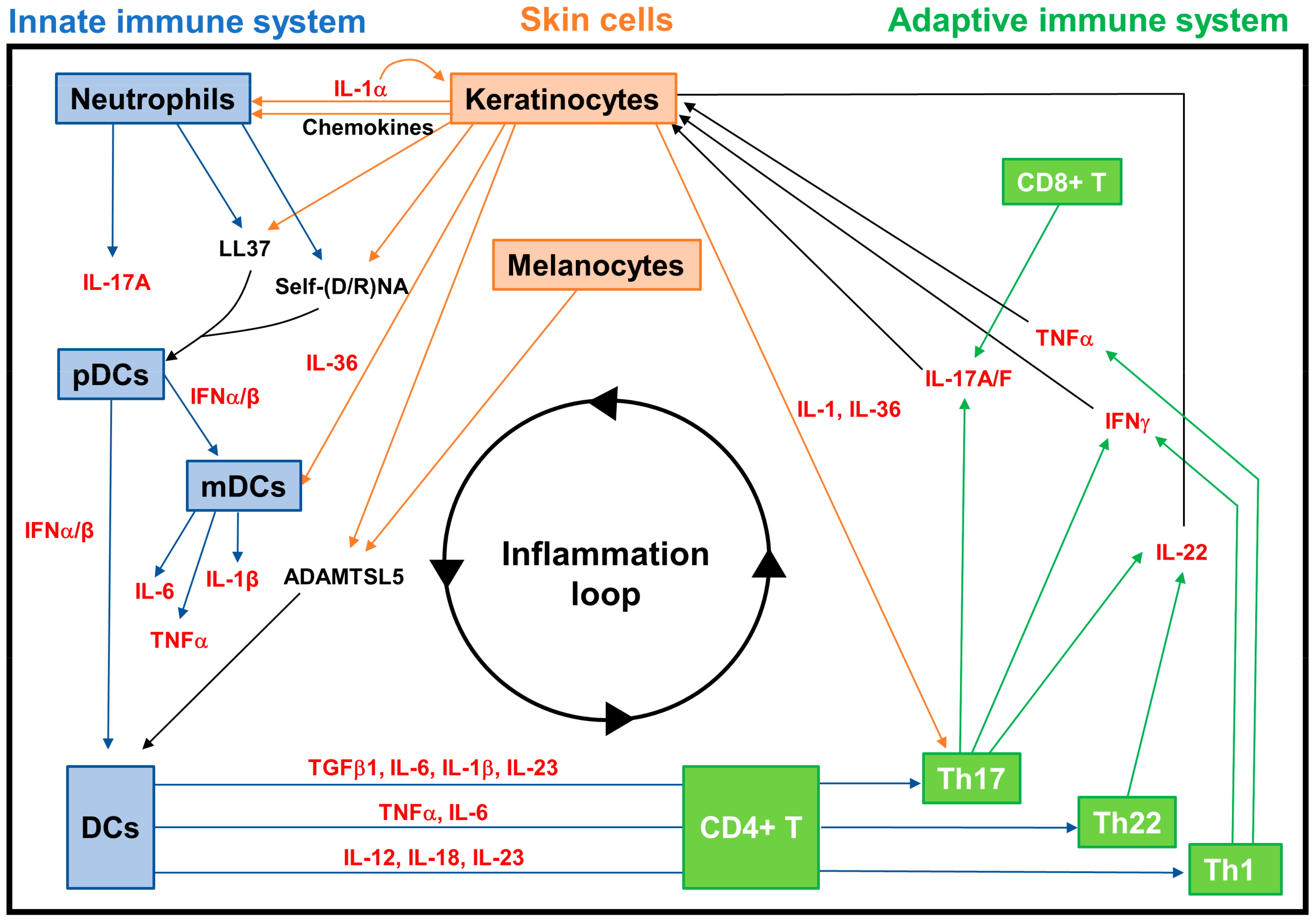
3.2.2. Neuroimmune Basis of Psoriasis
3.2.3. The Role of Serotonin in Psoriasis
4. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arck, P.C.; Slominski, A.; Theoharides, T.C.; Peters, E.M.; Paus, R. Neuroimmunology of stress: Skin takes center stage. J. Invest. Dermatol. 2006, 126, 1697–1704. [Google Scholar] [CrossRef]
- Fidalgo, S.; Ivanov, D.K.; Wood, S.H. Serotonin: From top to bottom. Biogerontology 2013, 14, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Mossner, R.; Lesch, K.P. Role of serotonin in the immune system and in neuroimmune interactions. Brain. Behav. Immun. 1998, 12, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Ahern, G.P. 5-HT and the immune system. Curr. Opin. Pharmacol. 2011, 11, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Bockaert, J.; Claeysen, S.; Becamel, C.; Dumuis, A.; Marin, P. Neuronal 5-HT metabotropic receptors: Fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006, 326, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Thorslund, K. Serotonergic Mechanisms in Psoriasis. Ph.D. Thesis, Karolinska Instituet, Stockholm, Sweden, 2012. [Google Scholar]
- Menezes, A.C.; Raposo, S.; Simoes, S.; Ribeiro, H.; Oliveira, H.; Ascenso, A. Prevention of photocarcinogenesis by agonists of 5-HT1A and antagonists of 5-HT2A receptors. Mol. Neurobiol. 2016, 53, 1145–1164. [Google Scholar] [CrossRef]
- Nordlind, K.; Azmitia, E.C.; Slominski, A. The skin as a mirror of the soul: Exploring the possible roles of serotonin. Exp. Dermatol. 2008, 17, 301–311. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Slominski, A.T. Neuroendocrinology of the skin. Dermatoendocrinology 2011, 3, 3–10. [Google Scholar] [CrossRef]
- Arck, P.; Paus, R. From the brain-skin connection: The neuroendocrine-immune misalliance of stress and itch. Neuroimmunomodulation 2006, 13, 347–356. [Google Scholar] [CrossRef]
- Chen, Y.; Maidof, R.; Lyga, J. Brain-skin connection: Impact of psychological stress on skin. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 2131–2152. [Google Scholar] [CrossRef]
- Harden, J.L.; Krueger, J.G.; Bowcock, A.M. The immunogenetics of psoriasis: A comprehensive review. J. Autoimmun. 2015, 64, 66–73. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Benhadou, F.; Mintoff, D.; del Marmol, V. Psoriasis: Keratinocytes or immune cells—Which is the trigger? Dermatology 2019, 235, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Zaid, A.; Carbone, F.R. Tissue-resident T cells: Dynamic players in skin immunity. Front. Immunol. 2014, 5, 332. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Paus, R.; Elias, P.M.; Tobin, D.J.; Feingold, K.R. Skin as an endocrine organ: Implications for its function. Drug Discov. Today Dis. Mech. 2008, 5, 137–144. [Google Scholar] [CrossRef]
- Slominski, A. Neuroendocrine system of the skin. Dermatology 2005, 211, 199–208. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Tobin, D.J. The cutaneous serotoninergic/melatoninergic system: Securing a place under the sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef]
- Luo, T.; Ma, Y.; Wei, W. Murine models of psoriasis and its applications in drug development. J. Pharmacol. Toxicol. Methods 2020, 101, 106657. [Google Scholar] [CrossRef]
- Kobayashi, T.; Naik, S.; Nagao, K. Choreographing immunity in the skin epithelial barrier. Immunity 2019, 50, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A. Skin Immunity. Arch. Immunol. Ther. Exp. (Warsz.) 2018, 66, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.S.; Röcken, M.; Ghoreschi, K. Cutaneous immunology: Basics and new concepts. Semin. Immunopathol. 2016, 38, 3–10. [Google Scholar] [CrossRef]
- Hugh, J.M.; Weinberg, J.M. Update on the pathophysiology of psoriasis. Cutis 2018, 102, 6–12. [Google Scholar] [PubMed]
- Romani, N.; Brunner, P.M.; Stingl, G. Changing views of the role of Langerhans cells. J. Invest. Dermatol. 2012, 132, 872–881. [Google Scholar] [CrossRef]
- Umnyakova, E.S.; Zharkova, M.S.; Berlov, M.N.; Shamova, O.V.; Kokryakov, V.N. Human antimicrobial peptides in autoimmunity. Autoimmunity 2020, 1–11. [Google Scholar] [CrossRef]
- Dubois, B.; Bridon, J.M.; Fayette, J.; Barthelemy, C.; Banchereau, J.; Caux, C.; Briere, F. Dendritic cells directly modulate B cell growth and differentiation. J. Leukoc. Biol. 1999, 66, 224–230. [Google Scholar] [CrossRef]
- Wollenberg, A.; Wagner, M.; Gunther, S.; Towarowski, A.; Tuma, E.; Moderer, M.; Rothenfusser, S.; Wetzel, S.; Endres, S.; Hartmann, G. Plasmacytoid dendritic cells: A new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J. Invest. Dermatol. 2002, 119, 1096–1102. [Google Scholar] [CrossRef]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Farkas, A.; Tonel, G.; Nestle, F.O. Interferon-alpha and viral triggers promote functional maturation of human monocyte-derived dendritic cells. Br. J. Dermatol. 2008, 158, 921–929. [Google Scholar] [CrossRef]
- Otsuka, A.; Kubo, M.; Honda, T.; Egawa, G.; Nakajima, S.; Tanizaki, H.; Kim, B.; Matsuoka, S.; Watanabe, T.; Nakae, S.; et al. Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS ONE 2011, 6, e25538. [Google Scholar] [CrossRef] [PubMed]
- Dress, R.J.; Wong, A.Y.; Ginhoux, F. Homeostatic control of dendritic cell numbers and differentiation. Immunol. Cell Biol. 2018, 96, 463–476. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, P.J.; Wang, X.; Leon-Ponte, M.; Griffiths, C.; Pingle, S.C.; Ahern, G.P. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood 2006, 107, 1010–1017. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef] [PubMed]
- Jonnakuty, C.; Gragnoli, C. What do we know about serotonin? J. Cell. Physiol. 2008, 217, 301–306. [Google Scholar] [CrossRef]
- Leon-Ponte, M.; Ahern, G.P.; O’Connell, P.J. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 2007, 109, 3139–3146. [Google Scholar] [CrossRef]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Thorslund, K.; El-Nour, H.; Nordlind, K. The serotonin transporter protein is expressed in psoriasis, where it may play a role in regulating apoptosis. Arch. Dermatol. Res. 2009, 301, 449–457. [Google Scholar] [CrossRef]
- Idzko, M.; Panther, E.; Stratz, C.; Muller, T.; Bayer, H.; Zissel, G.; Durk, T.; Sorichter, S.; Di Virgilio, F.; Geissler, M.; et al. The serotoninergic receptors of human dendritic cells: Identification and coupling to cytokine release. J. Immunol. 2004, 172, 6011–6019. [Google Scholar] [CrossRef]
- Kushnir-Sukhov, N.M.; Brown, J.M.; Wu, Y.; Kirshenbaum, A.; Metcalfe, D.D. Human mast cells are capable of serotonin synthesis and release. J. Allergy Clin. Immunol. 2007, 119, 498–499. [Google Scholar] [CrossRef]
- Kushnir-Sukhov, N.M.; Gilfillan, A.M.; Coleman, J.W.; Brown, J.M.; Bruening, S.; Toth, M.; Metcalfe, D.D. 5-Hydroxytryptamine induces mast cell adhesion and migration. J. Immunol. 2006, 177, 6422–6432. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Doe, J.M.; Horstmann, S.A.; Ahmad, S.; Ahmad, A.; Min, S.-J.; Reynolds, P.R.; Suram, S.; Gaydos, J.; Burnham, E.L.; et al. Neuroendocrine signaling via the serotonin transporter regulates clearance of apoptotic cells. J. Biol. Chem. 2014, 289, 10466–10475. [Google Scholar] [CrossRef]
- De las Casas-Engel, M.; Domínguez-Soto, A.; Sierra-Filardi, E.; Bragado, R.; Nieto, C.; Puig-Kroger, A.; Samaniego, R.; Loza, M.; Corcuera, M.T.; Gómez-Aguado, F.; et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J. Immunol. 2013, 190, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Dürk, T.; Panther, E.; Müller, T.; Sorichter, S.; Ferrari, D.; Pizzirani, C.; Di Virgilio, F.; Myrtek, D.; Norgauer, J.; Idzko, M. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int. Immunol. 2005, 17, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Albert, R.H.; Tretiakova, A.P.; Jameson, B.A. 5-HT(1B) receptors play a prominent role in the proliferation of T-lymphocytes. J. Neuroimmunol. 2006, 181, 68–81. [Google Scholar] [CrossRef]
- Chen, Y.; Leon-Ponte, M.; Pingle, S.C.; O’Connell, P.J.; Ahern, G.P. T lymphocytes possess the machinery for 5-HT synthesis, storage, degradation and release. Acta Physiol. (Oxf.) 2015, 213, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Aune, T.M.; McGrath, K.M.; Sarr, T.; Bombara, M.P.; Kelley, K.A. Expression of 5HT1a receptors on activated human T cells. Regulation of cyclic AMP levels and T cell proliferation by 5-hydroxytryptamine. J. Immunol. 1993, 151, 1175–1183. [Google Scholar]
- Lee, M.S.; Hanspers, K.; Barker, C.S.; Korn, A.P.; McCune, J.M. Gene expression profiles during human CD4+ T cell differentiation. Int. Immunol. 2004, 16, 1109–1124. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Akundi, R.S.; Seidel, M.; Geyer, V.; Haus, U.; Muller, W.; Stratz, T.; Candelario-Jalil, E. Expression of 5-HT3A receptors in cells of the immune system. Scand. J. Rheumatol. Suppl. 2004, 119, 9–11. [Google Scholar] [CrossRef]
- Magrini, E.; Szabò, I.; Doni, A.; Cibella, J.; Viola, A. Serotonin-mediated tuning of human helper T cell responsiveness to the chemokine CXCL12. PLoS ONE 2011, 6, e22482. [Google Scholar] [CrossRef][Green Version]
- Meredith, E.J.; Holder, M.J.; Chamba, A.; Challa, A.; Drake-Lee, A.; Bunce, C.M.; Drayson, M.T.; Pilkington, G.; Blakely, R.D.; Dyer, M.J.; et al. The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: Probing a potential anti-tumor target for psychotropics. FASEB J. 2005, 19, 1187–1189. [Google Scholar] [CrossRef]
- Iken, K.; Chheng, S.; Fargin, A.; Goulet, A.C.; Kouassi, E. Serotonin upregulates mitogen-stimulated B lymphocyte proliferation through 5-HT1A receptors. Cell. Immunol. 1995, 163, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Chiaravalli, A.M.; Mian, M.; Zucca, E.; Tibiletti, M.G.; Capella, C.; Bertoni, F. Serotonin receptor 3A expression in normal and neoplastic B cells. Pathobiology 2010, 77, 129–135. [Google Scholar] [CrossRef]
- Slominski, A.; Pisarchik, A.; Zbytek, B.; Tobin, D.J.; Kauser, S.; Wortsman, J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell. Physiol. 2003, 196, 144–153. [Google Scholar] [CrossRef]
- Lundeberg, L.; El-Nour, H.; Mohabbati, S.; Morales, M.; Azmitia, E.; Nordlind, K. Expression of serotonin receptors in allergic contact eczematous human skin. Arch. Dermatol. Res. 2002, 294, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Roumier, A.; Béchade, C.; Maroteaux, L. Serotonin and the immune system. In Serotonin; Pilowsky, P.M., Ed.; Academic Press: Boston, MA, USA, 2019; pp. 181–196. [Google Scholar] [CrossRef]
- Muller, T.; Durk, T.; Blumenthal, B.; Grimm, M.; Cicko, S.; Panther, E.; Sorichter, S.; Herouy, Y.; Di Virgilio, F.; Ferrari, D.; et al. 5-Hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS ONE 2009, 4, e6453. [Google Scholar] [CrossRef] [PubMed]
- Holst, K.; Guseva, D.; Schindler, S.; Sixt, M.; Braun, A.; Chopra, H.; Pabst, O.; Ponimaskin, E. The serotonin receptor 5-HT(7)R regulates the morphology and migratory properties of dendritic cells. J. Cell Sci. 2015, 128, 2866–2880. [Google Scholar] [CrossRef]
- Inoue, M.; Okazaki, T.; Kitazono, T.; Mizushima, M.; Omata, M.; Ozaki, S. Regulation of antigen-specific CTL and Th1 cell activation through 5-hydroxytryptamine 2A receptor. Int. Immunopharmacol. 2011, 11, 67–73. [Google Scholar] [CrossRef]
- Laberge, S.; Cruikshank, W.W.; Beer, D.J.; Center, D.M. Secretion of IL-16 (lymphocyte chemoattractant factor) from serotonin-stimulated CD8+ T cells in vitro. J. Immunol. 1996, 156, 310–315. [Google Scholar]
- Serafeim, A.; Grafton, G.; Chamba, A.; Gregory, C.D.; Blakely, R.D.; Bowery, N.G.; Barnes, N.M.; Gordon, J. 5-Hydroxytryptamine drives apoptosis in biopsylike Burkitt lymphoma cells: Reversal by selective serotonin reuptake inhibitors. Blood 2002, 99, 2545–2553. [Google Scholar] [CrossRef]
- Schrocksnadel, K.; Wirleitner, B.; Winkler, C.; Fuchs, D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta 2006, 364, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Blakely, R.D.; Hewlett, W.A. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 2006, 31, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Endoh, M.; Fujiwara, N.; Nawa, T. Receptors and transporter for serotonin in Merkel cell-nerve endings in the rat sinus hair follicle. An immunohistochemical study. Arch. Histol. Cytol. 2005, 68, 19–28. [Google Scholar] [CrossRef][Green Version]
- El-Nour, H.; Lundeberg, L.; Boman, A.; Abramowski, D.; Holst, M.; Nordlind, K. The expression and functional significance of the serotonin(2C) receptor in murine contact allergy. Exp. Dermatol. 2007, 16, 644–650. [Google Scholar] [CrossRef]
- Weisshaar, E.; Ziethen, B.; Gollnick, H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm. Res. 1997, 46, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Armstrong, E.J.; Armstrong, A.W. Corticosteroids and wound healing: Clinical considerations in the perioperative period. Am. J. Surg. 2013, 206, 410–417. [Google Scholar] [CrossRef]
- Padgett, D.A.; Glaser, R. How stress influences the immune response. Trends Immunol. 2003, 24, 444–448. [Google Scholar] [CrossRef]
- Eberle, F.C.; Brück, J.; Holstein, J.; Hirahara, K.; Ghoreschi, K. Recent advances in understanding psoriasis. F1000Research 2016, 5, F1000 Faculty Rev-770. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; van der Walt, J.M.; Ashcroft, D.M.; Flohr, C.; Naldi, L.; Nijsten, T.; Augustin, M. The global state of psoriasis disease epidemiology: A workshop report. Br. J. Dermatol. 2017, 177, e4–e7. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.A.; Loser, K. Novel insights into the pathogenesis of psoriasis. Clin. Immunol. 2018, 186, 43–45. [Google Scholar] [CrossRef]
- Feldman, S.R.; Burudpakdee, C.; Gala, S.; Nanavaty, M.; Mallya, U.G. The economic burden of psoriasis: A systematic literature review. Expert Rev. Pharmacoecon. Outcomes Res. 2014, 14, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sarkar, M.K.; Tsoi, L.C.; Gudjonsson, J.E. Psoriasis: A mixed autoimmune and autoinflammatory disease. Curr. Opin. Immunol. 2017, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Lee, K.M.; Ucmak, D.; Brodsky, M.; Atanelov, Z.; Farahnik, B.; Abrouk, M.; Nakamura, M.; Zhu, T.H.; Liao, W. Erythrodermic psoriasis: Pathophysiology and current treatment perspectives. Psoriasis (Auckland, N.Z.) 2016, 6, 93–104. [Google Scholar] [CrossRef]
- Omland, S.H.; Gniadecki, R. Psoriasis inversa: A separate identity or a variant of psoriasis vulgaris? Clin. Dermatol. 2015, 33, 456–461. [Google Scholar] [CrossRef]
- Fry, L.; Baker, B.S. Triggering psoriasis: The role of infections and medications. Clin. Dermatol. 2007, 25, 606–615. [Google Scholar] [CrossRef]
- Gudjonsson, J.E.; Thorarinsson, A.M.; Sigurgeirsson, B.; Kristinsson, K.G.; Valdimarsson, H. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: A prospective study. Br. J. Dermatol. 2003, 149, 530–534. [Google Scholar] [CrossRef]
- Koo, J.; Lebwohl, A. Psycho dermatology: The mind and skin connection. Am. Fam. Physician 2001, 64, 1873–1878. [Google Scholar]
- Dowlatshahi, E.A.; Wakkee, M.; Herings, R.M.; Hollestein, L.M.; Nijsten, T. Increased antidepressant drug exposure in psoriasis patients: A longitudinal population-based cohort study. Acta Derm. Venereol. 2013, 93, 544–550. [Google Scholar] [CrossRef]
- Oliveira, M.d.F.S.P.d.; Rocha, B.d.O.; Duarte, G.V. Psoriasis: Classical and emerging comorbidities. An. Bras. Dermatol. 2015, 90, 9–20. [Google Scholar] [CrossRef]
- Mueller, W.; Herrmann, B. Cyclosporin A for psoriasis. N. Engl. J. Med. 1979, 301, 555–556. [Google Scholar] [CrossRef]
- Bos, J.D.; Van Joost, T.H.; Powles, A.V.; Meinardi, M.M.H.M.; Heule, F.; Fry, L. Use of cyclosporin in psoriasis. Lancet 1989, 334, 1500–1502. [Google Scholar] [CrossRef]
- Albanesi, C.; Madonna, S.; Gisondi, P.; Girolomoni, G. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front. Immunol. 2018, 9, 1549. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schakel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef]
- Wang, A.; Bai, Y. Dendritic cells: The driver of psoriasis. J. Dermatol. 2019, 47, 104–113. [Google Scholar] [CrossRef]
- Alexander, H.; Nestle, F.O. Pathogenesis and immunotherapy in cutaneous psoriasis: What can rheumatologists learn? Curr. Opin. Rheumatol. 2017, 29, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gungor, S.; Rezigue, M. Nanocarriers mediated topical drug delivery for psoriasis treatment. Curr. Drug Metab. 2017, 18, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Hannen, R.; Udeh-Momoh, C.; Upton, J.; Wright, M.; Michael, A.; Gulati, A.; Rajpopat, S.; Clayton, N.; Halsall, D.; Burrin, J.; et al. Dysfunctional skin-derived glucocorticoid synthesis is a pathogenic mechanism of psoriasis. J. Invest. Dermatol. 2017, 137, 1630–1637. [Google Scholar] [CrossRef]
- Generali, E.; Ceribelli, A.; Stazi, M.A.; Selmi, C. Lessons learned from twins in autoimmune and chronic inflammatory diseases. J. Autoimmun. 2017, 83, 51–61. [Google Scholar] [CrossRef]
- Mohd Affandi, A.; Khan, I.; Ngah Saaya, N. Epidemiology and clinical features of adult patients with psoriasis in malaysia: 10-year review from the malaysian psoriasis registry (2007–2016). Dermatol. Res. Pract. 2018, 2018, 4371471. [Google Scholar] [CrossRef]
- Ran, D.; Cai, M.; Zhang, X. Genetics of psoriasis: A basis for precision medicine. Precis. Clin. Med. 2019, 2, 120–130. [Google Scholar] [CrossRef]
- Capon, F. The genetic basis of psoriasis. Int. J. Mol. Sci. 2017, 18, 2526. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pradhan, D.; Puri, P.; Ramesh, V.; Aggarwal, S.; Nayek, A.; Jain, A.K. Genomic alterations driving psoriasis pathogenesis. Gene 2019, 683, 61–71. [Google Scholar] [CrossRef]
- Wiśniewski, A.; Matusiak, Ł.; Szczerkowska-Dobosz, A.; Nowak, I.; Kuśnierczyk, P. HLA-C*06:02-independent, gender-related association of PSORS1C3 and PSORS1C1/CDSN single-nucleotide polymorphisms with risk and severity of psoriasis. Mol. Genet. Genom. MGG 2018, 293, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef]
- Bettelli, E.; Oukka, M.; Kuchroo, V.K. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007, 8, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Schon, M.P. Adaptive and innate immunity in psoriasis and other inflammatory disorders. Front. Immunol. 2019, 10, 1764. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Morales, J.M.G.; Puig, L.; Dauden, E.; Canete, J.D.; Pablos, J.L.; Martin, A.O.; Juanatey, C.G.; Adan, A.; Montalban, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621. [Google Scholar] [CrossRef]
- Lande, R.; Chamilos, G.; Ganguly, D.; Demaria, O.; Frasca, L.; Durr, S.; Conrad, C.; Schroder, J.; Gilliet, M. Cationic antimicrobial peptides in psoriatic skin cooperate to break innate tolerance to self-DNA. Eur. J. Immunol. 2015, 45, 203–213. [Google Scholar] [CrossRef]
- Arakawa, A.; Siewert, K.; Stohr, J.; Besgen, P.; Kim, S.M.; Ruhl, G.; Nickel, J.; Vollmer, S.; Thomas, P.; Krebs, S.; et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J. Exp. Med. 2015, 212, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, K.M.; Kunjravia, N.; Krueger, J.G.; Fuentes-Duculan, J. Cutaneous expression of a disintegrin-like and metalloprotease domain containing thrombospondin type 1 motif-like 5 (ADAMTSL5) in psoriasis goes beyond melanocytes. J. Pigment. Disord. 2016, 3, 244. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-G.; Kim, D.S.; Kim, H.-P.; Lee, M.-G. The pathophysiological role of dendritic cell subsets in psoriasis. BMB Rep. 2014, 47, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ketikoglou, I.; Karatapanis, S.; Elefsiniotis, I.; Kafiri, G.; Moulakakis, A. Extensive psoriasis induced by pegylated interferon alpha-2b treatment for chronic hepatitis B. Eur. J. Dermatol. 2005, 15, 107–109. [Google Scholar]
- Foster, A.M.; Baliwag, J.; Chen, C.S.; Guzman, A.M.; Stoll, S.W.; Gudjonsson, J.E.; Ward, N.L.; Johnston, A. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J. Immunol. 2014, 192, 6053–6061. [Google Scholar] [CrossRef]
- Li, N.; Yamasaki, K.; Saito, R.; Fukushi-Takahashi, S.; Shimada-Omori, R.; Asano, M.; Aiba, S. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36γ induction in human epidermal keratinocytes. J. Immunol. 2014, 193, 5140–5148. [Google Scholar] [CrossRef]
- Schön, M.P.; Erpenbeck, L. The Interleukin-23/Interleukin-17 axis links adaptive and innate immunity in psoriasis. Front. Immunol. 2018, 9, 1323. [Google Scholar] [CrossRef]
- Fitch, E.; Harper, E.; Skorcheva, I.; Kurtz, S.E.; Blauvelt, A. Pathophysiology of psoriasis: Recent advances on IL-23 and Th17 cytokines. Curr. Rheumatol. Rep. 2007, 9, 461–467. [Google Scholar] [CrossRef]
- Muhr, P.; Renne, J.; Schaefer, V.; Werfel, T.; Wittmann, M. Primary human keratinocytes efficiently induce IL-1-dependent IL-17 in CCR6+ T cells. Exp. Dermatol. 2010, 19, 1105–1107. [Google Scholar] [CrossRef]
- Chen, X.; Oppenheim, J.J. Th17 cells and Tregs: Unlikely allies. J. Leukoc. Biol. 2014, 95, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, S.; Bouguermouh, S.; Rubio, M.; Baba, N.; Bissonnette, R.; Sarfati, M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J. Allergy Clin. Immunol. 2015, 136, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H. The role of IL-22 and Th22 cells in human skin diseases. J. Dermatol. Sci. 2013, 72, 3–8. [Google Scholar] [CrossRef]
- Grine, L.; Dejager, L.; Libert, C.; Vandenbroucke, R.E. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015, 26, 25–33. [Google Scholar] [CrossRef]
- Gisondi, P.; Del Giglio, M.; Girolomoni, G. Treatment approaches to moderate to severe psoriasis. Int. J. Mol. Sci. 2017, 18, 2427. [Google Scholar] [CrossRef]
- Kurd, S.K.; Troxel, A.B.; Crits-Christoph, P.; Gelfand, J.M. The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Arch. Dermatol. 2010, 146, 891–895. [Google Scholar] [CrossRef]
- Egeberg, A.; Thyssen, J.P.; Wu, J.J.; Skov, L. Risk of first-time and recurrent depression in patients with psoriasis: A population-based cohort study. Br. J. Dermatol. 2019, 180, 116–121. [Google Scholar] [CrossRef]
- Galecki, P.; Mossakowska-Wojcik, J.; Talarowska, M. The anti-inflammatory mechanism of antidepressants—SSRIs, SNRIs. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 291–294. [Google Scholar] [CrossRef]
- Tsao, C.W.; Lin, Y.S.; Chen, C.C.; Bai, C.H.; Wu, S.R. Cytokines and serotonin transporter in patients with major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 899–905. [Google Scholar] [CrossRef]
- D’Erme, A.M.; Zanieri, F.; Campolmi, E.; Santosuosso, U.; Betti, S.; Agnoletti, A.F.; Cossidente, A.; Lotti, T. Therapeutic implications of adding the psychotropic drug escitalopram in the treatment of patients suffering from moderate-severe psoriasis and psychiatric comorbidity: A retrospective study. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Thorslund, K.; Nordlind, K. Serotonergic drugs–A possible role in the treatment of psoriasis? Drug News Perspect. 2007, 20, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Thorslund, K.; Svensson, T.; Nordlind, K.; Ekbom, A.; Fored, C.M. Use of serotonin reuptake inhibitors in patients with psoriasis is associated with a decreased need for systemic psoriasis treatment: A population-based cohort study. J. Intern. Med. 2013, 274, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, G.; Xiang, J.; Yin, D.; Chi, R. Immunohistochemical study of serotonin in lesions of psoriasis. Int. J. Dermatol. 2004, 43, 408–411. [Google Scholar] [CrossRef]
- Younes, S.F.; Bakry, O.A. Immunohistochemical Evaluation of Role of Serotonin in Pathogenesis of Psoriasis. J. Clin. Diagn. Res. 2016, 10, Ec05–Ec09. [Google Scholar] [CrossRef]
- Nordlind, K.; Thorslund, K.; Lonne-Rahm, S.B.; Mohabbati, S.; Berki, T.; Morales, M.; Azmitia, E.C. Expression of serotonergic receptors in psoriatic skin. Arch. Dermatol. Res. 2006, 298, 99–106. [Google Scholar] [CrossRef]
- Morita, T.; McClain, S.P.; Batia, L.M.; Pellegrino, M.; Wilson, S.R.; Kienzler, M.A.; Lyman, K.; Olsen, A.S.; Wong, J.F.; Stucky, C.L.; et al. HTR7 mediates serotonergic acute and chronic itch. Neuron 2015, 87, 124–138. [Google Scholar] [CrossRef]
- Sevimoglu, T.; Arga, K.Y. Computational systems biology of psoriasis: Are we ready for the age of omics and systems biomarkers? OMICS 2015, 19, 669–687. [Google Scholar] [CrossRef]
- Chularojanamontri, L.; Charoenpipatsin, N.; Silpa-Archa, N.; Wongpraparut, C.; Thongboonkerd, V. Proteomics in psoriasis. Int. J. Mol. Sci. 2019, 20, 1141. [Google Scholar] [CrossRef]
- Yan, D.; Afifi, L.; Jeon, C.; Trivedi, M.; Chang, H.W.; Lee, K.; Liao, W. The metabolomics of psoriatic disease. Psoriasis (Auckland, N.Z.) 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Zhao, Y.; Jhamb, D.; Shu, L.; Arneson, D.; Rajpal, D.K.; Yang, X. Multi-omics integration reveals molecular networks and regulators of psoriasis. BMC Syst. Biol. 2019, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gaiteri, C.; Bodea, L.G.; Wang, Z.; McElwee, J.; Podtelezhnikov, A.A.; Zhang, C.; Xie, T.; Tran, L.; Dobrin, R.; et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 2013, 153, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, J.; Freudenberg, J.M.; Meng, Q.; Rajpal, D.K.; Yang, X. Network-based identification and prioritization of key regulators of coronary artery disease loci. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Chan, K.H.K.; Zhang, G.; Huan, T.; Kurt, Z.; Zhao, Y.; Codoni, V.; Tregouet, D.A.; Yang, J.; Wilson, J.G.; et al. Shared genetic regulatory networks for cardiovascular disease and type 2 diabetes in multiple populations of diverse ethnicities in the United States. PLoS Genet. 2017, 13, e1007040. [Google Scholar] [CrossRef]
- Chakraborty, S.; Hosen, I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-OMICS approach: A new frontier in cancer research. BioMed Res. Int. 2018, 2018, 14. [Google Scholar] [CrossRef]
- Gunter, N.V.; Yap, B.J.M.; Chua, C.L.L.; Yap, W.H. Combining understanding of immunological mechanisms and genetic variants toward development of personalized medicine for psoriasis patients. Front. Genet. 2019, 10, 395. [Google Scholar] [CrossRef]

| Cells | 5-HT Metabolism and Transport | 5-HT Receptors | |
|---|---|---|---|
| Innate immune system | Dendritic cells | SERT [40] | 5-HT1BR, 5-HT1ER, 5-HT2AR, 5-HT2BR, 5-HT3R, 5-HT4R, 5-HT7R [41] |
| Mast cells | TPH1 [42] | 5-HT1AR (main receptor in human and mice); human MCs also express mRNA for receptors 5-HT1B, 5-HT1E, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT4 and 5-HT7 [43] | |
| Macrophages | SERT [44] | 5-HT1AR, 5-HT2AR, 5-HT2BR, 5-HT2CR, 5-HT3R, 5-HT7R [45,46] | |
| Adaptive immune system | T cells | TPH1 [47], MAO, SERT [48] | 5-HT1AR [49], 5-HT1BR [47], 5-HT2BR [50]; 5-HT3AR (in activated CD4+ Th) [51,52] |
| B cells | SERT [53] | 5-HT1AR [54], 5-HT3AR [55] | |
| Skin cells | Keratinocytes | TPH1 [21] | 5-HT1AR, 5HT1BR, 5-HT2AR, 5-HT2BR, 5-HT2CR, 5-HT7R [56], 5-HT3R [57] |
| Melanocytes | TPH1 [21] | 5-HT1AR, 5-HT1BR, 5-HT2AR, 5-HT2BR, 5-HT2CR, 5-HT7R [56] |
| Type | Name | Action | |
|---|---|---|---|
| Cells | Innate immunocytes | Antigen-presenting cells (APCs) | Include Langerhans cells (LCs) in the epidermis, dendritic cells (DCs) and macrophages in the dermis. They present the antigen to T cells which recognize it via a T-cell receptor and become activated. |
| Mast cells (MCs) | Granulocyte cells that contain histamine and are involved in allergy reactions, but can also activate and recruit immune-competent cells; can be induced to become APCs. | ||
| Neutrophils | Most common type of leukocytes in the blood, early markers of inflammation; can be induced to become APCs. | ||
| Natural killer (NK) cells | Cytotoxic lymphocytes which do not require activation to kill cells that do not have markers (antigens). | ||
| Adaptive immunocytes | Conventional T cells | T lymphocytes: CD4+ Th cells, cytotoxic CD8+ T cells, which can become long-term resident memory T cells (TRMs). Depending on the cytokine milieu, naïve CD4+ T cells differentiate into Th1, Th2, Th17, Treg, etc. | |
| NK T cells | Innate-like T lymphocytes that share surface markers and functional characteristics with conventional T cells and NK cells. | ||
| B cells | B lymphocytes that express B cell receptors on their cell membrane that can bind to an antigen initiating the antibody production (humoral immunity). | ||
| Non-immunocytes | Keratinocytes (KCs) | Epidermal cells responsible for the protective barrier function of the skin. Upon invasion of the upper layer of the epidermis, they produce proinflammatory signals (IL-1 family cytokines, AMPs, chemokines) which mediate their crosstalk with innate and adaptive immune cells. | |
| Melanocytes | Epidermal cells that generate the autoantigen ADAMTS-like protein 5 (ADAMTSL5). | ||
| Signaling molecules | Cytokines | Interleukins (IL) | Th1-type proinflammatory ILs: IL-2; Th2-type anti-inflammatory interleukins: IL-4, IL-5, IL-6, IL-9, IL-10, IL-13; Th17-type pro-inflammatory IL-17; IL-1-type pro-inflammatory IL-36; Th22-type pro-inflammatory IL-22; IL-12 family pro-inflammatory IL-23. |
| Interferon (IFN)-α | Pro-inflammatory cytokine mainly produced by plasmacytoid dendritic cells (pDCs) during the early phases of psoriasis. | ||
| Interferon (IFN)-γ | Th1-type proinflammatory cytokines; mainly produced by NK and NK T cells, CD4+ Th1 and Th17 cells, and CD8+ cytotoxic T cells. | ||
| Tumor necrosis factor (TNF)-α | Proinflammatory cytokine produced by macrophages, monocytes, endothelial cells, neutrophils and activated lymphocytes. | ||
| Tumor necrosis factor (TNF)-β | Proinflammatory cytokine mainly produced by KCs. | ||
| Chemokines | CXCL9, CXCL10, CXCL11, CCL20, chemerin, etc. | Small cytokines which act as mediators of innate immune cells chemotaxis, may be produced by KCs and leukocytes. | |
| Autoantigens | LL37 | Produced by KCs, it complexes with self-nucleic acids derived from damaged KCs and neutrophils, and acts as an autoantigen, activating pDCs via TLRs, and also being recognized by CD4+ and CD8+ T cells. | |
| ADAMTSL5 | Produced by melanocytes and KCs, it is able to activate DCs and is recognized as an autoantigen by CD8+ T cells. |
| Changes in Psoriatic Skin | ||
|---|---|---|
| Serotonin | 5-HT | Expression increased in epithelial and adnexal structures of psoriatic skin [127]. |
| Expression increased in basal and suprabasal skin layers in psoriatic skin [128]. | ||
| 5-HT receptors | 5-HT1AR | Lower expression in psoriatic dermis [129]. |
| 5-HT2AR | Increased expression in psoriatic dermis [129]. | |
| 5-HT3R | Increased expression in the basal epidermis of noninvolved psoriatic skin [129]. | |
| Increased expression in sensory nerve endings [68]. | ||
| 5-HT transporter | SERT | Increased expression in DCs and other inflammatory cells in the epidermis [6,40]. |
| Increased expression in MCs and lymphocytes in the dermis [6,40]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.M.; Ascenso, A.; Ribeiro, H.M.; Marto, J. The Brain–Skin Connection and the Pathogenesis of Psoriasis: A Review with a Focus on the Serotonergic System. Cells 2020, 9, 796. https://doi.org/10.3390/cells9040796
Martins AM, Ascenso A, Ribeiro HM, Marto J. The Brain–Skin Connection and the Pathogenesis of Psoriasis: A Review with a Focus on the Serotonergic System. Cells. 2020; 9(4):796. https://doi.org/10.3390/cells9040796
Chicago/Turabian StyleMartins, Ana M., Andreia Ascenso, Helena M. Ribeiro, and Joana Marto. 2020. "The Brain–Skin Connection and the Pathogenesis of Psoriasis: A Review with a Focus on the Serotonergic System" Cells 9, no. 4: 796. https://doi.org/10.3390/cells9040796
APA StyleMartins, A. M., Ascenso, A., Ribeiro, H. M., & Marto, J. (2020). The Brain–Skin Connection and the Pathogenesis of Psoriasis: A Review with a Focus on the Serotonergic System. Cells, 9(4), 796. https://doi.org/10.3390/cells9040796








