NeuroHeal Treatment Alleviates Neuropathic Pain and Enhances Sensory Axon Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Cultures and Axonal Growth Analysis
2.2. Drugs
2.3. Animals and Surgery
2.4. Assessment of Mechanical Allodynia
2.5. Electrophysiological Test
2.6. Tissue Processing for Histology
2.7. Immunohistochemistry and Image Analysis
2.8. Western Blot
2.9. Statistical Analysis
3. Results
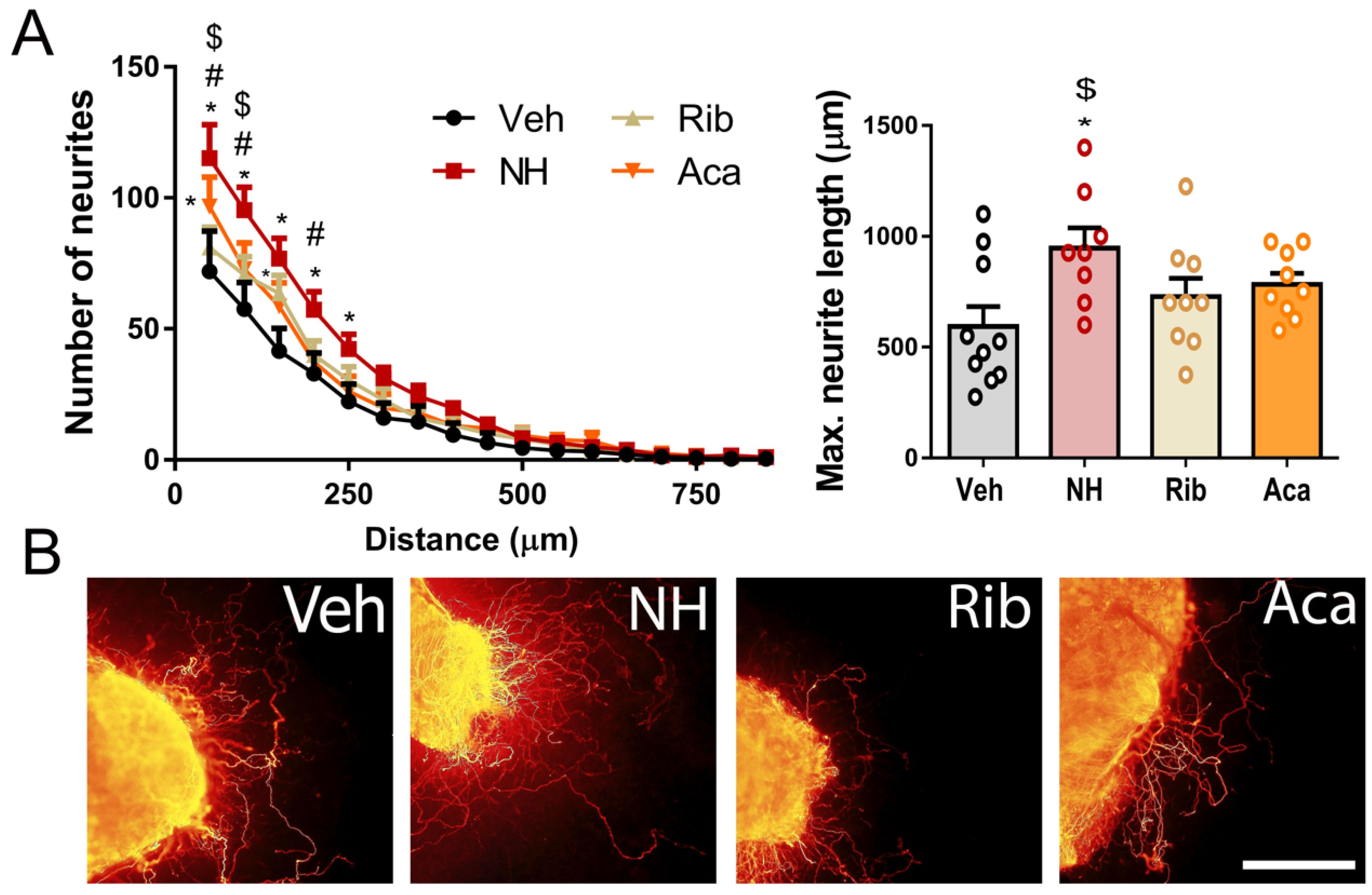
3.1. NeuroHeal Enhances Regeneration of Sensory and Motor Axons
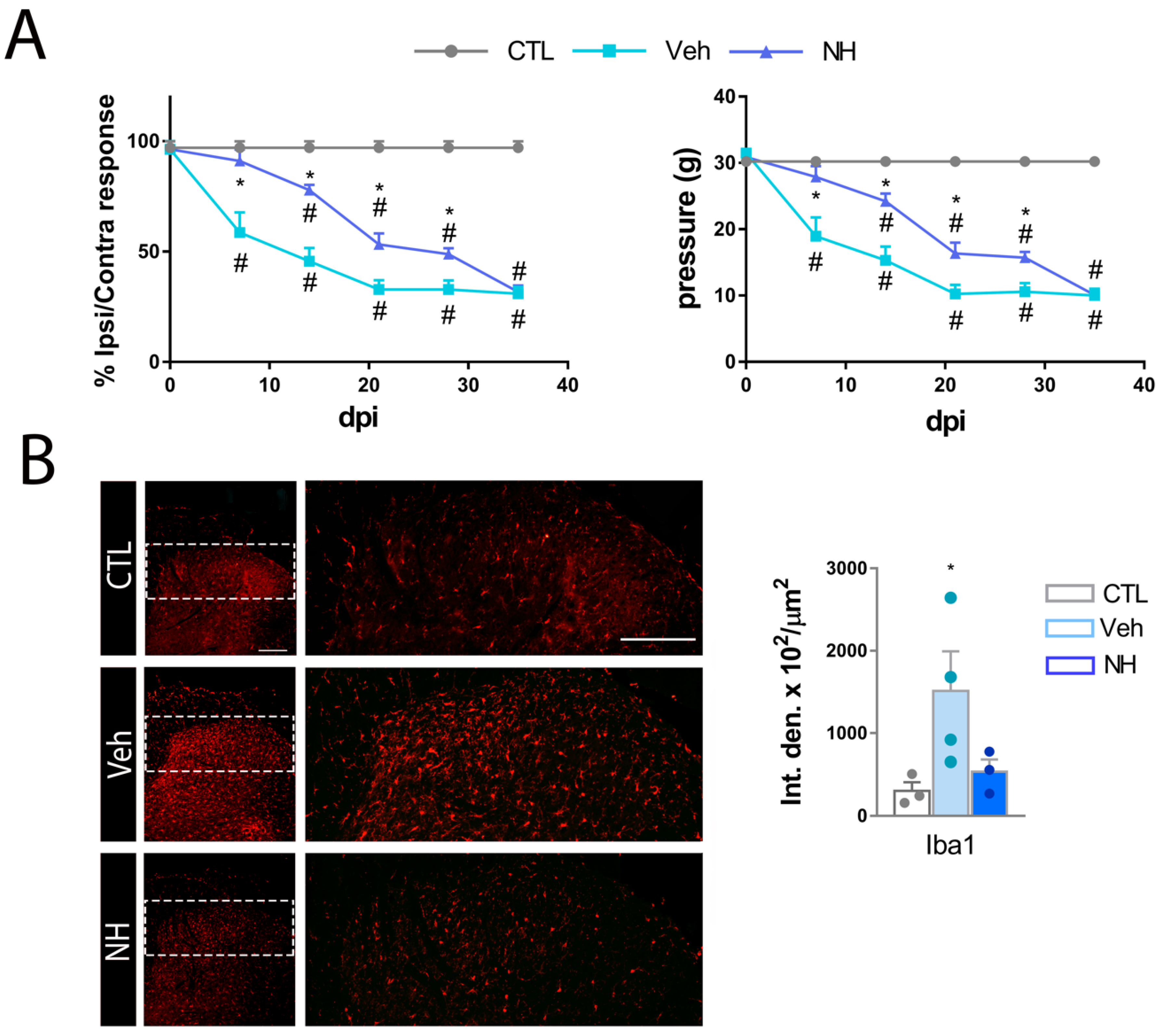
3.2. NeuroHeal Reduces Hyperalgesia after Peripheral Nerve Injury
3.3. NeuroHeal Reduces Mechanical Allodynia
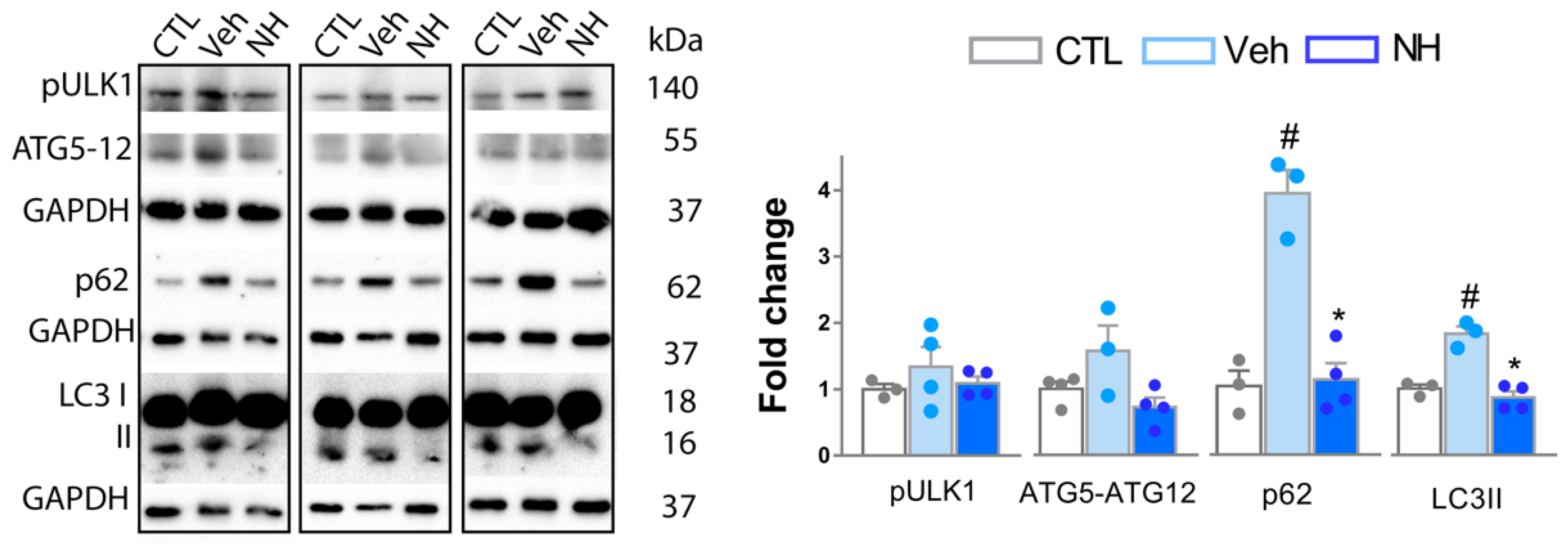
3.4. Autophagy Impairment is Resolved by NeuroHeal Treatment
3.5. NeuroHeal Increases KCC2 Levels in Dorsal Spinal Horn
4. Discussion
5. Study Limitations and Future Directions
6. Conclusions
7. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Munro, C.A.; Prasad, V.S.; Midha, R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J. Trauma 1998, 45, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Gordon, T.; Zochodne, D.W.; Power, H.A. Improving peripheral nerve regeneration: From molecular mechanisms to potential therapeutic targets. Exp. Neurol. 2014, 261, 826–835. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Prim. 2017, 3, 17002. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Khangura, R.K.; Sharma, J.; Bali, A.; Singh, N.; Jaggi, A.S. An integrated review on new targets in the treatment of neuropathic pain. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2019, 23, 1–20. [Google Scholar] [CrossRef]
- Berger, J.V.; Knaepen, L.; Janssen, S.P.M.; Jaken, R.J.P.; Marcus, M.A.E.; Joosten, E.A.J.; Deumens, R. Cellular and molecular insights into neuropathy-induced pain hypersensitivity for mechanism-based treatment approaches. Brain Res. Rev. 2011, 67, 282–310. [Google Scholar] [CrossRef]
- Fernandes, V.; Sharma, D.; Vaidya, S.; P.A, S.; Guan, Y.; Kalia, K.; Tiwari, V. Cellular and molecular mechanisms driving neuropathic pain: Recent advancements and challenges. Expert Opin. Ther. Targets 2018, 22, 131–142. [Google Scholar] [CrossRef]
- Dray, A. Neuropathic pain: Emerging treatments. Br. J. Anaesth. 2008, 101, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Aurilio, C.; Pota, V.; Pace, M.C.; Passavanti, M.B.; Barbarisi, M. Ionic channels and neuropathic pain: Physiopathology and applications. J. Cell. Physiol. 2008, 215, 8–14. [Google Scholar] [CrossRef]
- Chang, K.-T.; Lin, Y.-L.; Lin, C.-T.; Hong, C.-J.; Tsai, M.-J.; Huang, W.-C.; Shih, Y.-H.; Lee, Y.-Y.; Cheng, H.; Huang, M.-C. Leptin is essential for microglial activation and neuropathic pain after preganglionic cervical root avulsion. Life Sci. 2017, 187, 31–41. [Google Scholar] [CrossRef]
- Stokes, L.; Layhadi, J.A.; Bibic, L.; Dhuna, K.; Fountain, S.J. P2X4 Receptor Function in the Nervous System and Current Breakthroughs in Pharmacology. Front. Pharmacol. 2017, 8, 291. [Google Scholar] [CrossRef]
- Jensen, M.P.; Brownstone, R.M. Mechanisms of Spinal Cord Stimulation for the Treatment of Pain: Still in the Dark after 50 Years. Eur. J. Pain 2018. [Google Scholar] [CrossRef]
- Cobianchi, S.; Arbat-Plana, A.; Lopez-Alvarez, V.M.; Navarro, X. Neuroprotective Effects of Exercise Treatments After Injury: The Dual Role of Neurotrophic Factors. Curr. Neuropharmacol. 2017, 15, 495–518. [Google Scholar] [CrossRef]
- Brooks, K.G.; Kessler, T.L. Treatments for neuropathic pain. Clin. Pharm. 2017, 1–22. [Google Scholar]
- Romeo-Guitart, D.; Leiva-Rodriguez, T.; Espinosa-Alcantud, M.; Sima, N.; Vaquero, A.; Dominguez-Martin, H.; Ruano, D.; Casas, C. SIRT1 activation with neuroheal is neuroprotective but SIRT2 inhibition with AK7 is detrimental for disconnected motoneurons. Cell Death Dis. 2018, 9, 531. [Google Scholar] [CrossRef]
- Romeo-Guitart, D.; Forés, J.; Herrando-Grabulosa, M.; Valls, R.; Leiva-Rodríguez, T.; Galea, E.; González-Pérez, F.; Navarro, X.; Petegnief, V.; Bosch, A.; et al. Neuroprotective Drug for Nerve Trauma Revealed Using Artificial Intelligence. Sci. Rep. 2018, 8, 1879. [Google Scholar] [CrossRef]
- Torres-Espín, A.; Santos, D.; González-Pérez, F.; del Valle, J.; Navarro, X. Neurite-J: An Image-J plug-in for axonal growth analysis in organotypic cultures. J. Neurosci. Methods 2014, 236, 26–39. [Google Scholar] [CrossRef]
- Casals-Diaz, L.; Vivo, M.; Navarro, X. Nociceptive responses and spinal plastic changes of afferent C-fibers in three neuropathic pain models induced by sciatic nerve injury in the rat. Exp. Neurol. 2009, 217, 84–95. [Google Scholar] [CrossRef]
- Cobianchi, S.; de Cruz, J.; Navarro, X. Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp. Neurol. 2014, 255, 1–11. [Google Scholar] [CrossRef]
- Romeo-Guitart, D.; Forés, J.; Navarro, X.; Casas, C. Boosted Regeneration and Reduced Denervated Muscle Atrophy by NeuroHeal in a Pre-clinical Model of Lumbar Root Avulsion with Delayed Reimplantation. Sci. Rep. 2017, 7, 12028. [Google Scholar] [CrossRef] [PubMed]
- Romeo-Guitart, D.; Leiva-Rodríguez, T.; Forés, J.; Casas, C. Improved Motor Nerve Regeneration by SIRT1/Hif1a-Mediated Autophagy. Cells 2019, 8, 1354. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Gwon, D.H.; Kang, D.-W.; Hwang, T.W.; Shin, N.; Kwon, H.H.; Shin, H.J.; Yin, Y.; Kim, J.-J.; Hong, J.; et al. TLR4-mediated autophagic impairment contributes to neuropathic pain in chronic constriction injury mice. Mol. Brain 2018, 11, 11. [Google Scholar] [CrossRef]
- Berliocchi, L.; Russo, R.; Maiarù, M.; Levato, A.; Bagetta, G.; Corasaniti, M.T. Autophagy impairment in a mouse model of neuropathic pain. Mol. Pain 2011, 7, 83. [Google Scholar] [CrossRef]
- Jung, K.T.; Lim, K.J. Autophagy: Can It be a New Experimental Research Method of Neuropathic Pain? Korean J. Pain 2015, 28, 229–230. [Google Scholar] [CrossRef]
- Romeo-Guitart, D.; Marcos-DeJuana, C.; Navarro, X.; Casas, C. Novel Neuroprotective Therapy with NeuroHeal by Autophagy Induction for Damaged Neonatal Motoneurons. Theranostics 2020. In press. [Google Scholar]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Arozena, A.A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Bravo-Hernández, M.; Corleto, J.A.; Barragán-Iglesias, P.; González-Ramírez, R.; Pineda-Farias, J.B.; Felix, R.; Calcutt, N.A.; Delgado-Lezama, R.; Marsala, M.; Granados-Soto, V. The α5 subunit containing GABAA receptors contribute to chronic pain. Pain 2016, 157, 613–626. [Google Scholar] [CrossRef]
- Ferrini, F.; Trang, T.; Mattioli, T.-A.M.; Laffray, S.; Del’Guidice, T.; Lorenzo, L.-E.; Castonguay, A.; Doyon, N.; Zhang, W.; Godin, A.G.; et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat. Neurosci. 2013, 16, 183–192. [Google Scholar] [CrossRef]
- Coull, J.A.M.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef]
- Smith, P.A. BDNF: No gain without pain? Neuroscience 2014, 283, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Senkler, C.; Yang, A.; Soto, F.; Liang, B.T. P2X 4 Receptor Is a Glycosylated Cardiac Receptor Mediating a Positive Inotropic Response to ATP *. 2002, 277, 15752–15757. [Google Scholar]
- Romeo-Guitart, D.; Casas, C. Network-centric medicine for peripheral nerve injury: Treating the whole to boost endogenous mechanisms of neuroprotection and regeneration. Neural Regen. Res. 2019, 14, 1122–1128. [Google Scholar] [PubMed]
- Piao, Z.; Wang, W.; Xu, X.; Wang, Q.; Huo, X.; Han, M.; Piao, Y. Autophagy of neuron axon during regeneration of rat sciatic nerves. Di Yi Jun Yi Da Xue Xue Bao 2004, 24, 361–364. [Google Scholar]
- He, M.; Ding, Y.; Chu, C.; Tang, J.; Xiao, Q.; Luo, Z.-G. Autophagy induction stabilizes microtubules and promotes axon regeneration after spinal cord injury. Proc. Natl. Acad. Sci. 2016, 113, 11324–11329. [Google Scholar] [CrossRef]
- Clarke, J.-P.; Mearow, K. Autophagy inhibition in endogenous and nutrient-deprived conditions reduces dorsal root ganglia neuron survival and neurite growth in vitro. J. Neurosci. Res. 2016, 94, 653–670. [Google Scholar] [CrossRef]
- Sakamoto, K.; Ozaki, T.; Ko, Y.-C.; Tsai, C.-F.; Gong, Y.; Morozumi, M.; Ishikawa, Y.; Uchimura, K.; Nadanaka, S.; Kitagawa, H.; et al. Glycan sulfation patterns define autophagy flux at axon tip via PTPRsigma-cortactin axis. Nat. Chem. Biol. 2019, 15, 699–709. [Google Scholar] [CrossRef]
- Zhou, X.; Babu, J.R.; da Silva, S.; Shu, Q.; Graef, I.A.; Oliver, T.; Tomoda, T.; Tani, T.; Wooten, M.W.; Wang, F. Unc-51-like kinase 1/2-mediated endocytic processes regulate filopodia extension and branching of sensory axons. Proc. Natl. Acad. Sci. USA 2007, 104, 5842–5847. [Google Scholar] [CrossRef]
- Xie, W.; Strong, J.A.; Zhang, J.-M. Active Nerve Regeneration with Failed Target Reinnervation Drives Persistent Neuropathic Pain. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Patel, M.K.; Kaye, A.D.; Urman, R.D. Tanezumab: Therapy targeting nerve growth factor in pain pathogenesis. J. Anaesthesiol. Clin. Pharmacol. 2018, 34, 111–116. [Google Scholar]
- da Silva, J.T.; Evangelista, B.G.; Venega, R.A.G.; Seminowicz, D.A.; Chacur, M. Anti-NGF treatment can reduce chronic neuropathic pain by changing peripheral mediators and brain activity in rats. Behav. Pharmacol. 2019, 30, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Rodriguez, T.; Romeo-Guitart, D.; Marmolejo-Martinez-Artesero, S.; Herrando-Grabulosa, M.; Bosch, A.; Fores, J.; Casas, C. ATG5 overexpression is neuroprotective and attenuates cytoskeletal and vesicle-trafficking alterations in axotomized motoneurons. Cell Death Dis. 2018, 9, 626. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013, 19, 983. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-S.; Jing, P.-B.; Wang, J.-A.; Zhang, R.; Jiang, B.-C.; Gao, Y.-J.; Zhang, Z.-J. Increased autophagic activity in dorsal root ganglion attenuates neuropathic pain following peripheral nerve injury. Neurosci. Lett. 2015, 599, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yi, M.-H.; Ko, Y.; Kim, H.-W.; Seo, J.H.; Lee, Y.H.; Lee, W.; Kim, D.W. Expression of LC3 and Beclin 1 in the spinal dorsal horn following spinal nerve ligation-induced neuropathic pain. Brain Res. 2013, 1519, 31–39. [Google Scholar] [CrossRef]
- Marinelli, S.; Nazio, F.; Tinari, A.; Ciarlo, L.; D’Amelio, M.; Pieroni, L.; Vacca, V.; Urbani, A.; Cecconi, F.; Malorni, W.; et al. Schwann cell autophagy counteracts the onset and chronification of neuropathic pain. Pain 2014, 155, 93–107. [Google Scholar] [CrossRef]
- Lee, I.H. Mechanisms and disease implications of sirtuin-mediated autophagic regulation. Exp. Mol. Med. 2019, 51, 102. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Zhang, M.-X.; Zhou, S.-S.; Li, H.; Gao, J.; Du, L.; Yin, X.-X. SIRT1 attenuates neuropathic pain by epigenetic regulation of mGluR1/5 expressions in type 2 diabetic rats. Pain 2017, 158, 130–139. [Google Scholar] [CrossRef]
- Shao, H.; Xue, Q.; Zhang, F.; Luo, Y.; Zhu, H.; Zhang, X.; Zhang, H.; Ding, W.; Yu, B. Spinal SIRT1 activation attenuates neuropathic pain in mice. PLoS ONE 2014, 9, e100938. [Google Scholar] [CrossRef]
- Tang, X.; Drotar, J.; Li, K.; Clairmont, C.D.; Brumm, A.S.; Sullins, A.J.; Wu, H.; Liu, X.S.; Wang, J.; Gray, N.S.; et al. Pharmacological enhancement of KCC2 gene expression exerts therapeutic effects on human Rett syndrome neurons and Mecp2 mutant mice. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Khanna, G.; Bijjem, K. Effect of Acamprosate in experimental models of peripheral neuropathic and inflammatory pain in wistar rats. Int. J. Pharm. Sci. reserach 2015, 6, 5102–5114. [Google Scholar]
- Puskarjov, M.; Ahmad, F.; Kaila, K.; Blaesse, P. Activity-dependent cleavage of the K-Cl cotransporter KCC2 mediated by calcium-activated protease calpain. J. Neurosci. 2012, 32, 11356–11364. [Google Scholar] [CrossRef] [PubMed]
- Milicevic, I.; Pekovic, S.; Subasic, S.; Mostarica-Stojkovic, M.; Stosic-Grujicic, S.; Medic-Mijacevic, L.; Pejanovic, V.; Rakic, L.; Stojiljkovic, M. Ribavirin reduces clinical signs and pathological changes of experimental autoimmune encephalomyelitis in Dark Agouti rats. J. Neurosci. Res. 2003, 72, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Lavrnja, I.; Savic, D.; Bjelobaba, I.; Dacic, S.; Bozic, I.; Parabucki, A.; Nedeljkovic, N.; Pekovic, S.; Rakic, L.; Stojiljkovic, M. The effect of ribavirin on reactive astrogliosis in experimental autoimmune encephalomyelitis. J. Pharmacol. Sci. 2012, 119, 221–232. [Google Scholar] [CrossRef]
- Liao, S.-H.; Li, Y.; Lai, Y.-N.; Liu, N.; Zhang, F.-X.; Xu, P.-P. Ribavirin attenuates the respiratory immune responses to influenza viral infection in mice. Arch. Virol. 2017, 162, 1661–1669. [Google Scholar] [CrossRef]
- Dai, J.-P.; Wang, Q.-W.; Su, Y.; Gu, L.-M.; Zhao, Y.; Chen, X.-X.; Chen, C.; Li, W.-Z.; Wang, G.-F.; Li, K.-S. Emodin Inhibition of Influenza A Virus Replication and Influenza Viral Pneumonia via the Nrf2, TLR4, p38/JNK and NF-kappaB Pathways. Molecules 2017, 22, 1754. [Google Scholar] [CrossRef]
- Clark, A.K.; Staniland, A.A.; Marchand, F.; Kaan, T.K.Y.; McMahon, S.B.; Malcangio, M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J. Neurosci. 2010, 30, 573–582. [Google Scholar] [CrossRef]



| Gastrocnemius CMAP Amplitude (mV) | ||||||
| dpi | Veh | NH 0.05× | NH 0.2× | NH 1× | NH 1.7× | NH 3× |
| 15 | 1.93 ± 0.4 | 1.02 ± 0.11 | 3.273 ± 0.61 | 0.92 ± 0.26 | 2.783 ± 0.83 | 1.04 ± 0.20 |
| 25 | 9.99 ± 1.25 | 10.51 ± 0.09 | 13.79 ± 1.08 | 14.22 ± 1.47 | 14.24 ± 0.47 | 10.8 ± 1.19 |
| 35 | 24.4 ± 1.59 | 29.94 ± 1.52 * | 31.43 ± 1.46 * | 28.79 ± 2.51 | 29.08 ± 1.25 * | 25.25 ± 2.80 |
| Plantar Interossei CMAP Amplitude (mV) | ||||||
| dpi | Veh | NH 0.05× | NH 0.2× | NH 1× | NH 1.7× | NH 3× |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 0.015 ± 0.015 | 0.132 ± 0.05 | 0.29 ± 0.06 | 0.313 ± 0.184 | 0.252 ± 0.109 | 0.02 ± 0.02 |
| 35 | 0.828 ± 0.09 | 1.20 ± 0.10 * | 1.292 ± 0.11 * | 1.335 ± 0.16 * | 1.13 ± 0.08 * | 0.684 ± 0.13 |
| Pressure (g) | ||||
|---|---|---|---|---|
| Male | Female | |||
| dpi | Veh | NH 0.2× | Veh | NH 0.2× |
| 7 | 32.8 ± 1.17 | 31.29 ± 1.18 | 33.31 ± 0.09 | 29.86 ± 0.64 |
| 14 | 31.5 ± 0.56 | 31.70 ± 0.73 | 31.96 ± 1.14 | 30.25 ± 0.91 |
| 21 | 31.25 ± 0.42 | 30.69 ± 0.25 | 30.875 ± 0.40 | 30.55 ± 1.33 |
| 28 | 32.72 ± 0.24 | 31.84 ± 0.71 | 31.74 ± 0.23 | 31.28 ± 1.40 |
| 35 | 31.88 ± 0.33 | 32.24 ± 0.55 | 32.65 ± 0.36 | 31.47 ± 0.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo-Guitart, D.; Casas, C. NeuroHeal Treatment Alleviates Neuropathic Pain and Enhances Sensory Axon Regeneration. Cells 2020, 9, 808. https://doi.org/10.3390/cells9040808
Romeo-Guitart D, Casas C. NeuroHeal Treatment Alleviates Neuropathic Pain and Enhances Sensory Axon Regeneration. Cells. 2020; 9(4):808. https://doi.org/10.3390/cells9040808
Chicago/Turabian StyleRomeo-Guitart, David, and Caty Casas. 2020. "NeuroHeal Treatment Alleviates Neuropathic Pain and Enhances Sensory Axon Regeneration" Cells 9, no. 4: 808. https://doi.org/10.3390/cells9040808
APA StyleRomeo-Guitart, D., & Casas, C. (2020). NeuroHeal Treatment Alleviates Neuropathic Pain and Enhances Sensory Axon Regeneration. Cells, 9(4), 808. https://doi.org/10.3390/cells9040808





