The Molecular Mechanisms Underlying Prostaglandin D2-Induced Neuritogenesis in Motor Neuron-Like NSC-34 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Western Blotting
2.4. Neurite Outgrowth Assay
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Live/Dead Assay
2.7. Statistical Analyses
3. Results
3.1. PGD2 Affects Neurite Outgrowth
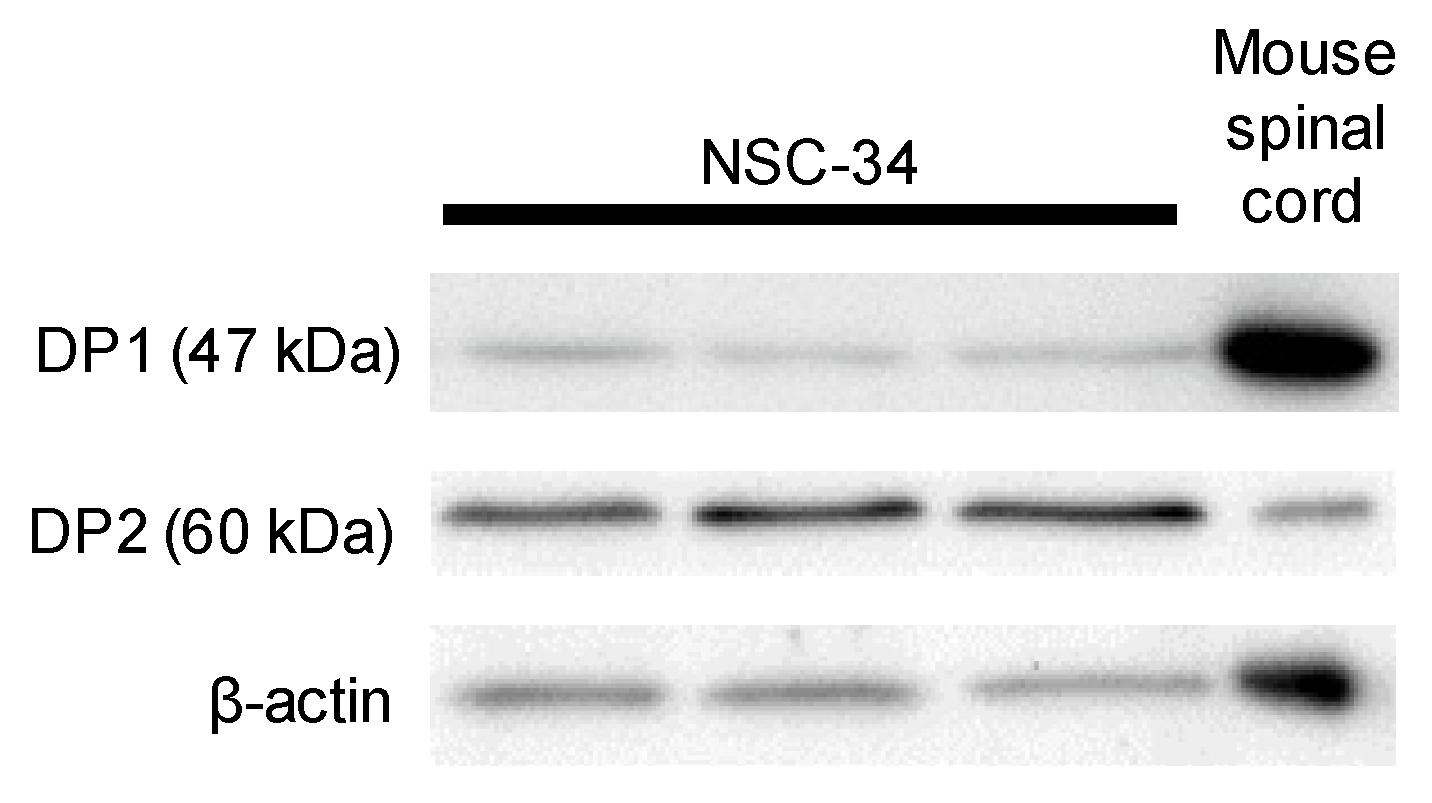
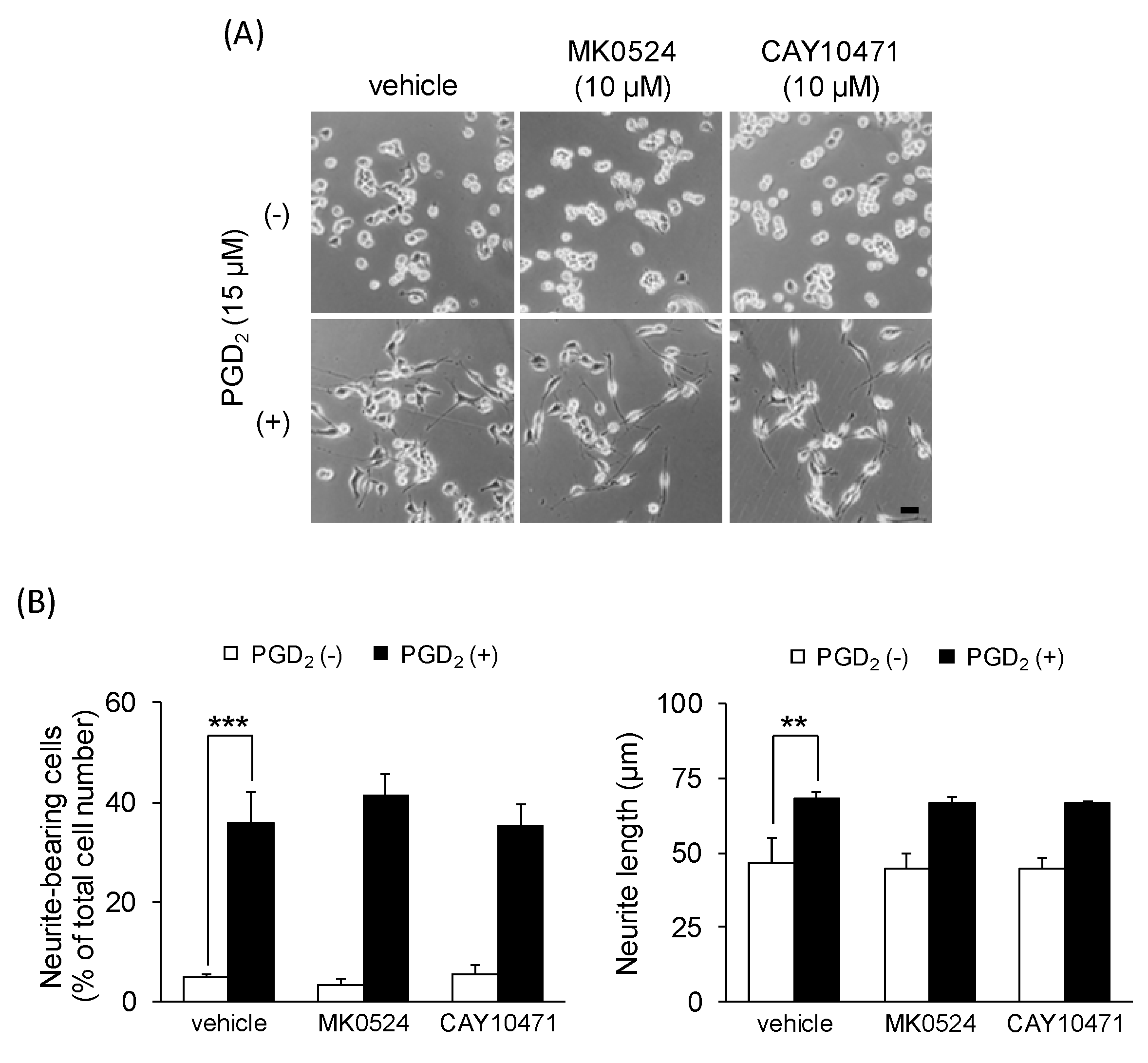
3.2. DP1 and DP2 Agonists Do Not Affect Neurite Outgrowth
3.3. 15d-PGJ2 Affects Neurite Outgrowth
3.4. The PPARγ Antagonist Affects PGD2-Induced Neurite Outgrowth
3.5. PGD2 and 15d-PGJ2 Increase the Expression of Motor Neuron-Specific Protein Marker Islet-1
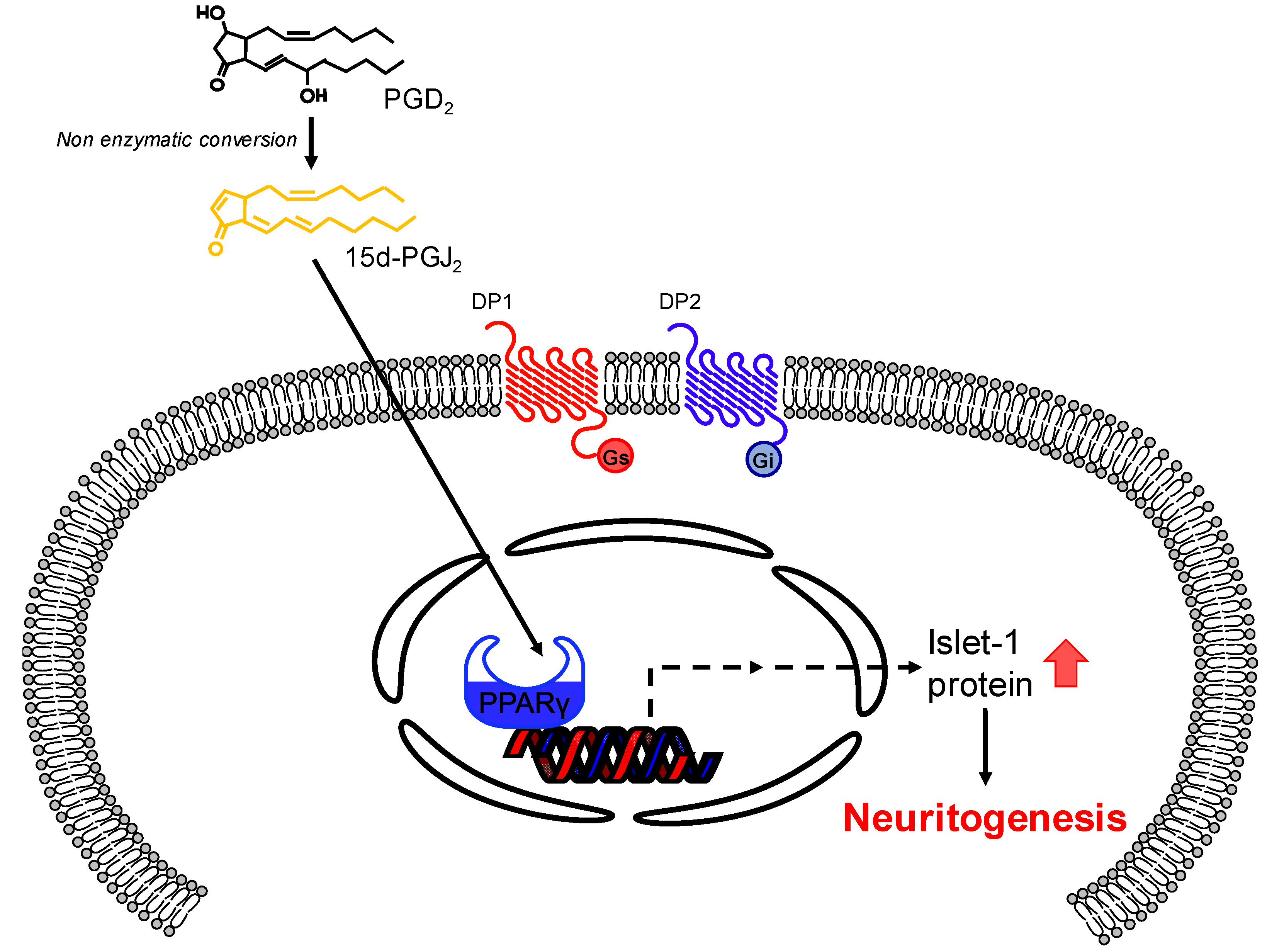
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arimura, N.; Kaibuchi, K. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 2007, 8, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Stifani, N. Motor neurons and the generation of spinal motor neuron diversity. Front. Cell. Neurosci. 2014, 8, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.K. Therapeutic opportunities and challenges of induced pluripotent stem cells-derived motor neurons for treatment of amyotrophic lateral sclerosis and motor neuron disease. Neural Regen. Res. 2017, 12, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Cashman, N.R.; Durham, H.D.; Blusztajn, J.K.; Oda, K.; Tabira, T.; Shaw, I.T.; Dahrouge, S.; Antel, J.P.; Durham, H.D.; Tabira, T.; et al. Neuroblastoma × spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev. Dyn. 1992, 194, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Maier, O.; Böhm, J.; Dahm, M.; Brück, S.; Beyer, C.; Johann, S.; Böhm, J.; Beyer, C.; Dahm, M.; Brück, S.; et al. Differentiated NSC-34 motoneuron-like cells as experimental model for cholinergic neurodegeneration. Neurochem. Int. 2013, 62, 1029–1038. [Google Scholar] [CrossRef]
- Johann, S.; Dahm, M.; Kipp, M.; Zahn, U.; Beyer, C. Regulation of choline acetyltransferase expression by 17 β-oestradiol in NSC-34 cells and in the spinal cord. J. Neuroendocrinol. 2011, 23, 839–848. [Google Scholar] [CrossRef]
- Keilhoff, G.; Mbou, R.P.; Lucas, B.; Schild, L. The differentiation of spinal cord motor neurons is associated with changes of the mitochondrial phospholipid cardiolipin. Neuroscience 2019, 400, 169–183. [Google Scholar] [CrossRef]
- Keilhoff, G.; Lucas, B.; Pinkernelle, J.; Steiner, M.; Fansa, H. Effects of cerebrolysin on motor-neuron-like NSC-34 cells. Exp. Cell Res. 2014, 327, 234–255. [Google Scholar] [CrossRef]
- Kupershmidt, L.; Weinreb, O.; Amit, T.; Mandel, S.; Carri, M.T.; Youdim, M.B.H. Neuroprotective and neuritogenic activities of novel multimodal iron-chelating drugs in motor-neuron-like NSC-34 cells and transgenic mouse model of amyotrophic lateral sclerosis. FASEB J. 2009, 23, 3766–3779. [Google Scholar] [CrossRef]
- Yagi, H.; Ohkawara, B.; Nakashima, H.; Ito, K.; Tsushima, M.; Ishii, H.; Noto, K.; Ohta, K.; Masuda, A.; Imagama, S.; et al. Zonisamide enhances neurite elongation of primary motor neurons and facilitates peripheral nerve regeneration in vitro and in a mouse model. PLoS ONE 2015, 10, e0142786. [Google Scholar] [CrossRef]
- Phillis, J.W.; Horrocks, L.A.; Farooqui, A.A. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: Their role and involvement in neurological disorders. Brain Res. Rev. 2006, 52, 201–243. [Google Scholar] [CrossRef] [PubMed]
- Yagami, T.; Koma, H.; Yamamoto, Y. Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol. Neurobiol. 2016, 53, 4754–4771. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Narumiya, S. Prostaglandin E receptors. J. Biol. Chem. 2007, 282, 11613–11617. [Google Scholar] [CrossRef]
- Hiruma, H.; Ichikawa, T.; Kobayashi, H.; Hoka, S.; Takenaka, T.; Kawakami, T. Prostaglandin E2 enhances axonal transport and neuritogenesis in cultured mouse dorsal root ganglion neurons. Neuroscience 2000, 100, 885–891. [Google Scholar] [CrossRef]
- Mitani, K.; Sekiguchi, F.; Maeda, T.; Tanaka, Y.; Yoshida, S.; Kawabata, A. The prostaglandin E2/EP4 receptor/cyclic AMP/T-type Ca2+ channel pathway mediates neuritogenesis in sensory neuron-like ND7/23 cells. J. Pharmacol. Sci. 2016, 130, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Nango, H.; Kosuge, Y.; Miyagishi, H.; Sugawa, K.; Ito, Y.; Ishige, K. Prostaglandin E2 facilitates neurite outgrowth in a motor neuron-like cell line, NSC-34. J. Pharmacol. Sci. 2017, 135, 64–71. [Google Scholar] [CrossRef]
- Urade, Y.; Eguchi, N. Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins Other Lipid Mediat. 2002, 68–69, 375–382. [Google Scholar] [CrossRef]
- Urade, Y.; Kitahama, K.; Ohishi, H.; Kaneko, T.; Mizuno, N.; Hayaishi, O. Dominant expression of mRNA for prostaglandin D synthase in leptomeninges, choroid plexus, and oligodendrocytes of the adult rat brain. Proc. Natl. Acad. Sci. USA 1993, 90, 9070–9074. [Google Scholar] [CrossRef]
- Mohri, I.; Eguchi, N.; Suzuki, K.; Urade, Y.; Taniike, M. Hematopoietic prostaglandin D synthase is expressed in microglia in the developing postnatal mouse brain. Glia 2003, 42, 263–274. [Google Scholar] [CrossRef]
- Liang, X.; Wu, L.; Hand, T.; Andreasson, K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J. Neurochem. 2005, 92, 477–486. [Google Scholar] [CrossRef]
- Ogorochi, T.; Narumiya, S.; Mizuno, N.; Yamashita, K.; Miyazaki, H.; Hayaishi, O. Regional distribution of prostaglandins D2, E2, and F2 alpha and related enzymes in postmortem human brain. J. Neurochem. 1984, 43, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Kihara, Y.; Matsushita, T.; Kita, Y.; Uematsu, S.; Akira, S.; Kira, J.-I.; Ishii, S.; Shimizu, T. Targeted lipidomics reveals mPGES-1-PGE2 as a therapeutic target for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2009, 106, 21807–21812. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.; Heinemann, A.; Hoefler, G.; Peskar, B.A.; Schuligoi, R. Effect of endotoxin treatment on the expression and localization of spinal cyclooxygenase, prostaglandin synthases, and PGD2 receptors. J. Neurochem. 2008, 104, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Lenhard, J.M.; Willson, T.M.; Patel, I.; Morris, D.C.; Lehmann, J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 1995, 83, 813–819. [Google Scholar] [CrossRef]
- Park, K.S.; Da Lee, R.; Kang, S.-K.; Han, S.Y.; Park, K.L.; Yang, K.H.; Song, Y.S.; Park, H.J.; Lee, Y.M.; Yun, Y.P.; et al. Neuronal differentiation of embryonic midbrain cells by upregulation of peroxisome proliferator-activated receptor-gamma via the JNK-dependent pathway. Exp. Cell Res. 2004, 297, 424–433. [Google Scholar] [CrossRef]
- Kosuge, Y.; Miyagishi, H.; Yoneoka, Y.; Yoneda, K.; Nango, H.; Ishige, K.; Ito, Y. Pathophysiological role of prostaglandin E2-induced up-regulation of the EP2 receptor in motor neuron-like NSC-34 cells and lumbar motor neurons in ALS model mice. Neurochem. Int. 2017, 119, 2–9. [Google Scholar] [CrossRef]
- Kosuge, Y.; Nango, H.; Kasai, H.; Yanagi, T.; Mawatari, T.; Nishiyama, K.; Miyagishi, H.; Ishige, K.; Ito, Y. Generation of Cellular Reactive Oxygen Species by Activation of the EP2 Receptor Contributes to Prostaglandin E2-Induced Cytotoxicity in Motor Neuron-Like NSC-34 Cells. Oxid. Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Meijering, E.; Jacob, M.; Sarria, J.-C.F.; Steiner, P.; Hirling, H.; Unser, M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry 2004, 58A, 167–176. [Google Scholar] [CrossRef]
- Miyagishi, H.; Kosuge, Y.; Takano, A.; Endo, M.; Nango, H.; Yamagata-Murayama, S.; Hirose, D.; Kano, R.; Tanaka, Y.; Ishige, K.; et al. Increased expression of 15-hydroxyprostaglandin dehydrogenase in spinal astrocytes during disease progression in a model of amyotrophic lateral sclerosis. Cell Mol. Neurobiol. 2017, 37, 445–452. [Google Scholar] [CrossRef]
- Shibata, T.; Kondo, M.; Osawa, T.; Shibata, N.; Kobayashi, M.; Uchida, K. 15-Deoxy-Δ 12,14 -prostaglandin J 2. J. Biol. Chem. 2002, 277, 10459–10466. [Google Scholar] [CrossRef] [PubMed]
- Straus, D.S.; Glass, C.K. Cyclopentenone prostaglandins: New insights on biological activities and cellular targets. Med. Res. Rev. 2001, 21, 185–210. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, H.-Y.; Liu, W.-X.; Jia, X.-X.; Zhang, J.-G.; Ma, C.-L.; Zhang, X.-J.; Yu, F.; Cong, B. Prostaglandin E2 restrains human Treg cell differentiation via E prostanoid receptor 2-protein kinase A signaling. Immunol. Lett. 2017, 191, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Tao, X.; Tang, K. Phosphoinositide 3-kinase/Akt signaling is essential for prostaglandin E2-induced osteogenic differentiation of rat tendon stem cells. Biochem. Biophys. Res. Commun. 2013, 435, 514–519. [Google Scholar] [CrossRef]
- Wong, C.T.; Ussyshkin, N.; Ahmad, E.; Rai-Bhogal, R.; Li, H.; Crawford, D.A. Prostaglandin E2 promotes neural proliferation and differentiation and regulates Wnt target gene expression. J. Neurosci. Res. 2016, 94, 759–775. [Google Scholar] [CrossRef]
- Almer, G.; Teismann, P.; Stevic, Z.; Halaschek-Wiener, J.; Deecke, L.; Kostic, V.; Przedborski, S.; Halaschek–Wiener, J.; Deecke, L.; Kostic, V.; et al. Increased levels of the pro-inflammatory prostaglandin PGE2 in CSF from ALS patients. Neurology 2002, 58, 1277–1279. [Google Scholar] [CrossRef]
- Reiber, H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin. Chim. Acta 2001, 310, 173–186. [Google Scholar] [CrossRef]
- Gallant, M.A.; Slipetz, D.; Hamelin, É.; Rochdi, M.D.; Talbot, S.; De Brum-Fernandes, A.J.; Parent, J.L. Differential regulation of the signaling and trafficking of the two prostaglandin D2 receptors, prostanoid DP receptor and CRTH2. Eur. J. Pharmacol. 2007, 557, 115–123. [Google Scholar] [CrossRef]
- Desai, S.; April, H.; Nwaneshiudu, C.; Ashby, B. Comparison of agonist-induced internalization of the Human EP2 and EP4 Prostaglandin Receptors: Role of the Carboxyl Terminus in EP4 Receptor Sequestration. Mol. Pharmacol. 2000, 58, 1279–1286. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Rose, M.E.; Pascoe, J.L.; Miller, T.M.; Ahmad, M.; Poloyac, S.M.; Hickey, R.W.; Graham, S.H. Prostaglandin D2 toxicity in primary neurons is mediated through its bioactive cyclopentenone metabolites. Neurotoxicology 2013, 39, 35–44. [Google Scholar] [CrossRef]
- Han, S.W.; Greene, M.E.; Pitts, J.; Wada, R.K.; Sidell, N. Novel expression and function of peroxisome proliferator-activated receptor gamma (PPARϒ) in human neuroblastoma cells. Clin. Cancer Res. 2001, 7, 98–104. [Google Scholar] [PubMed]
- Liu, H.; Rose, M.E.; Miller, T.M.; Li, W.; Shinde, S.N.; Pickrell, A.M.; Poloyac, S.M.; Graham, S.H.; Hickey, R.W. COX2-derived primary and cyclopentenone prostaglandins are increased after asphyxial cardiac arrest. Brain Res. 2013, 1519, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-Pereira, M.E.; Rockwell, P.; Schmidt-Glenewinkel, T.; Serrano, P. Neuroinflammation and J2 prostaglandins: Linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Front. Mol. Neurosci. 2015, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, M.; Frohnert, C.; Spillner, C.; Marcone, S.; Nath, A.; Lampe, T.; Fitzgerald, D.J.; Kehlenbach, R.H. The anti-inflammatory prostaglandin 15-deoxy-delta(12,14)-PGJ2 inhibits CRM1-dependent nuclear protein export. J. Biol. Chem. 2010, 285, 22202–22210. [Google Scholar] [CrossRef]
- Sawyer, N.; Cauchon, E.; Chateauneuf, A.; Cruz, R.P.G.; Nicholson, D.W.; Metters, K.M.; O’Neill, G.P.; Gervais, F.G. Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br. J. Pharmacol. 2002, 137, 1163–1172. [Google Scholar] [CrossRef]
- Hatanaka, M.; Shibata, N.; Shintani, N.; Haba, R.; Hayata, A.; Hashimoto, H.; Baba, A. 15d-prostaglandin J2 enhancement of nerve growth factor–induced neurite outgrowth is blocked by the chemoattractant receptor– homologous molecule expressed on T-Helper Type 2 Cells (CRTH2) antagonist CAY10471 in PC12 Cells. J. Pharmacol. Sci. 2010, 113, 89–93. [Google Scholar] [CrossRef]
- Shibata, T. 15-Deoxy-Δ12,14-prostaglandin J₂ as an electrophilic mediator. Biosci. Biotechnol. Biochem. 2015, 79, 1044–1049. [Google Scholar] [CrossRef]
- Jung, K.M.; Park, K.S.; Oh, J.H.; Jung, S.Y.; Yang, K.H.; Song, Y.S.; Son, D.J.; Park, Y.H.; Yun, Y.P.; Lee, M.K.; et al. Activation of p38 mitogen-activated protein kinase and activator Protein-1 during the promotion of neurite extension of PC-12 Cells by 15-deoxy-Δ 12,14 -prostaglandin J2. Mol. Pharmacol. 2003, 63, 607–616. [Google Scholar] [CrossRef]
- Shibata, T.; Takahashi, K.; Matsubara, Y.; Inuzuka, E.; Nakashima, F.; Takahashi, N.; Kozai, D.; Mori, Y.; Uchida, K. Identification of a prostaglandin D2 metabolite as a neuritogenesis enhancer targeting the TRPV1 ion channel. Sci. Rep. 2016, 6, 21261. [Google Scholar] [CrossRef]
- Shiraki, T.; Kamiya, N.; Shiki, S.; Kodama, T.S.; Kakizuka, A.; Jingami, H. α,β-Unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2005, 280, 14145–14153. [Google Scholar] [CrossRef]
- Ericson, J.; Thor, S.; Edlund, T.; Jessell, T.M.; Yamada, T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science 1992, 256, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Li, D.; Louis, K.R.; Li, X.; Yang, H.; Sun, Q.; Crandall, S.R.; Tsang, S.; Zhou, J.; Cox, C.L.; et al. High-efficiency motor neuron differentiation from human pluripotent stem cells and the function of Islet-1. Nat. Commun. 2014, 5, 3449–3461. [Google Scholar] [CrossRef] [PubMed]
- Kanakasabai, S.; Pestereva, E.; Chearwae, W.; Gupta, S.K.; Ansari, S.; Bright, J.J. PPARγ agonists promote oligodendrocyte differentiation of neural stem cells by modulating stemness and differentiation genes. PLoS ONE 2012, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pestereva, E.; Kanakasabai, S.; Bright, J.J. PPARγ agonists regulate the expression of stemness and differentiation genes in brain tumour stem cells. Br. J. Cancer 2012, 106, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Hemendinger, R.A.; Armstrong, E.J.; Radio, N.; Brooks, B.R. Neurotoxic injury pathways in differentiated mouse motor neuron-neuroblastoma hybrid (NSC-34D) cells in vitro-limited effect of riluzole on thapsigargin, but not staurosporine, hydrogen peroxide and homocysteine neurotoxicity. Toxicol. Appl. Pharmacol. 2012, 258, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, S.; Wang, P.; Wang, W. Transient mitochondrial permeability transition mediates excitotoxicity in glutamate-sensitive NSC34D motor neuron-like cells. Exp. Neurol. 2015, 271, 122–130. [Google Scholar] [CrossRef]
- Eggett, C.J.; Crosier, S.; Manning, P.; Cookson, M.R.; Menzies, F.M.; McNeil, C.J.; Shaw, P.J. Development and characterisation of a glutamate-sensitive motor neurone cell line. J. Neurochem. 2000, 74, 1895–1902. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Ostwal, P.; Sumitha, R.; Shruthi, S.; Varghese, A.M.; Mishra, P.; Manohari, S.G.; Sagar, B.C.; Sathyaprabha, T.N.; Nalini, A.; et al. Role of VEGF and VEGFR2 receptor in reversal of ALS-CSF induced degeneration of NSC-34 motor neuron cell line. Mol. Neurobiol. 2015, 51, 995–1007. [Google Scholar] [CrossRef]
- Madji Hounoum, B.; Vourc’h, P.; Felix, R.; Corcia, P.; Patin, F.; Guéguinou, M.; Potier-Cartereau, M.; Vandier, C.; Raoul, C.; Andres, C.R.; et al. NSC-34 motor neuron-like cells are unsuitable as experimental model for glutamate-mediated excitotoxicity. Front. Cell. Neurosci. 2016, 10, 1–12. [Google Scholar] [CrossRef]
- Miyagishi, H.; Kosuge, Y.; Yoneoka, Y.; Ozone, M.; Endo, M.; Osada, N.; Ishige, K.; Kusama-Eguchi, K.; Ito, Y. Prostaglandin E2-induced cell death is mediated by activation of EP2 receptors in motor neuron-like NSC-34 cells. J. Pharmacol. Sci. 2013, 121, 347–350. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nango, H.; Kosuge, Y.; Yoshimura, N.; Miyagishi, H.; Kanazawa, T.; Hashizaki, K.; Suzuki, T.; Ishige, K. The Molecular Mechanisms Underlying Prostaglandin D2-Induced Neuritogenesis in Motor Neuron-Like NSC-34 Cells. Cells 2020, 9, 934. https://doi.org/10.3390/cells9040934
Nango H, Kosuge Y, Yoshimura N, Miyagishi H, Kanazawa T, Hashizaki K, Suzuki T, Ishige K. The Molecular Mechanisms Underlying Prostaglandin D2-Induced Neuritogenesis in Motor Neuron-Like NSC-34 Cells. Cells. 2020; 9(4):934. https://doi.org/10.3390/cells9040934
Chicago/Turabian StyleNango, Hiroshi, Yasuhiro Kosuge, Nana Yoshimura, Hiroko Miyagishi, Takanori Kanazawa, Kaname Hashizaki, Toyofumi Suzuki, and Kumiko Ishige. 2020. "The Molecular Mechanisms Underlying Prostaglandin D2-Induced Neuritogenesis in Motor Neuron-Like NSC-34 Cells" Cells 9, no. 4: 934. https://doi.org/10.3390/cells9040934
APA StyleNango, H., Kosuge, Y., Yoshimura, N., Miyagishi, H., Kanazawa, T., Hashizaki, K., Suzuki, T., & Ishige, K. (2020). The Molecular Mechanisms Underlying Prostaglandin D2-Induced Neuritogenesis in Motor Neuron-Like NSC-34 Cells. Cells, 9(4), 934. https://doi.org/10.3390/cells9040934






