PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Culture Medium
2.3. Cell Culture Plate Coating
2.4. Evaluation of Cell Culture Plate Coatings
2.5. Length and Density of Neurites
2.6. Neurite Outgrowth (Spectrofluorimetric Evaluation)
2.7. Expression of Neuronal Biomarkers
2.8. Statistical Analysis
3. Results
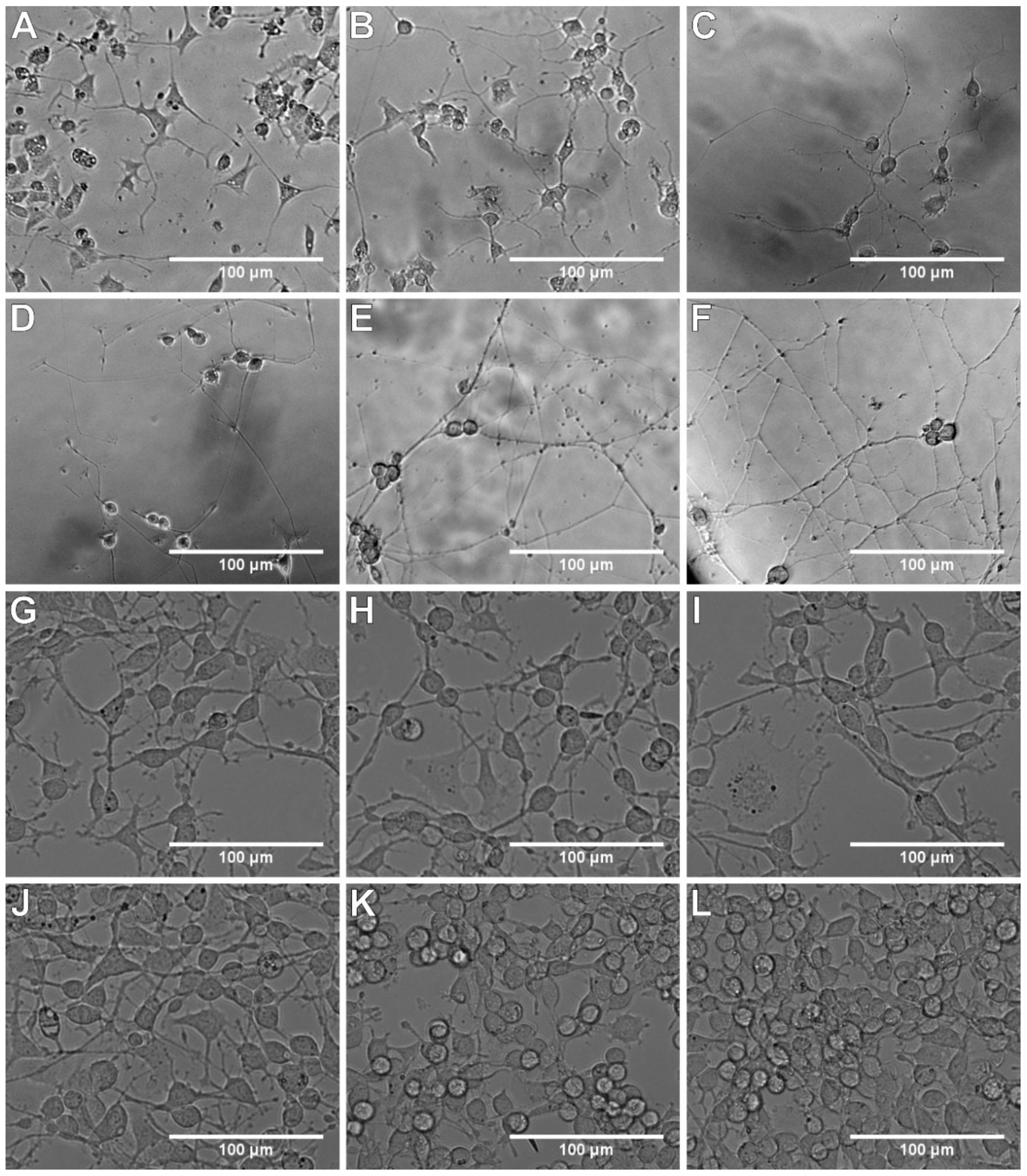
3.1. Evaluation of Cell Culture Plate Coatings
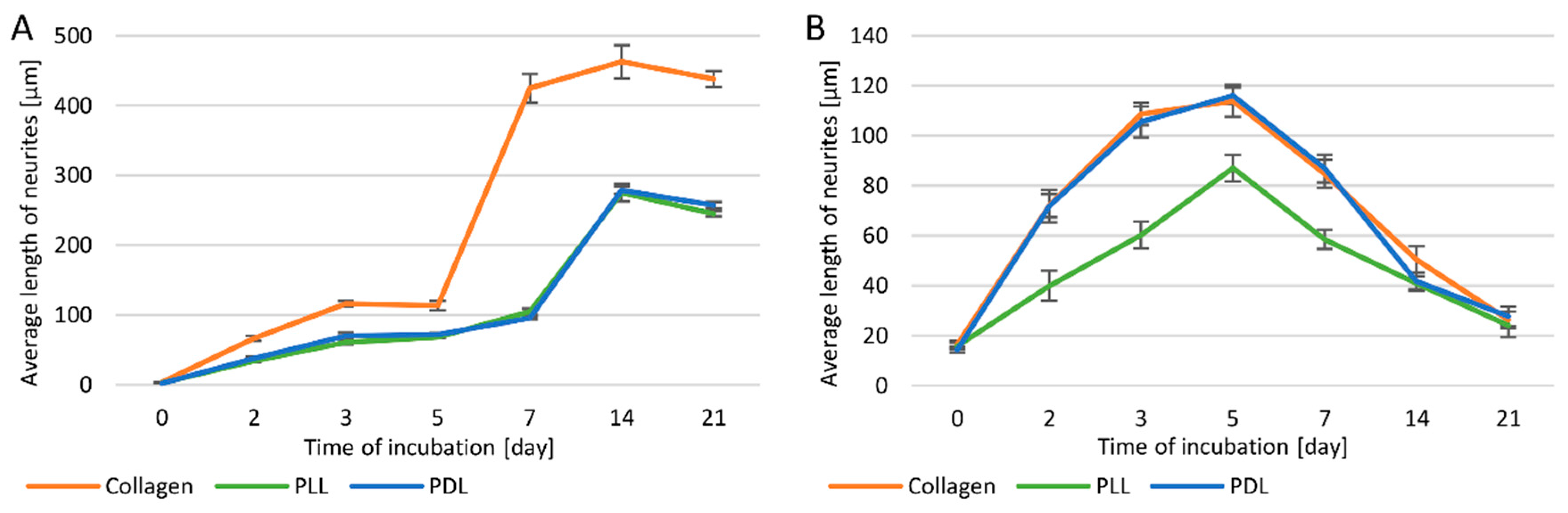
3.2. Length of Neurites
3.3. Neurite Density
3.4. Neurite Outgrowth (Spectrofluorimetric Evaluation)
3.5. Expression of Neuronal Biomarkers
3.6. Effect of NGF Origin and Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bal-Price, A.; Pistollato, F.; Sachana, M.; Bopp, S.K.; Munn, S.; Worth, A. Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods. Toxicol. Appl. Pharmacol. 2018, 354, 7–18. [Google Scholar] [CrossRef]
- Llorens, J.; Li, A.A.; Ceccatelli, S.; Suñol, C.; Cristina, S. Strategies and tools for preventing neurotoxicity: To test, to predict and how to do it. Neurotoxicology 2012, 33, 796–804. [Google Scholar] [CrossRef]
- Duan, X.-H.N.; Wang, W.-L.; Dai, R.; Yan, H.-W.; Han, C.-N.; Liu, L.-S. Current Situation of PC12 Cell Use in Neuronal Injury Study. Int. J. Biotechnol. Wellness Ind. 2015, 4, 61–66. [Google Scholar] [CrossRef]
- Westerink, R.H.S.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2007, 192, 273–285. [Google Scholar] [CrossRef]
- De Rios, C.L.; Cano-Abad, M.F.; Villarroya, M.; López, M.G. Chromaffin cells as a model to evaluate mechanisms of cell death and neuroprotective compounds. Pflugers Arch. 2017, 470, 187–198. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Maruta, R.; Takaki, K.; Kotani, E.; Kato, Y.; Yoshimura, R.; Endo, Y.; Whitty, C.; Pernstich, C.; Gandhi, R.; et al. Sustained Neurotrophin Release from Protein Nanoparticles Mediated by Matrix Metalloproteinases Induces the Alignment and Differentiation of Nerve Cells. Biomolecules 2019, 9, 510. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, J.; Zheng, W. Artemisinin protects PC12 cells against β-amyloid-induced apoptosis through activation of the ERK1/2 signaling pathway. Redox Biol. 2017, 12, 625–633. [Google Scholar] [CrossRef]
- Grau, C.M.; Greene, L.A. Use of PC12 Cells and Rat Superior Cervical Ganglion Sympathetic Neurons as Models for Neuroprotective Assays Relevant to Parkinson’s Disease. Breast Cancer 2012, 846, 201–211. [Google Scholar] [CrossRef]
- Zhang, W.; Benmohamed, R.; Arvanites, A.C.; Morimoto, R.I.; Ferrante, R.J.; Kirsch, N.R.; Silverman, R.B. Cyclohexane 1,3-diones and their inhibition of mutant SOD1-dependent protein aggregation and toxicity in PC12 cells. Bioorg. Med. Chem. 2011, 20, 1029–1045. [Google Scholar] [CrossRef]
- Hillion, J.A.; Takahashi, K.; Maric, D.; Ruetzler, C.; Barker, J.L.; Hallenbeck, J.M. Development of an Ischemic Tolerance Model in a PC12 Cell Line. Br. J. Pharmacol. 2005, 25, 154–162. [Google Scholar] [CrossRef]
- Das, K.P.; Freudenrich, T.M.; Mundy, W.R. Assessment of PC12 cell differentiation and neurite growth: A comparison of morphological and neurochemical measures. Neurotoxicol. Teratol. 2004, 26, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.J.; Zahid, S. The Role of Synapsins in Neurological Disorders. Neurosci. Bull. 2017, 34, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.S.; Dos Santos, N.A.G.; Bernardes, C.P.; Sisti, F.M.; Amaral, L.; Fontana, A.C.K.; Dos Santos, A.C. Caffeic Acid Phenethyl Ester (CAPE) Protects PC12 Cells from Cisplatin-Induced Neurotoxicity by Activating the NGF-Signaling Pathway. Neurotox. Res. 2017, 34, 32–46. [Google Scholar] [CrossRef]
- Wakulik, K.; Wiatrak, B.; Szczukowski, Ł.; Bodetko, D.; Szandruk-Bender, M.; Dobosz, A.; Świątek, P.; Gąsiorowski, K. Effect of Novel Pyrrolo[3,4-d]pyridazinone Derivatives on Lipopolysaccharide-Induced Neuroinflammation. Int. J. Mol. Sci. 2020, 21, 2575. [Google Scholar] [CrossRef]
- Udomruk, S.; Kaewmool, C.; Pothacharoen, P.; Phitak, T.; Kongtawelert, P. Sesamin suppresses LPS-induced microglial activation via regulation of TLR4 expression. J. Funct. Foods 2018, 49, 32–43. [Google Scholar] [CrossRef]
- Jeon, C.-Y.; Jin, J.-K.; Koh, Y.-H.; Chun, W.; Choi, I.-G.; Kown, H.-J.; Kim, Y.-S.; Park, J.-B. Neurites from PC12 cells are connected to each other by synapse-like structures. Synapse 2010, 64, 765–772. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, B.; Que, H.; Lin, Q.; Lv, S.; Liu, S. Neurons derived from PC12 cells have the potential to develop synapses with primary neurons from rat cortex. Acta Neurobiol. Exp. 2006, 66, 105–112. [Google Scholar]
- Biederer, T.; Scheiffele, P. Mixed-culture assays for analyzing neuronal synapse formation. Nat. Protoc. 2007, 2, 670–676. [Google Scholar] [CrossRef]
- Andreyeva, A.; Nieweg, K.; Horstmann, K.; Klapper, S.; Müller-Schiffmann, A.; Korth, C.; Gottmann, K. C-terminal fragment of N-cadherin accelerates synapse destabilization by amyloid-β. Brain 2012, 135, 2140–2154. [Google Scholar] [CrossRef]
- ATCC PC-12 Adh (ATCC CRL-1721.1). Available online: https://www.atcc.org/products/all/CRL-1721.1.aspx (accessed on 1 March 2020).
- ATCC PC-12 (ATCC CRL-1721). Available online: https://www.atcc.org/products/all/CRL-1721.aspx (accessed on 1 March 2020).
- Nimiya, Y.; Wang, W.; Du, Z.; Sukamtoh, E.; Zhu, J.; Decker, E.A.; Zhang, G. Front cover: Redox modulation of curcumin stability: Redox active antioxidants increase chemical stability of curcumin. Mol. Nutr. Food Res. 2016, 60, 487–494. [Google Scholar] [CrossRef]
- Heusinkveld, H.J.; Westerink, R.H. Comparison of different in vitro cell models for the assessment of pesticide-induced dopaminergic neurotoxicity. Toxicol. Vitr. 2017, 45, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Minase, T.; Ishima, T.; Itoh, K.; Hashimoto, K. Potentiation of nerve growth factor-induced neurite outgrowth by the ROCK inhibitor Y-27632: A possible role of IP3 receptors. Eur. J. Pharmacol. 2010, 648, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Cao, Q.; Sun, Z.; Chen, J.; Zheng, Q.; Xiao, F. A novel method of neural differentiation of PC12 cells by using Opti-MEM as a basic induction medium. Int. J. Mol. Med. 2017, 41, 195–201. [Google Scholar] [CrossRef]
- Chen, C.-C.; Bates, R.; Carlson, J. Effect of environmental and cultural conditions on medium pH and explant growth performance of Douglas-fir (Pseudotsuga menziesii) shoot cultures. F1000Research 2015, 3, 298. [Google Scholar] [CrossRef]
- Michl, J.; Park, K.C.; Swietach, P. Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun. Boil. 2019, 2, 144. [Google Scholar] [CrossRef]
- McKee, T.J.; Komarova, S.V. Is it time to reinvent basic cell culture medium? Am. J. Physiol. Physiol. 2017, 312, C624–C626. [Google Scholar] [CrossRef]
- Voorde, J.V.; Ackermann, T.; Pfetzer, N.; Sumpton, D.; Mackay, G.; Kalna, G.; Nixon, C.; Blyth, K.; Gottlieb, E.; Tardito, S. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci. Adv. 2019, 5, eaau7314. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Wandiyanto, J.V.; Linklater, D.; Perera, P.G.T.; Orlowska, A.; Truong, V.K.; Thissen, H.; Ghanaati, S.; Baulin, V.A.; Crawford, R.; Juodkazis, S.; et al. Pheochromocytoma (PC12) Cell Response on Mechanobactericidal Titanium Surfaces. Materials 2018, 11, 605. [Google Scholar] [CrossRef]
- Libério, M.S.; Sadowski, M.; Soekmadji, C.; Davis, R.A.; Nelson, C.C. Differential Effects of Tissue Culture Coating Substrates on Prostate Cancer Cell Adherence, Morphology and Behavior. PLoS ONE 2014, 9, e112122. [Google Scholar] [CrossRef]
- Orlowska, A.; Perera, P.G.T.; Al Kobaisi, M.; Dias, A.; Nguyen, H.K.D.; Ghanaati, S.; Baulin, V.A.; Crawford, R.; Ivanova, E.P. The Effect of Coatings and Nerve Growth Factor on Attachment and Differentiation of Pheochromocytoma Cells. Materials 2017, 11, 60. [Google Scholar] [CrossRef]
- Keshmirian, J.; Bray, G.; Carbonetto, S. The extracellular matrix modulates the response of PC12 cells to nerve growth factor: Cell aggregation versus neurite outgrowth on 3-dimensional laminin substrata. J. Neurocytol. 1989, 18, 491–504. [Google Scholar] [CrossRef]
- Tamplenizza, M.; Lenardi, C.; Maffioli, E.M.; Nonnis, S.; Negri, A.; Forti, S.; Sogne, E.; De Astis, S.; Matteoli, M.; Schulte, C.; et al. Nitric oxide synthase mediates PC12 differentiation induced by the surface topography of nanostructured TiO2. J. Nanobiotechnol. 2013, 11, 35. [Google Scholar] [CrossRef]
- Phan, C.-W.; Wong, W.-L.; David, P.; Naidu, M.; Sabaratnam, V. Pleurotus giganteus (Berk.) Karunarathna & K.D. Hyde: Nutritional value and in vitro neurite outgrowth activity in rat pheochromocytoma cells. BMC Complement. Altern. Med. 2012, 12, 102. [Google Scholar] [CrossRef]
- Mazia, D.; Schatten, G.; Sale, W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J. Cell Bol. 1975, 66, 198–200. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, M.; Zhang, N.; Yin, H.; Shu, B.; Duan, W. A pilot study on searching for peri-nuclear NeuN-positive cells. PeerJ 2020, 8, e8254. [Google Scholar] [CrossRef]
- Fujita, K.; Lazarovici, P.; Guroff, G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ. Health Perspect. 1989, 80, 127–142. [Google Scholar] [CrossRef]
- Kinarivala, N.; Shah, K.; Abbruscato, T.J.; Trippier, P.C. Passage Variation of PC12 Cells Results in Inconsistent Susceptibility to Externally Induced Apoptosis. ACS Chem. Neurosci. 2016, 8, 82–88. [Google Scholar] [CrossRef]
- Sakagami, H.; Hara, Y.; Shi, H.; Iwama, S.; Nakagawa, M.; Suzuki, H.; Tanaka, K.; Abe, T.; Tamura, N.; Takeshima, H.; et al. Change in Anticancer Drug Sensitivity During Neuronal Differentiation of PC12 Cells. Vivo 2018, 32, 765–770. [Google Scholar] [CrossRef]
- Lü, X.; Zhang, H.; Huang, Y.; Zhang, Y. A proteomics study to explore the role of adsorbed serum proteins for PC12 cell adhesion and growth on chitosan and collagen/chitosan surfaces. Regen. Biomater. 2018, 5, 261–273. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Mater. 2020, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Flier, L.; Carbonetto, S. Identification of a cell-surface protein involved in PC12 cell- substratum adhesion and neurite outgrowth on laminin and collagen. J. Neurosci. 1989, 9, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Convertino, D.; Luin, S.; Marchetti, L.; Coletti, C. Peripheral Neuron Survival and Outgrowth on Graphene. Front. Mol. Neurosci. 2018, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Fujii, D.; Massoglia, S.; Savion, N.; Gospodarowicz, D. Neurite outgrowth and protein synthesis by PC12 cells as a function of substratum and nerve growth factor. J. Neurosci. 1982, 2, 1157–1175. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Stachowiak, E.K.; Birkaya, B.; Terranova, C.; Capacchietti, M.; Claus, P.; Aletta, J.M.; Stachowiak, M.K. NGF-Induced Cell Differentiation and Gene Activation Is Mediated by Integrative Nuclear FGFR1 Signaling (INFS). PLoS ONE 2013, 8, e68931. [Google Scholar] [CrossRef]
- Geetha, T.; Rege, S.; Mathews, S.E.; Meakin, S.; White, M.F.; Babu, J.R. Nerve Growth Factor Receptor TrkA, a New Receptor in Insulin Signaling Pathway in PC12 Cells. J. Boil. Chem. 2013, 288, 23807–23813. [Google Scholar] [CrossRef]
- Sierra-Fonseca, J.A.; Najera, O.; Martinez-Jurado, J.; Walker, E.M.; Varela-Ramirez, A.; Khan, A.; Miranda, M.; Lamango, N.S.; Roychowdhury, S. Nerve growth factor induces neurite outgrowth of PC12 cells by promoting Gβγ-microtubule interaction. BMC Neurosci. 2014, 15, 132. [Google Scholar] [CrossRef]
- Brett, J.; Schmidt, A.M.; Du Yan, S.; Zou, Y.S.; Weidman, E.; Pinsky, D.; Nowygrod, R.; Neeper, M.; Przysiecki, C.; Shaw, A.; et al. Survey of the Distribution of a Newly Characterized Receptor for Advanced Glycation End Products in Tissues. Am. J. Pathol. 1993, 143, 1699–1712. [Google Scholar]
- Drubin, D.G.; Feinstein, S.C.; Shooter, E.M.; Kirscher, M.W. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubulo assembly-promoting factors. J. Cell Biol. 1985, 101, 1799–1807. [Google Scholar] [CrossRef]
- Shmueli, O.; Gdalyahu, A.; Sorokina, K.; Nevo, E.; Avivi, A.; Reiner, O. DCX in PC12 cells: CREB-mediated transcription and neurite outgrowth. Hum. Mol. Genet. 2001, 10, 1061–1070. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, Y.-P.; Hou, Z.; Huang, C.; Zhu, H.; Zhang, C.-Q.; Yin, Q. Novel Insights into NeuN: From Neuronal Marker to Splicing Regulator. Mol. Neurobiol. 2015, 53, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Greenamyre, J.T. NeuN is not a reliable marker of dopamine neurons in rat substantia nigra. Neurosci. Lett. 2009, 464, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Lee, T.-J.; Lim, J.M.; Lim, J.S.; Han, A.M.; Choi, C.Y.; Kwon, Y.H.K.; Kim, B.-S. The effect of the controlled release of nerve growth factor from collagen gel on the efficiency of neural cell culture. Biomaterials 2009, 30, 126–132. [Google Scholar] [CrossRef] [PubMed]
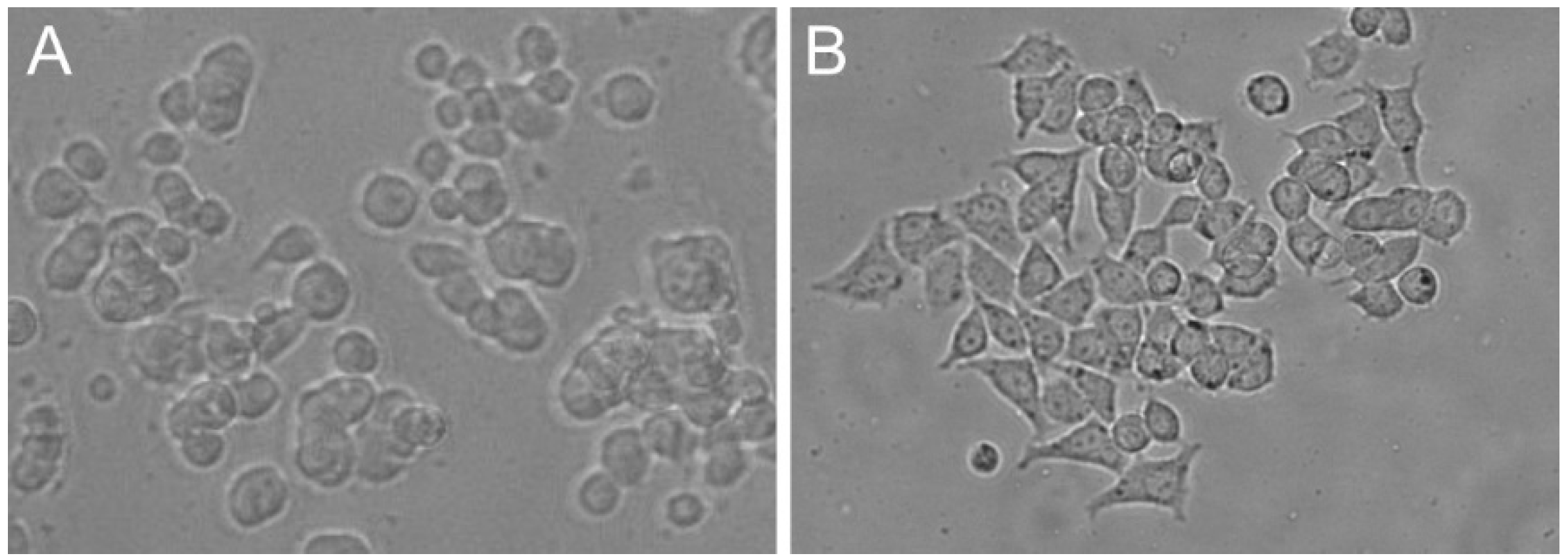





| Feature | PC12 Cell Line (ATCC CRL-1721) | PC12 Adh Cell Line (ATCC CRL-1721.1) |
|---|---|---|
| Cell type | Cluster of floating cells | Adherent cells |
| Morphology | Small and irregular shape | Polygonal shape |
| Culture medium | RPMI-1640 with 10% DHS, 5% FBS | Ham’s F-12K with 15% DHS, 2.5% FBS |
| Differentiation/neurite outgrowth | Differentiation with NGF or Opti-MEM with 0.5% FBS | Neurite outgrowth promoted by Rho kinase (ROCK) inhibition |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. https://doi.org/10.3390/cells9040958
Wiatrak B, Kubis-Kubiak A, Piwowar A, Barg E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells. 2020; 9(4):958. https://doi.org/10.3390/cells9040958
Chicago/Turabian StyleWiatrak, Benita, Adriana Kubis-Kubiak, Agnieszka Piwowar, and Ewa Barg. 2020. "PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions" Cells 9, no. 4: 958. https://doi.org/10.3390/cells9040958
APA StyleWiatrak, B., Kubis-Kubiak, A., Piwowar, A., & Barg, E. (2020). PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells, 9(4), 958. https://doi.org/10.3390/cells9040958






